Abstract
Tissue plasminogen activator (tPA) is a serine protease expressed in several brain regions and reported to be involved in the control of emotional and cognitive functions. Nevertheless, little is known about the structure-function relationships of these tPA-dependent behaviors. Here, by using a new model of constitutive tPA-deficient mice (tPAnull), we first show that tPA controls locomotor activity, spatial cognition and anxiety. To investigate the brain structures involved in these tPA-dependent behavioral phenotypes, we next generated tPAflox mice allowing conditional tPA deletion (cKO) following stereotaxic injections of adeno-associated virus driving Cre-recombinase expression (AAV-Cre-GFP). We demonstrate that tPA removal in the dentate gyrus of the hippocampus induces hyperactivity and partial spatial memory deficits. Moreover, the deletion of tPA in the central nucleus of the amygdala, but not in the basolateral nucleus, induces hyperactivity and reduced anxiety-like level. Importantly, we prove that these behaviors depend on the tPA present in the adult brain and not on neurodevelopmental disorders. Also, interestingly, our data show that tPA from Protein kinase-C delta-positive (PKCδ) GABAergic interneurons of the lateral/ capsular part of adult mouse central amygdala controls emotional functions through neuronal activation of the medial central amygdala. Together, our study brings new data about the critical central role of tPA in behavioral modulations in adult mice.
Similar content being viewed by others
Introduction
In humans, emotional and cognitive impairments are symptoms of several psychiatric diseases, exacerbated by concomitant events such as stress, aging and/or chronic diseases, leading to a dramatic deterioration in the quality of life [1]. The precise molecular and cellular mechanisms that underlie these abnormal behaviors are still not well understood, and deserve further works aimed at developing accurate targeted therapies.
The tissue-type plasminogen activator (tPA) is a pleiotropic serine protease initially characterized for its ability to activate plasminogen into plasmin and thus to promote vascular and tissue fibrinolysis [2, 3]. In the central nervous system, tPA is expressed in many brain areas including the hippocampus, the entorhinal cortex and the amygdala [4,5,6,7,8] and reported to influence physiological mechanisms such as synaptic plasticity [9,10,11,12]. Thus, tPA contributes, either directly or indirectly, to the modulation of some learning and memory processes [8, 10, 13,14,15,16,17] as well as emotional responses [4, 18,19,20,21], and locomotor activity [22, 23]. tPA-dependent effects on synaptic plasticity can be attributed to the modulation of N-Methyl D-Aspartate receptor, the type I Low Density Lipoprotein Related Receptor (LRP-1) signaling or to the plasmin-dependent conversion of the pro-Brain Derived Neurotrophic Factor (pro-BDNF) into its mature form [8,9,10, 12, 15, 24, 25]. Up to now, all the studies aiming at investigating the role of tPA in behaviors were performed from constitutive tPA-deficient mice, without information about whether these impairments could be linked to developmental alterations or not. Similarly, structure-function respective relationships in these tPA-dependent functions were indirectly determined in the majority of cases. In addition, all the data today available were obtained from C57BL/6J tPA-deficient mice harboring the 129-derived chromosomal segment that co-segregates with the targeted tPA (Plat) allele, leading to a differential expression of a set of genes which could interfere with tPA’s functions [26].
In the present study, we have first characterized the behavioral phenotype of a novel tPA-deficient mouse strain (named tPAnull mice) generated by a unique deletion of the exon-3 of the Plat gene, to avoid possible off-target effects. Then, the region-specific tPA’s deletion was induced through stereotaxic delivery of AAV-Cre-GFP either in the dentate gyrus of the hippocampus (DG), the amygdala (basolateral amygdala; BLA and central amygdala; CeA) or the prefrontal cortex (PFC) of adult tPAflox mice (flanking exon-3 of the Plat gene), also newly generated by our group. Using these new experimental models, we first found that the conditional tPA deletion in the dentate gyrus of adult mice leads to hyperactivity and mild cognitive deficits. Then, we revealed that deletion of the tPA contained in PKCδ-positive GABAergic neurons of the adult CeA promotes hyperactivity as well as reduced anxiety-like behavior. Finally, we demonstrate that tPA expressed by PKCδ interneurons of the lateral/ capsular part (CeL/C) of the CeA modulates neuronal activity within the central medial amygdala (CeM).
Materials and methods
Animals
Experiments were performed on 8 weeks old males tPAnull mice, tPAflox mice and their wild-type (WT) littermates (20–25 g; Animal care facilities: Centre Universitaire de Ressources Biologiques, Normandy University, Caen, France; Approval #A14118015). Animals were housed with a 12-h light/12-h dark cycle in standard polypropylene cages (22 × 37 × 19 cm, Charles River, L’Arbresle, France; 5 mice per cage). All experiments were conducted in accordance with the French ethical law (Decree 2013-118; Approval #10959) and the European Community Council guidelines (2010/63/EU).
Generation of transgenic mice
tPAflox mice (C57BL/6NTac background) in which exon-3 of Plat gene is flanked by loxP sites was generated as previously described (see [27]). tPAnull mice in which exon-3 of Plat gene is constitutively excised through the Cre-lox system were generated by the Mouse Clinical Institute (ICS, Illkrich, France) by crossing tPAflox mice with the ubiquitous Cre-expressing mouse line, Rosa26Cre [28].
Mice were genotyped by PCR analysis and southern blots, using tail genomic DNA samples, for the presence of loxP sequences and the presence of the third exon.
