Abstract
Acetylcholine (ACh) levels are elevated in actively depressed subjects. Conversely, antagonism of either nicotinic or muscarinic ACh receptors can have antidepressant effects in humans and decrease stress-relevant behaviors in rodents. Consistent with a role for ACh in mediating maladaptive responses to stress, brain ACh levels increase in response to stressful challenges, whereas systemically blocking acetylcholinesterase (AChE, the primary ACh degradative enzyme) elicits depression-like symptoms in human subjects, and selectively blocking AChE in the hippocampus increases relevant behaviors in rodents. We used an ACh sensor to characterize stress-evoked ACh release, then used chemogenetic, optogenetic and pharmacological approaches to determine whether cholinergic inputs from the medial septum/diagonal bands of Broca (MSDBB) or ChAT-positive neurons intrinsic to the hippocampus mediate stress-relevant behaviors in mice. Chemogenetic inhibition or activation of MSDBB cholinergic neurons did not result in significant behavioral effects, while inhibition attenuated the behavioral effects of physostigmine. In contrast, optogenetic stimulation of septohippocampal terminals or selective chemogenetic activation of ChAT-positive inputs to hippocampus increased stress-related behaviors. Finally, stimulation of sparse ChAT-positive hippocampal neurons increased stress-related behaviors in one ChAT-Cre line, which were attenuated by local infusion of cholinergic antagonists. These studies suggest that ACh signaling results in maladaptive behavioral responses to stress if the balance of signaling is shifted toward increased hippocampal engagement.
Similar content being viewed by others
Introduction
Depressed individuals have high brain levels of acetylcholine (ACh) compared to control subjects in human imaging experiments [1,2,3]. This is consistent with human studies showing that depression can be precipitated by hyperactivation of the cholinergic system by administration of physostigmine, an inhibitor of acetylcholinesterase (AChE: the primary enzyme that hydrolyzes ACh; [4, 5]. In contrast, decreasing cholinergic signaling with antagonists of muscarinic [6, 7] or nicotinic acetylcholine receptors [8, 9] have some efficacy for treatment of mood disorders. In animal models local knock down of AChE in hippocampus increases stress-sensitive behaviors and decreases resilience to social defeat stress. Conversely, local hippocampal knock down of cholinergic receptor subtypes (M1 muscarinic, α7 or α2 nicotinic receptors) can reverse the behavioral effects of systemic AChE antagonism [10].
The hippocampus is involved in adaptation to stress [11, 12], and receives significant cholinergic input from the medial septum and the diagonal band of Broca (MSDBB) [13, 14]. These projections impinge onto both glutamatergic and GABAergic neurons, and can potentiate synaptic plasticity [15]. Stress activates the septo-hippocampal cholinergic pathway [16, 17], whereas cholinergic neurons of the posterior septum (projecting to habenula) fire during coping behaviors [18]. ChAT-positive interneurons are also found in the dentate gyrus and CA1 field of the hippocampus [19, 20]. These interneurons control oscillatory activity, and may regulate adult neurogenesis, which can be critical for antidepressant response [21].
Pharmacological and molecular genetic studies suggest that elevated hippocampal ACh contributes to stress reactivity, however, it is not known whether specific cholinergic inputs underlie stress-relevant behaviors or whether ACh exerts a similar neuromodulatory effect in hippocampus regardless of its source. Similarly, it remains unknown whether decreasing the activity of cholinergic neurons innervating hippocampus is sufficient to provide resilience to stress-related behaviors.
In this study, we first used a genetically-encoded fluorescent ACh sensor to determine the dynamics of ACh release in the hippocampus following exposure to an acute stressor. We then used targeted chemogenetic and optogenetic approaches to determine whether specific pools of cholinergic neurons and their projections contribute to behavioral responses to stressful challenges. Finally, we combined chemogenetic and pharmacological interventions to confirm that behavioral consequences of chemogenetic manipulations were mediated through ACh receptors.
Materials and methods
Animals (see Supplementary information)
Adult male C57BL/6 J mice, BAC-ChAT-Cre and ChAT-IRES-Cre male mice were used throughout this study. Because we did not perform social defeat in the optogenetic study, we were able to use both male and female mice in this experiment. Both transgenic ChAT-Cre mouse strains are widely used, but differences between lines could affect the outcome of experimental manipulations. We therefore used two independent ChAT-Cre lines to control for any differences due to the targeting strategy. Although the pattern of Cre expression is essentially similar, there are subtle differences between lines [20]. Importantly, because the BAC-ChAT backbone construct contains the VAChT coding region, it is possible that this strain has altered cholinergic tone, as was described for the BAC-ChAT-ChR2 line [22]. In contrast, the ChAT-KI-Cre line has reduced ChAT expression from the recombined chromosome.
All procedures were carried out as approved by the Yale University Institutional Animal Care and Use Committee.
Drugs (see Supplementary information)
Viral constructs and Stereotaxic surgeries (see Supplementary Information).
Optogenetic experiments
Animals were first connected to the fiber and were left to rest in a holding cage for a few seconds before the start of the experiment. Stimulation with 473 nm blue light was started at the beginning of the experiment, and continued throughout the duration of the paradigm with the following parameters: as described in [23]: 20 sec ON, 20 sec OFF, 20 mW, 12 Hz. These parameters were previously shown to induce efficient neuronal stimulation of terminals fields from medial septum to hippocampus. Behavioral assays were scored “live” during each session.
Fiber photometry
GRAB 3.0 ACh fluorescence was measured using a standard two-channel, single-site fiber photometry system (Doric Lenses) as described in [24]. The final pre-processed ACh fluorescence signal is expressed as (ΔF/F) within a 95% bootstrapped confidence interval (95% BCi). Detailed processing described in Supplementary Information.
Electrophysiological recording of DREADD efficacy (Supplementary materials and Supplementary Fig. 1).
Behavioral assays
A battery of behavioral tests was used following chemogenetic, optogenetic, and pharmacological manipulations. The design of the battery of tests was established following the RDoC matrix and recommendations on stress-relevant behaviors [25]. See Supplementary Table 1 and Supplementary Information for specific details.
Tissue processing and verification of construct expression and targeting (See Supplementary Information).
Statistical analyses
No outliers were removed from analyses; however, data from animals with off-target infusions were not included in statistical analyses and are represented in red in the scatterplots. Statistical analyses were performed using GraphPad Prism 8 and Staview (SAS) software. Values are expressed as means ± standard error of the mean (SEM). Analysis of variance (ANOVA) was used when comparing means between groups and α was set at 95%. When ANOVA reached significance, and equal variances was assumed (as defined by Brown-Forsythe test), posthoc multiple-comparisons analyses were performed by t-tests with Bonferroni corrections.
Results
Exposure to an acute stressor increases ACh levels in both dorsal and ventral hippocampus
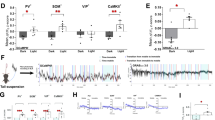
Since the dorsal and ventral hippocampus have been suggested to respond to physiological and emotional stressors differentially, we determined the time course of ACh signaling across these broad hippocampal divisions in response to an acute stressor in freely moving mice to identify any temporal or qualitative differences that might be relevant to stress-dependent behavioral outcomes. We found that two levels of foot shock (0.4 and 0.7 mA) induced measurable ACh transients in both dorsal and ventral hippocampus that outlasted the duration of the footshock (Fig. 1A). ACh transients in ventral hippocampus were similar across shock intensities, with a sustained increase over 20 sec followed by a decrease below baseline after shock termination. In the dorsal hippocampus, ACh amplitude was greater at 0.7 mA compared to 0.4 mA, but returned to baseline similar to ventral hippocampus. These data demonstrate that a physical stressor induces a sustained and measurable ACh release in the hippocampus, that lasts for more than 10 sec. Conversely, restraint stress, either acutely or repeated for several days, did not alter AChE activity in the hippocampus (See Supplementary Information and Supplementary Fig. 2), suggesting that stress-induced cholinergic engagement is more driven by local neuronal activity-dependent ACh release rather than reduced enzymatic hydrolysis of ACh.
A genetically-encoded fluorescent ACh sensor (AAV1-GRABACh3.0) was infused into the dorsal and ventral hippocampus to record ACh-induced fluorescent transients (A) in mice implanted with an optic fiber (insets). Animals experienced two foot shocks, 0.4 mA and 0.7 mA, for 1 sec. Traces represent the average fluorescence 50 sec before and after shock onset in ventral or dorsal hippocampus. Shaded areas represent bootstrapped 95% confidence intervals; n = 9 mice per group. To determine the behavioral consequences of increasing ACh levels in hippocampus, BAC-ChAT-Cre and ChAT-KI-Cre male mice were infused with AAV2-DREADD-carrying virus into the medial septum with some diffusion in the diagonal band (B) allowing modulation of the activity of MSDBB cholinergic neurons with CNO (10 mg/kg, i.p.) (C). Time spent in the light side of the light-dark box (BAC-ChAT-Cre (D), ChAT-KI-Cre (H)). Time spent immobile in the tail suspension test (BAC-ChAT-Cre (E), ChAT-KI-Cre (I) or forced swim test (BAC-ChAT-Cre (F), ChAT-KI-Cre (J)). Ratio of interaction (time with CD1/time without CD1) following 8 bouts of social defeat (BAC-ChAT-Cre (G), ChaT-KI-Cre (K)). Results are expressed as Mean ± SEM. *: p < 0.05; **: p < 0.01. BAC-Chat-Cre: Control, n = 10; Gq, n = 9; Gi, n = 12. ChAT-KI-Cre: Control, n = 11; Gq, n = 13; Gi, n = 11. Red dots represent data from animals with mistargeted placements (provided for reference and not included in calculation of means or SEM (see example in supp. Fig)).