Behavioral assessment
Behavioral tests were performed 3 weeks after the AAV injections in the 11–13 week old mice.
Open-field test
The open-field test (OF) was used to assess general locomotor activity and anxiety-like behavior as previously described [29, 30].
Barnes maze test
The Barnes maze test (BM) was used to assess long-term reference memory [31, 32].
c-Fos analysis
For c-Fos quantification, the number of c-Fos positive cells was counted manually in five independent animals for which the AAV injections were validated by the GFP expression in the CeA (Supplementary Fig. 1) using ImageJ software. Three to four slices per mouse, spanning the CeL/C, the CeM and the bed nucleus of the stria terminalis (BNST) in each of the two hemispheres were used for quantifications.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (version 8.0). Analysis of the datasets via parametric approaches turned out to be inappropriate in some cases due to violation of normality (Shapiro–Wilk tests). Therefore, parametric or non-parametric approaches were used whenever appropriated.
Results
Constitutive tPAnull mice display hyperactivity, reduced anxiety, and impaired spatial memory
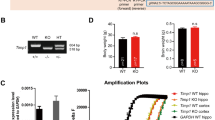
To avoid possible off-target effects of tPA knockout strategies [26], we used here a new strain of tPA-deficient mouse (tPAnull; Fig. 1a, b) subjected to a battery of behavioral tests (n = 20 per group).
a, b Generation of tPAnull mice and the corresponding tPAflox mice. c–e tPAnull mice display locomotor hyperactivity in the open-field test. Intergroup comparisons of the velocity (c) and distance (d); Mann–Whitney U tests, *p < 0.05, **p < 0.01. Averaged heat maps and representative trajectories of tPAnull and WT mice (e). f–i tPAnull mice show decreased anxious responses in the open-field test. Intergroup comparisons of entries (f), time (g) and distance in the center (h), or peripheral (i), area; Mann–Whitney U tests, *p < 0.05, **p < 0.01. j–l tPAnull mice display impaired cognition in the Barnes maze test. Intergroup comparisons of the number of errors (j); Student’s t test, **p < 0.01. Intragroup comparisons of the time spent in areas (k); Wilcoxon test, #p < 0.05. Comparison with chance level (5.5 %), Wilcoxon signed-rank test, $p < 0.05. Averaged heat maps of tPAnull (n = 10) and WT mice (n = 10) from the same training group (l). n = 20 per genotype. Data are presented as mean ± SD.
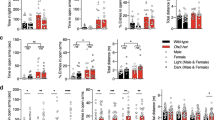
The OF was first used to measure general locomotion (Fig. 1c–e) and anxiety-related behavior (Fig. 1f–i). Mutant mice displayed significant higher velocity (Fig. 1c, +17.5%, p < 0.01) and distance traveled (Fig. 1d, +17.5%, p < 0.05) than WT animals. Moreover, mutant mice showed an increase in number of entries (Fig. 1f, +31.65%, p < 0.01), time spent (Fig. 1g, +20%, p < 0.05) and distance traveled (Fig. 1h, +12.8%, p < 0.05) in the anxiogenic center area of the apparatus compared to controls. Note that the locomotor behavior cannot explain the observed results as the total distance was similar for both groups in periphery (Fig. 1i, p > 0.05). Thus, data from OF indicate hyperactivity and reduced anxiety in tPAnull mice. We then used the BM to study possible spatial cognition deficits due to the lack of tPA. During the training session, both groups showed good acquisition performance in spite of the greater traveled distance exhibited by mutant mice (Supplementary Fig. 2a, p < 0.001). Interestingly, tPAnull mice did not succeed in the probe test as they made more errors to find the target area than control mice (Fig. 1j, +89%; p < 0.01). Furthermore, they spent similar time in the target area compared to others (Fig. 1k, l, p > 0.05; Supplementary Fig. 3a’, p > 0.05) unlike control mice that explored more time the target than any other areas (Fig. 1k, l, p < 0.05; Supplementary Fig. 3a, p < 0.001). Consistent with these results, the time spent in the target area was significantly higher than chance level in WT mice (Fig. 1k, l; p < 0.05) contrary to tPAnull mice (Fig. 1k, l; p > 0.05). These data show that the tPA deficiency leads to an impairment of long-term spatial memory.
Altogether, we reveal here that tPA is involved in a range of behavioral functions, including locomotor activity, anxiety and spatial memory.
Region-specific deletion of tPA reproduces a subset of phenotypes observed in tPAnull mice
To characterize the respective structure-function relationships in the tPA-dependent behaviors characterized above, we induced the deletion of the target protein in several brain areas (DG, BLA, CeA, PFC; Supplementary Fig. 4) of adult mice.