Anterograde expression of DREADDs in MSDBB cholinergic neurons has no significant effect on stress-related behaviors at baseline, but DREADD-mediated inhibition reverses the behavioral effects induced by systemic physostigmine administration
Since a physical stressor induced a transient ACh increase across dorsal and ventral hippocampal regions, we used a chemogenetic approach to determine whether stimulating or inhibiting MSDBB, the primary cholinergic input to hippocampus, might alter stress-related behaviors. Neither stimulation (with Cre-specific Gq-DREADD) nor inhibition (with Cre-specific Gi-DREADD) of septal cholinergic neurons (Fig. 1B, C) significantly altered behavioral outcomes of Bac-ChAT-Cre mice in the light/dark box (LD; Fig. 1D), the tail suspension (TST; Fig. 1E), the forced swim (FST; Fig. 1F) or the social defeat tests (SD; Fig. 1G) compared to control animals, and locomotor activity level was similar between treatment groups and strains (all F < 1). The same behavioral pattern was observed in ChAT-KI-Cre mice for the LD (Fig. H), the TST (Fig. I), the FST (Fig. J), and the SD (Fig. K).
We next determined whether chemogenetic inhibition of MSDBB cholinergic neurons could reverse the stress-related effects of the cholinesterase blocker physostigmine (which prolongs ACh signaling by blocking ACh breakdown). The effect of the DREADD was evaluated independently in either physostigmine-treated or non-physostigmine-treated animals because the latter experimental group did not receive any injections and were naïve to behavioral tests before DREADD testing. Inhibition of septal cholinergic neurons with CNO after infusion of a Gi-DREADD into the MSDBB of either BAC-ChAT-Cre or ChAT-KI-Cre mice reversed the effect of physostigmine treatment in the LD (Fig. 1C, BAC-Chat-Cre F(1, 22) = 8.75, p = 0.007; Fig. 1G, ChAT-KI-Cre: F(1, 20) = 11.62, p = 0.003), the TST (Fig. 1D, BAC-Chat-Cre: F(1, 22) = 4.29, p = 0.05; Fig. 1H, Chat-KI-Cre: F(1, 19) = 4.54, p = 0.046) and the FST (Fig. 1E, BAC-Chat-Cre: F(1, 22) = 7.5, p = 0.012; Fig. 1I, Chat-KI-Cre: F(1, 20) = 7.5, p = 0.01).
Thus, the lack of significant behavioral effects of DREADD-induced modulation of the MSDBB cholinergic could be due to effects of ACh in multiple downstream brain areas with disparate control over stress-related behaviors that mask specific effects of septo-hippocampal pathway activation, although the current data are not sufficient to address this possibility. In contrast, decreasing activity of the MSDBB is sufficient to block stress-relevant behaviors due to physostigmine-induced prolongation of ACh signaling.
Activation of septo-hippocampal neuronal terminals using channelrhodopsin increases susceptibility to stress across behavioral paradigms
To test the possibility that MSDBB inhibition or stimulation did not alter stress-related behaviors because hippocampal ACh effects were counteracted by ACh effects in other brain areas, we used an optogenetic approach to stimulate MSDBB terminals locally in hippocampus (Fig. 2A, B, C)) No significant differences in behavior were detected between stimulated and control mice in the LD (Fig. 2D). However, in the TST, light stimulation led to greater time spent immobile in the ChR2 group compared to the mCherry control group (Fig. 2E; two-way ANOVA (F(1, 33) = 4.94, p = 0.03) although only male mice showed significant changes in behavior (t(16) = 15.9, p = 0.02), whereas female mice showed a non-significant trend toward more immobility. Finally, in the novelty-suppressed feeding test (NSFT), ChR2-expressing mice stimulated with light showed a significantly greater time to initiate a feeding episode (Fig. 2F; F(1, 33) = 4.6, p = 0.04; males and females considered together as no interaction between sex and stimulation was detected).
AAV2-ChR2 was infused into the MSDBB (A), and an optic fiber was placed above the dorsal hippocampus (B) allowing stimulation of the septo-hippocampal pathway (C). Time spent in the light side of the light-dark box (D). Time spent immobile in the tail suspension test (E). Time to first feeding episode in the novelty-suppressed feeding test (F). Results are expressed as Mean ± SEM. *: p < 0.05. Control males, n = 9; ChR2 males, n = 9. Control females, n = 7; ChR2 females, n = 6.
This experiment confirmed that stimulation of MSDBB terminals in the hippocampus is sufficient to increase stress-relevant behaviors.
Stimulation following retrograde infection of ChAT-positive neurons projecting to the hippocampus with an excitatory DREADD increases stress susceptibility across a number of behavioral paradigms
In order to target the cholinergic inputs from MSDBB to hippocampus selectively and engage cholinergic terminals more widely throughout the hippocampal structure, we infused a retrograde virus carrying Cre-dependent Gq- or Gi-DREADD bilaterally into the hippocampus of ChAT -Cre mice (Fig. 3A). Using this strategy we were able to modulate the activity of a broader set of cholinergic neurons projecting to the hippocampus (Fig. 3B).
BAC-ChAT-Cre and ChAT-KI-Cre male mice were infused with a Gq-DREADD-carrying retrograde virus into the hippocampus (A) allowing activation of hippocampal cholinergic projections (with CNO 10 mg/kg) (B). Time spent in the light side of the light-dark box (BAC-ChAT-Cre (C), ChAT-KI-Cre (G)). Time spent immobile in the tail suspension test (BAC-ChAT-Cre (D), ChAT-KI-Cre (H)) or forced swim test (BAC-ChAT-Cre (E), ChAT-KI-Cre (I)). Ratio of interaction (time with CD1/time without CD1) following eight social defeats (BAC-ChAT-Cre (F), ChaT-KI-Cre (J)). Results are expressed as Mean ± SEM. *: p < 0.05; **: p < 0.01; ***: p < 0.001. BAC-Chat-Cre: Control, n = 9; Gq, n = 9. ChAT-KI-Cre: Control, n = 9; Gq, n = 10.