First, AAV-Cre-GFP was bilaterally injected in the dentate gyrus of newly generated tPAflox mice (Fig. 2a) for a conditional deletion of tPA in the hippocampal mossy fibers (cKO-DG, n = 19). The same AAV construct was injected in WT littermate mice (Control, n = 20). The efficacy of the recombination was confirmed by immunohistochemistry for GFP and tPA to unmask the specific depletion of endogenous tPA in the hippocampal mossy fibers (Fig. 2b). These data were confirmed by fibrin-agar zymography showing reduced levels of active tPA in hippocampal homogenates of the injected tPAflox mice compared to the injected tPAWT animals (Fig. 2c, n = 4 per group, p < 0.05). As performed for the tPAnull mice (Fig. 1), the same set of behavioral tests was conducted with the cKO-DG and control mice three weeks post-AAV-Cre-GFP injections. In the OF, both the velocity and the distance traveled in the arena were significantly increased in cKO-DG mice compared to controls (Fig. 2d–f; velocity: +12.7%, p < 0.05; distance traveled: +17%, p < 0.05). This locomotor hyperactivity of conditional mice was particularly seen in the periphery (Fig. 2j, p < 0.01). By contrast, the anxiety-like behavior was similar in the two groups with the same number of entries, time spent and distance traveled in the center area (Fig. 2g–i, p > 0.05). In the cognitive task, both groups displayed an equal number of errors to discriminate the target box (Fig. 2k, p > 0.05; Supplementary Fig. 3). However, cKO-DG mice performance was equivalent to random exploration (Fig. 2l, p > 0.05; compared with chance level) unlike that of the control mice which was significantly greater than chance level (Fig. 2l, $$$: p < 0.001, compared with chance level). Altogether, these data demonstrate that tPA present in the hippocampus of adult mice is involved in the modulation of locomotor activity and partly in spatial memory.
a Bilateral stereotaxic injections of AAV-Cre-GFP in the dentate gyrus of WT and tPAflox mice. b Immunostaining for GFP (green), tPA (red) and DAPI (blue); Coronal sections; White scale bar: 500 µm (High magnification scale bar: 100 µm). c Fibrin-agar zymography showing tPA activity in the hippocampus of Control and cKO-DG mice; Mann–Whitney U test, *p < 0.05, n = 4 per group. d–f cKO-DG mice show locomotor hyperactivity in the open-field test. Intergroup comparisons of the velocity (d) and distance (e); Mann–Whitney U tests, *p < 0.05. Averaged heat maps and representative trajectories of Control and cKO-DG mice (f). g–j tPA deletion in the hippocampus does not affect anxious responses in the open-field test. Intergroup comparisons of entries (g), time (h) and distance in the center (i), or peripheral (j), area; Mann–Whitney U test, **p < 0.01. k–m cKO-DG mice display moderate spatial deficits in the Barnes maze test. Intergroup comparisons of the number of errors (k); Student’s t test, p > 0.05. Intragroup comparisons of the time spent in areas (l); Wilcoxon test, #p < 0.05, ###p < 0.001. Comparison with chance level (5.5 %), Wilcoxon signed-rank test, $$$p < 0.001. Averaged heat maps of Control (n = 6) and cKO-DG (n = 6) mice from the same training group (m). Control mice: n = 19, cKO-DG mice, n = 20. Data are presented as mean ± SD.
The targeted suppression of tPA in the PFC, which is known to modulate decision making and anxiety responses [33,34,35,36], did not affect locomotor activity, anxiety-like behaviors or spatial cognition (Supplementary Fig. 5).
Then, AAV-Cre-GFP was bilaterally injected in the CeA of the tPAflox mice for a conditional deletion of tPA (Fig. 3a; cKO-CeA, n = 19). The same AAV construct was injected in the WT littermate mice (Control, n = 20). The efficacy of the recombination was confirmed as above (Fig. 3b, c, n = 4 per group, p < 0.05). In the OF, cKO-CeA mice displayed a hyperactive phenotype (Fig. 3d–f; velocity: +9.4%, p < 0.05; distance traveled: +9.4%, p < 0.05). A reduced anxiety-like behavior was also observed in conditional mutant mice compared to controls (Fig. 3g–j; entries to center area: +31.5%, p < 0.05; time in the center area: +35.5%, p < 0.05; distance traveled in the center area: +43.2%, p < 0.01; distance traveled in periphery p > 0.05). In the BM, the conditional knockout mice succeeded in the task since spatial performance was similar in both groups (Fig. 3k–m).
a Bilateral stereotaxic injections of AAV-Cre-GFP in the central amygdala of WT and tPAflox mice. b Immunostaining for GFP (green) and DAPI (blue); Coronal sections; White scale bar: 500 µm. c Fibrin-agar zymography showing tPA activity in the central amygdala of Control and cKO-CeA mice; Mann–Whitney U test, *p < 0.05, n = 4 per group. d–f cKO-CeA mice show locomotor hyperactivity in the open-field test. Intergroup comparisons of the velocity (d) and distance (e); Student’s t tests, *p < 0.05. Averaged heat maps and representative trajectories of Control and cKO-CeA mice (f). g–j tPA deletion in the central amygdala induces decreased anxious responses in the open-field test. Intergroup comparisons of entries (g), time (h) and distance in the center (i), or peripheral (j), area; Student’s t tests, *p < 0.05, **p < 0.01. k–m cKO-CeA mice display similar cognition performance to controls in the Barnes maze test. Intergroup comparisons of the number of errors (k); Mann–Whitney U test, p > 0.05. Intragroup comparisons of the time spent in areas (l); Wilcoxon test, ##p < 0.01, ###p < 0.001. Comparison with chance level (5.5%), Wilcoxon signed-rank test, $$p < 0.01, $$$p < 0.001. Averaged heat maps of Control (n = 8) and cKO-CeA (n = 8) mice from the same training group (m). Control mice: n = 20, cKO-CeA mice, n = 19. Data are presented as mean ± SD.
In a second time, parallel bilateral injections of AAV-Cre-GFP were performed in the basolateral part of the amygdala (BLA) of tPAflox mice and their WT littermates (Fig. 4a; cKO-BLA: n = 18; Control: n = 18). The site of injection and efficacy of the recombination was confirmed as above (Fig. 4b, c, n = 4 per group, p < 0.05). In the OF, the locomotor activities were equal in cKO-BLA and control mice (Fig. 4d–f; p > 0.05). Likewise, the anxious responses were similar for the two groups (Fig. 4g–j; p > 0.05). The BM test revealed that both groups displayed analogous performance (Fig. 4k–m). Altogether, these data reveal that the tPA present in the CeA of adult mice, but not in the BLA, is involved in the control of anxiety-like behavior, with tPA promoting anxiety. Furthermore, we demonstrate that central amygdalar tPA modulates locomotor activity.
a Bilateral stereotaxic injections of AAV-Cre-GFP in the basolateral amygdala of WT and tPAflox mice. b Immunostaining for GFP (green) and DAPI (blue); Coronal sections; White scale bar: 500 µm. c Fibrin-agar zymography showing tPA activity in the basolateral amygdala of Control and cKO-BLA mice; Mann–Whitney U test, *p < 0.05, n = 4 per group. d–f Deletion of tPA in the basolateral amygdala does not affect locomotor activity in the open-field test. Intergroup comparisons of the velocity (d) and distance (e); Mann–Whitney U tests, p > 0.05. Averaged heat maps and representative trajectories of Control and cKO-BLA mice (f). g–j tPA deletion in basolateral amygdala does not affect anxious phenotype in the open-field test. Intergroup comparisons of entries (g), time (h) and distance in the center (i), or peripheral (j), area; Mann–Whitney U tests, p > 0.05. k–m cKO-BLA mice display similar cognition performance to controls in the Barnes maze test. Intergroup comparisons of the number of errors (k); Mann–Whitney U test, p > 0.05. Intragroup comparisons of the time spent in areas (l); Wilcoxon test, ##p < 0.01, ###p < 0.001. Comparison with chance level (5.5%), Wilcoxon signed-rank test, $$p < 0.01. Averaged heat maps of Control (n = 10) and cKO-BLA (n = 10) mice from the same training group (m). n = 18 for both genotypes. Data are presented as mean ± SD.
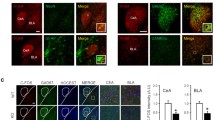
In order to determine which cell types of the CeA are involved in the functions of tPA described above, immunohistochemistry was performed from coronal brain sections of WT mice using a set of neuronal markers (protein kinase-C delta: PKCδ; somatostatin: SOM; vesicular GABA transporter: VGAT; vesicular glutamate transporter 2: VGLUT2) combined with tPA immunostaining. Our data revealed positive neuronal fibers for tPA colocalized with VGAT but not with VGLUT2 (Fig. 5a). Co-immunostaining of these tPA-positive GABAergic interneurons revealed that they were also positive for the PKCδ in the central lateral/capsular part (CeL/C), but not for SOM (Fig. 5b, c; p = 0.05). The expression of tPA mRNA (Plat) was also confirmed by fluorescent in situ hybridization which shows that Plat mRNA are abundantly present in PKCδ neurons and in a lesser extent in SOM neurons (Fig. 5d, e; p < 0.001). In order to assess the effect of tPA expressed by CeA’s PKCδ interneurons on neuronal activation, we then investigated c-Fos expression in cKO-CeA and control mice. Quantifications were performed in the CeL/C (where PKCδ interneurons are found). Therefore c-Fos measurements were also performed in the central medial part (CeM) of CeA, and in the BNST which were previously described as output of CeA PKCδ neurons [37, 38]. We found a selective decrease of c-Fos-positive cells in the CeM of cKO-CeA mice compared to controls (Fig. 5f; p < 0.05). In contrast, tPA deletion in the CeA had no significant effect in the CeL/C nor in the BNST (Fig. 5f; p > 0.05). Altogether, these data show that the tPA expressed in CeL/C PKCδ neurons influences neuronal activation of the CeM.
a Immunostaining for tPA (red), vGAT (cyan) and vGLUT2 (green) in the CeA of WT mice reveal the colocalization of tPA and vGAT interneurons. Arrows indicates the colocalization of tPA and vGAT along a tPA-positive neurite. White scale bar: 50 µm. b Immunostaining for tPA (red), PKCδ (cyan) and somatostatin (SOM; green) interneurons in the central amygdala lateral/capsular part (CeL/C) of WT mice reveal the colocalization of tPA and PKCδ interneurons. Arrows indicates the colocalization tPA ans PKCδ staining along the along a tPA-positive neurite. White scale bar: 50 µm. c Quantification of the percentage of tPA colocalized volume over PKCδ or SOM immunostainings in the CeA (n = 3 coronal slices from 3 WT mice; unpaired t-test; p = 0.05). d Quantification of the number of tPA mRNA (Plat) dots per cell showing that Plat mRNA are abundantly present in PKCδ neurons and in a lesser extent in SOM neurons in the CeA (n = 85 PKC δ-positive neurons; n = 37 SOM-positive neurons; Mann–Whitney U tests, p < 0.001; data from two coronal slices). e Representative images of Plat mRNA (red dots) colocalization with PKCδ positive neurons (cyan) and SOM (green) positive neurons in the CeA. f Quantification of c-Fos-positive cells in the lateral/capsular (CeL/C) and the medial (CeM) parts of the CeA, and in the dorsal part of the bed nucleus of the stria terminalis (BNST) of Control and cKO-CeA mice; Mann–Whitney U tests, *p < 0.05, n = 5 for both genotypes. Data are presented as mean ± SD.