Contrary to what was observed when using an anterograde Cre-dependent DREADD strategy in the MSDBB, rg-Gq-DREADD-mediated stimulation of septal cholinergic neurons projecting to the hippocampus consistently increased stress-related behaviors in the LD, the TST, FST and SD in two lines of ChAT-Cre mice. In the LD, rg-Gq-DREADD stimulation significantly decreased the time spent in the light side (Fig. 3C, BAC-ChAT-Cre: F(1, 16) = 6.6, p = 0.02; Fig. 3G, ChAT-KI-Cre: F(1, 16) = 18.46, p < 0.001) while increasing the time spent immobile in the TST (Fig. 3D, BAC-ChAT-Cre: F(1, 16) = 40.17, p < 0.001; Fig. 3H, ChAT-KI-Cre: F(1, 17) = 4.52, p = 0.048) or the FST (Fig. 3E, BAC-ChAT-Cre: F(1, 16) = 30.58, p < 0.001; Fig. 3I, ChAT-KI-Cre: F(1, 16) = 20.77, p < 0.001), and decreasing social interaction following SD (Fig. 3F, BAC-ChAT-Cre: F(1, 16) = 31.87, p < 0.001; Fig. 3J, ChAT-KI-Cre: F(1, 17) = 25.01, p < 0.001). These results suggest that activating cholinergic inputs to the hippocampus selectively is sufficient to increase a broad spectrum of stress-related behaviors, unlike broader stimulation of MSDBB cholinergic targets. These studies do not distinguish between the possibility that activation of ACh septo-hippocampal inputs is necessary for these behavioral changes, or whether increasing hippocampal ACh locally is sufficient.
DREADD-mediated activation of local ChAT-positive neurons in the hippocampus increases stress-related behaviors in BAC-ChAT-Cre mice
To determine whether extrinsic ACh inputs were required for modulation of stress-related behaviors or whether local ACh innervation might also contribute, we infused Cre-dependent AAV2-Gi- or Gq-coupled DREADDs into the hippocampus of two different lines of ChAT-Cre mice (Fig. 4A and B). The AAV2 serotype infects local cell bodies, but does not infect neuronal terminals, confining expression to local hippocampal ChAT-positive cells.
BAC-ChAT-Cre and ChAT-KI-Cre male mice were infused with AAV2 carrying Cre-dependent Gq- or Gi-DREADD in the hippocampus (A), allowing modulation of cholinergic neurons intrinsic to the hippocampus with CNO (10 mg/kg) (B). Time spent in the light side of the light-dark box (BAC-ChAT-Cre (C), ChAT-KI-Cre (G)). Time spent immobile in the tail suspension test (BAC-ChAT-Cre (D), ChAT-KI-Cre (H)) or forced swim test (BAC-ChAT-Cre (E), ChAT-KI-Cre (I)). Ratio of interaction (time with CD1/time without CD1) following eight social defeats (BAC-ChAT-Cre (F), ChaT-KI-Cre (J)). Results are expressed as Mean ± SEM. *: p < 0.05; **: p < 0.01; ***: p < 0.001. BAC-Chat-Cre: Control, n = 9; Gq, n = 14; Gi, n = 9. ChAT-KI-Cre: Control, n = 9; Gq, n = 11; Gi, n = 11.
Like rg-BAC-ChAT-Cre mice, animals infused with AAV2-Gq-DREADD into the hippocampus showed increased stress sensitivity across the battery of stress-relevant behaviors. Conversely, AAV2-Gi-DREADDs had no effects on behavior. Indeed, in the LD, there was an overall treatment effect for the time spent in the light side (Fig. 4C, F(2, 39) = 34.1, p < 0.001; one outlier was removed for not moving) with a significantly decreased time spent in the AAV2-Gq DREADD mice compared to control animals (t(21) = 3.7, p = 0.001. In the TST, there was also an overall effect (Fig. 4D, F(2, 40) = 11.4, p = 0.004) and posthoc analysis showed that AAV2-Gq DREADD significantly increased time spent immobile (t(22) = 2.9, p = 0.002), as opposed to Gi DREADD (NS). The same was observed for the time spent immobile in the FST (Fig. 4E, F(2, 40) = 18.5, p < 0.001; Gq DREADD vs control: t(22) = 3.5, p = 0.001). In the SD, stress reactivity as measured by a decrease of social interaction was also heightened in animals with AAV2-Gq DREADD stimulation whereas Gi DREADD did not show any significant effect (Fig.4F, F(2, 40) = 2.3, p = 0.07; Gq DREADD vs control: t(22) = 2.1, p = 0.046).