Discussion
In the present study, we reveal that tPA of PKCδ-positive GABAergic interneurons of the CeL/C in adult mouse is involved in the control of emotional functions through neuronal activation of CeM.
The current literature investigating roles of tPA, including its brain functions, were obtained using C57BL/6J tPA-deficient mice harboring the 129-derived chromosomal segment that co-segregates with the targeted Plat allele [26]. In this model, some others genes than tPA are altered (Arhgef18, Mcf2l and Mcph1) which can lead to confusion in the data interpretation [39, 40]. The tPAnull mouse strain generated by our group and used in the present study is the consequence of a specific deletion of the exon-3 of the Plat gene, with no possibly of off-target effects. Our behavioral experiments confirm most of the previous reports about the functional roles of tPA such as its involvement in spatial memory processes [8, 10, 15, 41] or locomotor activity [23]. Importantly, our study is the first to show that tPA deficiency also leads to decreased anxiety-like responses under basal conditions. Nevertheless, as the phenotypes found in tPAnull mice could be linked to neurodevelopmental disorders [27, 42, 43], we then completed our work by generating a novel model allowing conditional deletion of tPA at adult stages.
Using this new molecular tool, the tPAflox mice targeting the exon-3 of the Plat gene, we demonstrate tPA-dependent structure-function respective relationships. We thus evidence that tPA of the dentate gyrus, in adult mice, is involved in the regulation of locomotor activity and partly in spatial cognition. Furthermore, we show that tPA expressed in CeA of adult mice controls both locomotor activity and anxiety-like behaviors.
It was previously reported that tPA displays functions in learning and memory processes [8, 10, 13,14,15,16,17, 41]. For example, overexpression of tPA in the central nervous system has been associated with improved spatial performance when using both Morris Water Maze (MWM) and Homing hole board tests [13]. Accordingly, tPA deficiency has been shown to induce spatial deficits in both MWM and T-maze [8, 41], as well as alterations in responsiveness to a spatial change during an object placement test [10]. However, other studies using tPA-deficient mice did not report a particular phenotype when using MWM or BM [9, 44]. Regarding tPA and locomotor activity, data in literature are again contradictory. Indeed, Pothakos and collaborators reported a locomotor hyperactivity in tPA-deficient mice while Calabresi and collaborators did not [10, 23]. One notes that these studies were led on constitutive tPA-deficient mice, with distinct behavioral experimental conditions. In this study, we highlight for the first time that the tPA in the adult dentate gyrus plays a role in the modulation of locomotor activity. Since CA3 neurons are the major input of dentate gyrus granular cells, immunohistochemical analysis should be performed in a future study in order to assess whether tPA released from mossy fibers may modulate the activation of CA3 neurons and downstream locomotor effects. We also reveal moderate cognitive deficits in the BM following tPA removal in the dentate gyrus, suggesting that tPA synthesized in other brain areas could influence spatial processing. Indeed, tPA expression was also reported in CA1 and CA3 regions of the hippocampus and in parahippocampal regions such as the entorhinal cortex which are also involved spatial processing [7, 8, 45, 46]. In a previous work, we have showed that tPA plays a key role in entorhinal cortex-dependent processing of spatial information in a gender specific manner [8]. Our present study was only conducted in males to avoid heterogeneity related to the estrous cycle. Thus, we cannot exclude that the behavioral responses observed here could be differentially affected in tPAnull female mice.
tPA activity in the amygdala is critical for both stress-induced synaptic plasticity and anxiety-like behavior [4, 18, 47]. Accordingly, tPA-deficient mice exhibit reduced anxiety-like behavior under stress conditions [4, 18]. Our present data thus provide new insights about tPA and anxiety, demonstrating that conditional deletion of tPA in the adult amygdala central nucleus leads to reduced anxiety-related behavior, regardless of any stressor. It is important to note that CeA plays a key role in the integration of emotional sensory information coming from the BLA, and can modulate emotional responses through intrinsic circuit within the CeA and projections to different brain areas such as the BNST [48,49,50,51,52]. The CeA consists of CeL, CeC and CeM. One of the peculiarities of CeL and CeM neurons is that they synthesize GABA. Moreover, PKCδ-positive interneurons is one of the major neuronal subtype found in the CeL/C with SOM-positive interneurons [51, 53]. These two populations, largely non-overlapping, display distinct firing patterns and connectivity [38, 51, 54]. Indeed, PKCδ and SOM interneurons can form a local and reciprocal inhibitory circuit within the CeL while PKCδ neurons can also inhibit CeM neurons and send long range projections to the BNST [37, 38, 51, 54, 55]. Optogenetic approaches already reported that CeL PKCδ neurons modulate anxiety-like behaviors, with their activation that lead to differential responses depending on the experimental design [37, 56]. In addition, a recent study demonstrates that CeL PKCδ neuronal activity is necessary to induce the anxiolytic effects of benzodiazepines [57]. Here, we evidence that tPA is expressed by PKCδ-positive GABAergic interneurons in the CeA. Our data also reveal that tPA depletion in the CeA induces a decrease of c-Fos positive cells in the CeM demonstrating that tPA expressed by PKCδ interneurons in the CeL could influence CeM activation. In neurons, tPA is transported along dendrites and axons and can be released at the synapse in a constitutive or activity-dependent manner [58, 59]. tPA activity is increased in limbic regions such as the CeA, the medial amygdala (MeA) and the BNST under stress conditions [4, 18, 60]. Based on our present results, we can hypothesize that the tPA released from CeL PKCδ neurons influences anxiety responses with projections to the CeM. This result suggests that tPA expressed by PKCδ interneurons projecting on CeM neurons may induce a process of disinhibition by positively-modulating neurons of the CeM. However, we did not identify the exact molecular mechanism by which tPA can influence CeM neurons activation. In the brain, tPA is susceptible to have neuromodulatory effects through multiple pathways, including modulations of NMDA receptors or LRP receptors signaling [9, 25, 61], control of synaptic plasticity and behavioral responses through a plasmin-dependent conversion of pro-BDNF into its mature form [17, 24]. Interestingly, calcium imaging recently revealed that the anxiolytic effect of benzodiazepine was linked to a decreased activity of CeM+ neurons without effect on PKCδ+ neurons [57]. Since tPA can modulate NMDA-dependent calcium influx [12, 61], we can speculate that tPA deletion in PKCδ+ could reduce post-synaptic activation of CeM by decreasing NMDA receptor signaling. Further investigations will be necessary to assess whether tPA-dependent modulation of such pathways could affect central amygdala activation and emotional responses. Our study also shows that tPA deficiency in the CeA increases locomotor activity. Accordingly, a previous study revealed that CeL PKCδ neuronal activity can modulate locomotor activity [56]. In addition, targeting monosynaptic connections between the CeA and the mesencephalic locomotor region was reported to regulate spontaneous locomotion [62]. Further investigations will be needed to describe the projections of tPA-expressing PKCδ interneurons.