While there were changes across the battery of behaviors in BAC-ChAT-Cre mice infused with AAV2-Gq DREADD, there were no differences in behavior observed in the ChAT-KI-Cre line (Fig. 4G, H, I, J, all F < 1), suggesting a strain-specific effect of DREADD modulation. It is possible that this is due to different levels of Cre expression in the two lines, since BAC-ChAT-Cre mice carry multiple copies of the transgene, whereas the ChAT-KI-Cre line carries a single copy of Cre in the ChAT locus, with decreased expression from the ChAT promoter [26].
Together, the results suggest that increasing or prolonging ACh signaling in hippocampus, regardless of the location of the cholinergic cell bodies, is sufficient to induce heightened stress reactivity.
Local infusion of cholinergic antagonists into hippocampus reverses the effects of DREADD-mediated stimulation of hippocampal ChAT-Cre positive neurons
Hippocampal ChAT-positive interneurons are very sparse, can co-release other neurotransmitters, and Cre expression can be ectopic, all of which could contribute to the effect of DREADD-mediated stimulation we observed in the BAC-ChAT-Cre animals. We therefore infused either scopolamine (muscarinic antagonist) or mecamylamine (nicotinic antagonist) into the hippocampus of BAC-Chat-Cre mice that received Gq-DREADD stimulation of hippocampal ChAT-Cre positive cells (Fig. 5A and B) to determine whether the behavioral effects induced by AAV2-Gq-DREADD infusion were indeed due to ACh signaling. While CNO-mediated activation of the Gq-DREADD recapitulated the behavioral outcomes observed previously, local infusion of either cholinergic antagonist blunted the behavioral effects of the Gq-DREADD, while neither of the antagonists had any effects on their own in the different behavioral tests (all F < 1; supplementary Fig 3). In the LD box, there was an overall effect of Gq-DREADD stimulation on the time spent in the light side (Fig. 5C, F(1, 16 = 27.38, p < 0.001) although the decrease observed with either scopolamine or mecamylamine did not reach significance. In the TST and FST, Gq-DREADD stimulation significantly increased the time spent immobile (TST, Fig. 5D: F(1, 17 = 36.34, p < 0.001; FST, Fig. 5E: F(1, 17 = 21.59, p = 0.002) but local infusion of scopolamine decreased this effect significantly (TST scop vs control: p = 0.003; mec vs control: p = 0.003; FST scop vs control: p = 0.04; mec vs control: p = 0.02). These results confirm that local activation of ChAT-Cre positive neurons leads to behavioral effects via ACh signaling.
The effects of local infusion of cholinergic antagonists into the hippocampus were evaluated in BAC-ChAT-Cre male mice during Cre-dependent Gq-DREADD stimulation of the hippocampus with CNO (10 mg/kg), at baseline (+CSF), with mecamylamine (1 ng) or scopolamine (0.5 ng). Mice were infused with AAV2-Gq-DREADD into the hippocampus through a canula. After 3 weeks recovery, to allow for expression of the DREADD construct, animals were infused with either mecamylamine or scopolamine through the same guide canula 15 min before testing (A), allowing for activation of ChAT-positive neurons of the hippocampus by the DREADD construct and infusion of cholinergic antagonists into the same hippocampal area (B). Time spent in the light side of the light-dark box (A). Time spent immobile in the tail suspension (C) or forced swim test (D). Results are expressed as Mean ± SEM. *: p < 0.05; **: p < 0.01. Control, n = 9; Gq + CSF, n = 10; Gq + MEC, n = 9; Gq + SCOP, n = 11. Red dots represent data from mistargeted animals (provided for reference and not included in calculation of means or SEM).
Discussion
ACh is an essential neuromodulator for adaptation to environmental challenges [27,28,29]. Depressed patients have greater ACh levels throughout the brain [1] and anticholinesterase exposure can lead to symptoms of depression, even in individuals with no history of depression [30, 31]. Following stressful challenges, ACh levels increase in prefrontal cortex, amygdala and hippocampus [32, 33], and maladaptive behaviors can be observed by prolonging ACh signaling in hippocampus [34]. Exposure to stress also alters the function of cholinergic enzymes including AChE [35]. Thus, adequate regulation of the cholinergic circuitry innervating the hippocampus could constitute a critical mechanism for adaptative responses to stress.
We first assessed the effects of modulating activity of cholinergic neurons in the MSDBB, which provides ~65% of hippocampal ACh input [36]. Acute inhibition or activation of MSDBB ChAT-positive neurons did not alter the behavior of mice in behavioral assays of anxiolytic or antidepressant efficacy, or in response to social defeat stress, but did increase behavioral variability. One possibility is that additional projections emanating from the MSDBB to structures outside the hippocampus have opposing effects on stress-related behaviors [37], (Supplementary Information and Supplementary Figs. 4 and 5). Thus, cholinergic signaling in response to stress is likely to be homeostatic under normal circumstances, and only when that signaling is prolonged, as in depressed patients, or is increased selectively in particular postsynaptic regions are maladaptive behavioral responses observed.