In our study we cannot exclude that other subpopulations of interneurons, such as those expressing neuropeptide markers such as neuropeptide Y, corticotropin-releasing factor (CRF) or neurotensin found in the CeA, could synthesize tPA [53].
Indeed, in the amygdala, tPA was shown to promote the effect of CRF of neuronal activation and anxiety-related responses [18]. Given that reciprocal inhibition may occur between interneurons of the CeA, we can hypothesize that tPA may differentially influence neuronal activity in the CeA depending on its expression by CRF-positive interneurons or other interneuron subpopulations. Similarly, tPA detection in the CeA could also originate from projecting axons coming from other brain areas such as the BLA or the BNST, two brain areas which are well-known inputs of the CeA [38, 49, 50] and reported to express tPA [7, 60].
In conclusion, we demonstrate that tPA deficiency (tPAnull mice) leads to hyperactivity, spatial memory deficits and reduced anxious responses. Using a corresponding conditional knockout model allowing structural brain-specific deletion of tPA in the adult mice, we reveal that 1/ selective loss of tPA in the hippocampus leads to hyperactivity and moderate spatial memory deficits, 2/ tPA deficiency in the CeA induces both hyperactivity and anxiolytic-like phenotype. In addition, we unveil that the expression of tPA in PKCδ-positive GABAergic interneurons of the CeA is associated to tPA-dependent emotional behaviors involving the activation of neurons within the CeM.
References
Beaudreau SA, O’Hara R. Late-life anxiety and cognitive impairment: a review. Am J Geriatr Psychiatry. 2008;16:790–803.
Collen D, Lijnen HR. Basic and clinical aspects of fibrinolysis and thrombolysis. Blood. 1991;78:3114–24.
Akassoglou K, Kombrinck KW, Degen JL, Strickland S. Tissue plasminogen activator-mediated fibrinolysis protects against axonal degeneration and demyelination after sciatic nerve injury. J Cell Biol. 2000;149:1157–66.
Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci. 2003;6:168–74.
Louessard M, Lacroix A, Martineau M, Mondielli G, Montagne A, Lesept F, et al. Tissue plasminogen activator expression is restricted to subsets of excitatory pyramidal glutamatergic neurons. Mol Neurobiol. 2016;53:5000–12.
Teesalu T, Kulla A, Asser T, Koskiniemi M, Vaheri A. Tissue plasminogen activator as a key effector in neurobiology and neuropathology. Biochem Soc Trans. 2002;30:183–9.
Stevenson TK, Lawrence DA. Characterization of tissue plasminogen activator expression and trafficking in the adult murine brain. ENeuro. 2018;5:1–18.
Hébert M, Anfray A, Chevilley A, Martinez de Lizarrondo S, Quenault A, Louessard M, et al. Distant space processing is controlled by tPA-dependent NMDA receptor signaling in the entorhinal cortex. Cereb Cortex. 2017;27:4783–96.
Huang YY, Bach ME, Lipp HP, Zhuo M, Wolfer DP, Hawkins RD, et al. Mice lacking the gene encoding tissue-type plasminogen activator show a selective interference with late-phase long-term potentiation in both Schaffer collateral and mossy fiber pathways. Proc Natl Acad Sci USA. 1996;93:8699–704.
Calabresi P, Napolitano M, Centonze D, Marfia GA, Gubellini P, Teule MA, et al. Tissue plasminogen activator controls multiple forms of synaptic plasticity and memory. Eur J Neurosci. 2000;12:1002–12.
Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004;3:407–30.
Parcq J, Bertrand T, Montagne A, Baron AF, MacRez R, Billard JM, et al. Unveiling an exceptional zymogen: the single-chain form of tPA is a selective activator of NMDA receptor-dependent signaling and neurotoxicity. Cell Death Differ. 2012;19:1983–91.
Madani R, Hulo S, Toni N, Madani H, Steimer T, Muller D, et al. Enhanced hippocampal long-term potentiation and learning by increased neuronal expression of tissue-type plasminogen activator in transgenic mice. EMBO J. 1999;18:3007–12.
Pawlak R, Nagai N, Urano T, Napiorkowska-Pawlak D, Ihara H, Takada Y, et al. Rapid, specific and active site-catalyzed effect of tissue-plasminogen activator on hippocampus-dependent learning in mice. Neuroscience. 2002;113:995–1001.
Benchenane K, Castel H, Boulouard M, Bluthé R, Fernandez-Monreal M, Roussel BD, et al. Anti-NR1 N-terminal-domain vaccination unmasks the crucial action of tPA on NMDA-receptor-mediated toxicity and spatial memory. J Cell Sci. 2007;120:578–85.
Obiang P, Macrez R, Jullienne A, Bertrand T, Lesept F, Ali C, et al. GluN2D subunit-containing NMDA receptors control tissue plasminogen activator-mediated spatial memory. J Neurosci. 2012;32:12726–34.
Obiang P, Maubert E, Bardou I, Nicole O, Launay S, Bezin L, et al. Enriched housing reverses age-associated impairment of cognitive functions and tPA-dependent maturation of BDNF. Neurobiol Learn Mem. 2011;96:121–9.
Matys T, Pawlak R, Matys E, Pavlides C, McEwen BS, Strickland S. Tissue plasminogen activator promotes the effects of corticotropin- releasing factor on the amygdala and anxiety-like behavior. Proc Natl Acad Sci USA. 2004;101:16345–50.
Norris EH, Strickland S. Modulation of NR2B-regulated contextual fear in the hippocampus by the tissue plasminogen activator system. Proc Natl Acad Sci USA. 2007;104:13473–8.
Zhou Y, Maiya R, Norris EH, Kreek MJ, Strickland S. Involvement of tissue plasminogen activator in stress responsivity during acute cocaine withdrawal in mice. Stress. 2010;13:481–90.
Maiya R, Zhou Y, Norris EH, Kreek MJ, Strickland S. Tissue plasminogen activator modulates the cellular and behavioral response to cocaine. Proc Natl Acad Sci USA. 2009;106:1983–8.
Krizo JA, Moreland LE, Rastogi A, Mou X, Prosser RA, Mintz EM. Regulation of Locomotor activity in fed, fasted, and food-restricted mice lacking tissue-type plasminogen activator. BMC Physiol. 2018;18:1–9.
Pothakos K, Robinson JK, Gravanis I, Marsteller DA, Dewey SL, Tsirka SE. Decreased serotonin levels associated with behavioral disinhibition in tissue plasminogen activator deficient (tPA-/-) mice. Brain Res. 2010;1326:135–42.
Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–91.
Zhuo M, Holtzman DM, Li Y, Osaka H, DeMaro J, Jacquin M, et al. Role of tissue plasminogen activator receptor LRP in hippocampal long- term potentiation. J Neurosci. 2000;20:542–9.
Szabo R, Samson AL, Lawrence DA, Medcalf RL, Bugge TH. Passenger mutations and aberrant gene expression in congenic tissue plasminogen activator-deficient mouse strains. J Thromb Haemost. 2016;14:1618–28.
Pasquet N, Douceau S, Naveau M, Lesept F, Louessard M, Lebouvier L, et al. Tissue-type plasminogen Activator controlled corticogenesis through a mechanism dependent of NMDA receptors expressed on radial glial cells. Cereb Cortex. 2019;29:2482–98.
Birling MC, Dierich A, Jacquot S, Hérault Y, Pavlovic G. Highly-efficient, fluorescent, locus directed cre and FlpO deleter mice on a pure C57BL/6N genetic background. Genesis. 2012;50:482–9.
Bailey MT, Kinsey SG, Padgett DA, Sheridan JF, Leblebicioglu B. Social stress enhances IL-1β and TNF-α production by Porphyromonas gingivalis lipopolysaccharide-stimulated CD11b+ cells. Physiol Behav. 2009;98:351–8.
Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R. Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Brain Behav Immun. 2007;21:458–66.
Poucet B, Herrmann T, Buhot MC. Effects of short-lasting inactivations of the ventral hippocampus and medial septum on long-term and short-term acquisition of spatial information in rats. Behav Brain Res. 1991;44:53–65.
Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104.
van Holstein M, Floresco SB. Dissociable roles for the ventral and dorsal medial prefrontal cortex in cue-guided risk/reward decision making. Neuropsychopharmacology. 2020;45:683–93.
Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–70.
Liu WZ, Zhang WH, Zheng ZH, Zou JX, Liu XX, Huang SH, et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat Commun. 2020;11:1–15.
Hare BD, Duman RS. Prefrontal cortex circuits in depression and anxiety: contribution of discrete neuronal populations and target regions. Mol Psychiatry. 2020;25:2742–58.
Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-δ+ neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci. 2014;17:1240–8.
Ye J, Veinante P. Cell-type specific parallel circuits in the bed nucleus of the stria terminalis and the central nucleus of the amygdala of the mouse. Brain Struct Funct. 2019;224:1067–95.
Sasaki K, Arimoto K, Kankawa K, Terada C, Yamamori T, Watakabe A, et al. Rho guanine nucleotide exchange factors regulate horizontal axon branching of cortical upper layer neurons. Cereb Cortex. 2020;30:2506–18.