Consistent with the idea that prolonged ACh signaling in the hippocampus increases stress-relevant behaviors, the effects of systemic physostigmine administration were significantly decreased by Gi-DREADD-mediated inhibition of MSDBB ChAT-positive neurons. These results are consistent with previous work showing that decreasing signaling through ACh receptors in hippocampus has little effect at baseline, but can reverse the behavioral effects of physostigmine [10, 38]. A previous study showed that Gi-DREADD-mediated inhibition of the MS in ChAT-KI-Cre mice can decrease anxiety-like behavior in the elevated plus maze and the novelty-suppressed feeding paradigms [39] while we saw no effects at baseline in the current study. It is possible that test-specific effects underlie this difference. In addition, mice in the Zhang et al. study appear to show a high level of baseline stress, and control animals in that study were not treated with CNO. Both DREADD-expressing and control mice in chemogenetic experiments in the current study received CNO to control for any behavioral effects the drug might have on its own [40].
Anterograde infection tended to show more localized expression in septum compared to the broader, albeit weaker, fluorescence induced by retrograde infection from the hippocampus. While it remains difficult to assess DREADD efficacy solely based on anatomical diffusion, it is possible that more limited expression with anterograde infection or infection beyond the septum could explain the lack of effect in the anterograde DREADD infusion experiments. However, the fact that Gi DREADD anterograde infection could reverse physostigmine effects suggests that anterograde DREADD expression was sufficient to alter acetylcholine signaling in the hippocampus. Because of the possible behavioral effects of MSDBB projections to non-hippocampal regions, we used local optogenetic activation of hippocampal pre-synaptic terminals emanating from the MSDBB. Most of the behavioral effects of optical stimulation were modest, possibly due to a limited efficacy of stimulation of the terminal fibers [41], the duration of optogenetic-induced activation compared to sustained DREADD stimulation, or the infection of non-cholinergic neurons. Indeed, GABAergic and glutamatergic neurons also make up the MSDBB and can influence behaviors related to stress and anxiety [23, 42,43,44].
The most effective and consistent modulation of stress-related behaviors was observed when cholinergic inputs to hippocampus were targeted selectively by retrograde infection of ChAT-positive terminals using rg-DIO-DREADDs infused into two different ChAT-Cre mouse lines. Activation of ChAT-positive terminals with rg-Gq-DREADD induced anxiety-like and depression-like behaviors and increased susceptibility to social stress in both ChAT-Cre mouse lines. These results are line with the results of terminal optogenetic stimulation, although the behavioral changes were greater with rg-DREADD than with optogenetic terminal activation. As previously suggested, the pattern of DREADD stimulation and the broader DREADD expression (widely spread throughout the hippocampal structure and bilaterally infused), likely result in greater stimulation than what can be achieved by a (untilateral) light cone of the optical fiber [45].
The contrast between the increases in stress-related behaviors following retrograde infection of hippocampal ChAT-positive terminals and the lack of effect following anterograde expression of DREADDs in ChAT-positive MSDBB neurons also raises the question that the less prominent cholinergic pathways projecting to the hippocampus could also have significant effects on stress-related behaviors.
In addition to extrinsic ChAT-positive inputs to the hippocampus, the retrograde virus would also infect interneuronal synapses, which would lead to DREADD-related activation of hippocampal ChAT-positive neurons. To determine whether local ChAT-positive neurons could contribute to behavioral outcomes following manipulations of hippocampal ACh signaling, we injected anterograde, Cre-dependent DREADD constructs into the hippocampus of ChAT-Cre mice to target ChAT-positive interneurons. Activation of hippocampal ChAT-positive neurons also increased stress-related behaviors, suggesting either that the effect of retrograde Gq-DREADD stimulation could be mediated in part by cholinergic interneurons, or that the source of ACh input to the hippocampus is not critical, consistent with the idea that ACh signaling can occur by volume transmission [46]. While local ChAT-positive interneurons appear to have an effect on stress-related behaviors, the size of these behavioral effects was greater following retrograde infection of ChAT-positive neurons, including those in the MSDBB, suggesting that septo-hippocampal cholinergic inputs provide a significant contribution to stress reactivity.
One limitation of this study is that increases in stress-related behaviors following hippocampal infusion of anterograde Cre-dependent DREADD constructs was only seen in the BAC-ChAT-Cre mouse line and not in the ChAT-KI-Cre mouse line. The lack of significant behavioral effects of stimulating hippocampal ChAT-positive interneurons in the ChAT-KI-Cre mouse line could be due to differences in baseline behaviors across the two strains, different levels of ACh signaling in the hippocampus due to BAC construct [22] or cryptic splicing site in KI [47].
ChAT-positive interneurons in the hippocampus are very sparse and can co-release other neurotransmitters including GABA [48] and glutamate [20, 49], although ChAT-positive neurons in the hippocampus do not express GAD RNA and are therefore non-GABAergic [19]. In addition, Cre expression can be ectopic [50], all of which could potentially contribute to the effect of local hippocampal DREADD-mediated stimulation we observed in the BAC-ChAT-Cre mice. We therefore confirmed that hippocampal infusion of cholinergic antagonists could reverse the behavioral effects of DREADD-mediated stimulation of ChAT-positive neurons in the hippocampus. This indicates that despite the sparse nature of hippocampal ChAT-positive interneurons, local cholinergic transmission in the hippocampus is sufficient to increase stress sensitivity in several behavioral paradigms. Anatomical distribution suggests that cholinergic interneurons may act primarily through modulation of hippocampal inputs (via dentate gyrus) and outputs (via CA1) and open the possibility for further investigation.