Hayashi T, Yoshida T, Ra M, Taguchi R, Mishina M. IL1RAPL1 associated with mental retardation and autism regulates the formation and stabilization of glutamatergic synapses of cortical neurons through RhoA signaling pathway. PLoS ONE. 2013;8:1–12.
Oh SB, Byun CJ, Yun JH, Jo DG, Carmeliet P, Koh JY, et al. Tissue plasminogen activator arrests Alzheimer’s disease pathogenesis. Neurobiol Aging. 2014;35:511–9.
Seeds NW, Basham ME, Haffke SP. Neuronal migration is retarded in mice lacking the tissue plasminogen activator gene. Proc Natl Acad Sci USA. 1999;96:14118–23.
Lee SH, Ko HM, Kwon KJ, Lee J, Han SH, Han DW, et al. TPA regulates neurite outgrowth by phosphorylation of LRP5/6 in neural progenitor cells. Mol Neurobiol. 2014;49:199–215.
Pawlak R, Rao BSS, Melchor JP, Chattarji S, McEwen B, Strickland S. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc Natl Acad Sci USA. 2005;102:18201–6.
Sallés FJ, Strickland S, Sallé FJ, Strickland S. Localization and regulation of the tissue plasminogen activator-plasmin system in the hippocampus. J Neurosci. 2002;22:2125–34.
Hartley T, Lever C, Burgess N, O’keefe J. Space in the brain: how the hippocampal formation supports spatial cognition. Philos Trans R Soc B Biol Sci. 2014;369:1–11.
Bennur S, Shankaranarayana Rao BS, Pawlak R, Strickland S, McEwen BS, Chattarji S. Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience. 2007;144:8–16.
Gilpin NW, Herman MA, Roberto M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry. 2015;77:859–69.
Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–62.
Babaev O, Piletti Chatain C, Krueger-Burg D. Inhibition in the amygdala anxiety circuitry. Exp Mol Med. 2018;50:1–16.
Hunt S, Sun Y, Kucukdereli H, Klein R, Sah P. Intrinsic circuits in the lateral central amygdala. ENeuro. 2017;4:1–18.
Ahrens S, Wu MV, Furlan A, Hwang GR, Paik R, Li H, et al. A central extended amygdala circuit that modulates anxiety. J Neurosci. 2018;38:5567–83.
McCullough KM, Morrison FG, Hartmann J, Carlezon WA, Ressler KJ. Quantified coexpression analysis of central amygdala subpopulations. ENeuro. 2018;5:e0010-18.2018.
Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–6.
Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B, et al. Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci. 2013;16:332–9.
Botta P, Demmou L, Kasugai Y, Markovic M, Xu C, Fadok JP, et al. Regulating anxiety with extrasynaptic inhibition. Nat Neurosci. 2015;18:1493–1500.
Griessner J, Pasieka M, Böhm V, Grössl F, Kaczanowska J, Pliota P, et al. Central amygdala circuit dynamics underlying the benzodiazepine anxiolytic effect. Mol Psychiatry. 2021;26:534–44.
Lenoir S, Varangot A, Lebouvier L, Galli T, Hommet Y, Vivien D. Post-synaptic release of the neuronal tissue-type plasminogen activator (tPA). Front Cell Neurosci. 2019;13:1–15.
Lochner JE, Honigman LS, Grant WF, Gessford SK, Hansen AB, Silverman MA, et al. Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. J Neurobiol. 2006;66:564–77.
Matys T, Pawlak R, Strickland S. Tissue plasminogen activator in the bed nucleus of stria terminalis regulates acoustic startle. Neuroscience. 2005;135:715–22.
Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001;7:59–64.
Roseberry TK, Lalive AL, Margolin BD, Kreitzer AC. Locomotor suppression by a monosynaptic amygdala to brainstem circuit. BioRxiv. 2019. https://doi.org/10.1101/724252.
Acknowledgements
We are grateful to the molecular biology and viral production facility of MIRCen, François Jacob Institute of Biology, CEA for viral particles production. We thank Pr Gilles Bonvento for the help to setup the experimental guidelines regarding AAV manipulations. This work was supported by grants from the Ministère de l’Enseignement Supérieur et de la Recherche and INSERM (French National Institute for Health and Medical Research) (HCERES U1237-2017/2022).
Author information
Authors and Affiliations
Contributions
DV and VA designed and supervised the study. SD, EL, and DV performed the data analysis. SD, EL, YH, LL, and EM performed the experiments. AB and CJ designed and produced AAV constructs. SD, VA, and DV wrote the manuscript. All authors have read and have approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Douceau, S., Lemarchand, E., Hommet, Y. et al. PKCδ-positive GABAergic neurons in the central amygdala exhibit tissue-type plasminogen activator: role in the control of anxiety. Mol Psychiatry 27, 2197–2205 (2022). https://doi.org/10.1038/s41380-022-01455-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01455-4
- Springer Nature Limited
This article is cited by
-
Tissue-plasminogen activator effects on the phenotype of splenic myeloid cells in acute inflammation
Journal of Inflammation (2024)
-
The Janus face of endogenous neuronal tPA: promoting self-protection and worsening the death of neighboring neurons
Cell Death & Disease (2024)
-
Foxg1 Modulation of the Prkcd Gene in the Lateral Habenula Mediates Trigeminal Neuralgia-Associated Anxiety-Like Behaviors in Mice
Molecular Neurobiology (2024)
-
Modulations of the neuronal trafficking of tissue-type plasminogen activator (tPA) influences glutamate release
Cell Death & Disease (2023)