These studies suggest that engagement of MSDBB cholinergic signaling can result in homeostatic behavioral responses to stress, and only if hippocampal cholinergic signaling is increased selectively, in the absence of additional engagement of cholinergic targets, is there an increase in stress susceptibility. In addition, increasing activity of extrinsic or intrinsic ChAT-positive neurons is sufficient to increase stress susceptibility, supporting the idea of cholinergic volume transmission in regulating hippocampal activity relevant for stress-dependent behaviors.
References
Saricicek A, Esterlis I, Maloney KH, Mineur YS, Ruf BM, Muralidharan A, et al. Persistent beta2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. Am J psychiatry. 2012;169:851–9.
Hannestad JO, Cosgrove KP, DellaGioia NF, Perkins E, Bois F, Bhagwagar Z, et al. Changes in the cholinergic system between bipolar depression and euthymia as measured with [123I]5IA single photon emission computed tomography. Biol psychiatry. 2013;74:768–76.
Esterlis I, Hannestad JO, Bois F, Sewell RA, Tyndale RF, Seibyl JP, et al. Imaging changes in synaptic acetylcholine availability in living human subjects. J Nucl Med. 2013;54:78–82.
Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–5.
Risch SC, Cohen RM, Janowsky DS, Kalin NH, Sitaram N, Gillin JC, et al. Physostigmine induction of depressive symptomatology in normal human subjects. Psychiatry Res. 1981;4:89–94.
Drevets WC, Zarate CA, Jr., Furey ML. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol psychiatry 2012;73:1156–63.
Janowsky DS. Serendipity strikes again: scopolamine as an antidepressant agent in bipolar depressed patients. Curr Psychiatry Rep. 2011;13:443–5.
George TP, Sacco KA, Vessicchio JC, Weinberger AH, Shytle RD. Nicotinic antagonist augmentation of selective serotonin reuptake inhibitor-refractory major depressive disorder: a preliminary study. J Clin Psychopharmacol. 2008;28:340–4.
Mineur YS, Picciotto MR. Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends Pharm Sci. 2010;31:580–6.
Mineur YS, Ernstsen C, Islam A, Lefoli Maibom K, Picciotto MR. Hippocampal knockdown of alpha2 nicotinic or M1 muscarinic acetylcholine receptors in C57BL/6J male mice impairs cued fear conditioning. Genes, brain, Behav. 2020;19:e12677.
Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2009;3:68.
Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol psychiatry. 2000;48:755–65.
Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain. 1998;121:2249–57.
Mesulam MM. Cholinergic pathways and the ascending reticular activating system of the human brain. Ann N. Y Acad Sci. 1995;757:169–79.
Drever BD, Riedel G, Platt B. The cholinergic system and hippocampal plasticity. Behavioural brain Res. 2011;221:505–14.
Gilad GM. The stress-induced response of the septo-hippocampal cholinergic system. A vectorial outcome of psychoneuroendocrinological interactions. Psychoneuroendocrinology. 1987;12:167–84.
Gilad GM, Gilad VH, Tizabi Y. Aging and stress-induced changes in choline and glutamate uptake in hippocampus and septum of two rat strains differing in longevity and reactivity to stressors. International journal of developmental neuroscience: the official journal of the International Society for. Developmental Neurosci. 1990;8:709–13.
Yamaguchi T, Danjo T, Pastan I, Hikida T, Nakanishi S. Distinct roles of segregated transmission of the septo-habenular pathway in anxiety and fear. Neuron. 2013;78:537–44.
Frotscher M, Vida I, Bender R. Evidence for the existence of non-GABAergic, cholinergic interneurons in the rodent hippocampus. Neuroscience. 2000;96:27–31.
Yi F, Catudio-Garrett E, Gabriel R, Wilhelm M, Erdelyi F, Szabo G, et al. Hippocampal “cholinergic interneurons” visualized with the choline acetyltransferase promoter: anatomical distribution, intrinsic membrane properties, neurochemical characteristics, and capacity for cholinergic modulation. Front synaptic Neurosci. 2015;7:4.
Masiulis I, Yun S, Eisch AJ. The interesting interplay between interneurons and adult hippocampal neurogenesis. Mol Neurobiol. 2011;44:287–302.
Kolisnyk B, Guzman MS, Raulic S, Fan J, Magalhaes AC, Feng G, et al. ChAT-ChR2-EYFP mice have enhanced motor endurance but show deficits in attention and several additional cognitive domains. J Neurosci. 2013;33:10427–38.
Robinson J, Manseau F, Ducharme G, Amilhon B, Vigneault E, El Mestikawy S, et al. Optogenetic activation of septal glutamatergic neurons drive hippocampal theta rhythms. J Neurosci. 2016;36:3016–23.
Crouse RB, Kim K, Batchelor HM, Girardi EM, Kamaletdinova R, Chan J et al. Acetylcholine is released in the basolateral amygdala in response to predictors of reward and enhances the learning of cue-reward contingency. Elife 2020;9:e57335.
Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–18.
Chen E, Lallai V, Sherafat Y, Grimes NP, Pushkin AN, Fowler JP, et al. Altered baseline and nicotine-mediated behavioral and cholinergic profiles in chat-cre mouse lines. J Neurosci. 2018;38:2177–88.
Cope ZA, Lavadia ML, Joosen AJM, van de Cappelle CJA, Lara JC, Huval A et al. Converging evidence that short-active photoperiod increases acetylcholine signaling in the hippocampus. Cogn, affect & behav neurosci. 2020;20:1173–83
Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–29.
Neugebauer NM, Einstein EB, Lopez MB, McClure-Begley TD, Mineur YS, Picciotto MR. Morphine dependence and withdrawal induced changes in cholinergic signaling. Pharmacol, Biochem, Behav. 2013;109:77–83.
Harrison V, Mackenzie, Ross S. Anxiety and depression following cumulative low-level exposure to organophosphate pesticides. Environ Res. 2016;151:528–36.
Jaga K, Dharmani C. The interrelation between organophosphate toxicity and the epidemiology of depression and suicide. Rev Environ Health. 2007;22:57–73.
Imperato A, Puglisi-Allegra S, Casolini P, Angelucci L. Changes in brain dopamine and acetylcholine release during and following stress are independent of the pituitary-adrenocortical axis. Brain Res. 1991;538:111–7.
Imperato A, Puglisi-Allegra S, Casolini P, Zocchi A, Angelucci L. Stress-induced enhancement of dopamine and acetylcholine release in limbic structures: role of corticosterone. Eur J Pharmacol. 1989;165:337–8.
Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, et al. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci USA. 2013;110:3573–8.
Sternfeld M, Shoham S, Klein O, Flores-Flores C, Evron T, Idelson GH, et al. Excess “read-through” acetylcholinesterase attenuates but the “synaptic” variant intensifies neurodeterioration correlates. Proc Natl Acad Sci USA. 2000;97:8647–52.
Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524.
Di Liberto V, Frinchi M, Verdi V, Vitale A, Plescia F, Cannizzaro C, et al. Anxiolytic effects of muscarinic acetylcholine receptors agonist oxotremorine in chronically stressed rats and related changes in BDNF and FGF2 levels in the hippocampus and prefrontal cortex. Psychopharmacology. 2017;234:559–73.
Mineur YS, Mose TN, Blakeman S, Picciotto MR. Hippocampal alpha7 nicotinic ACh receptors contribute to modulation of depression-like behaviour in C57BL/6J mice. Br J Pharmacol. 2018;175:1903–14.
Zhang Y, Jiang YY, Shao S, Zhang C, Liu FY, Wan Y, et al. Inhibiting medial septal cholinergic neurons with DREADD alleviated anxiety-like behaviors in mice. Neurosci Lett. 2017;638:139–44.
Manvich DF, Webster KA, Foster SL, Farrell MS, Ritchie JC, Porter JH, et al. The DREADD agonist clozapine N-oxide (CNO) is reverse-metabolized to clozapine and produces clozapine-like interoceptive stimulus effects in rats and mice. Sci Rep. 2018;8:3840.
Packer AM, Roska B, Hausser M. Targeting neurons and photons for optogenetics. Nat Neurosci. 2013;16:805–15.
Degroot A, Treit D. Anxiety is functionally segregated within the septo-hippocampal system. Brain Res. 2004;1001:60–71.
Menard J, Treit D. Lateral and medial septal lesions reduce anxiety in the plus-maze and probe-burying tests. Physiol Behav. 1996;60:845–53.
Moor E, DeBoer P, Westerink BH. GABA receptors and benzodiazepine binding sites modulate hippocampal acetylcholine release in vivo. Eur J Pharmacol. 1998;359:119–26.
Pisanello M, Pisano F, Sileo L, Maglie E, Bellistri E, Spagnolo B, et al. Tailoring light delivery for optogenetics by modal demultiplexing in tapered optical fibers. Sci Rep. 2018;8:4467.
Disney AA, Higley MJ. Diverse spatiotemporal scales of cholinergic signaling in the neocortex. J Neurosci. 2020;40:720–5.
Bello SM, Perry MN, Smith CL. Know your model: a knockout does not always make a null. Lab Anim (NY). 2020;49:59–60.
Saunders A, Granger AJ, Sabatini BL. Corelease of acetylcholine and GABA from cholinergic forebrain neurons. Elife 2015;4:e06412.
Trudeau LE, El, Mestikawy S. Glutamate cotransmission in cholinergic, GABAergic and monoamine systems: contrasts and commonalities. Front Neural Circuits. 2018;12:113.
Harris JA, Hirokawa KE, Sorensen SA, Gu H, Mills M, Ng LL, et al. Anatomical characterization of Cre driver mice for neural circuit mapping and manipulation. Front Neural Circuits. 2014;8:76.
Bruno CA, O’Brien C, Bryant S, Mejaes JI, Estrin DJ, Pizzano C, et al. pMAT: An open-source software suite for the analysis of fiber photometry data. Pharmacol, Biochem, Behav. 2021;201:173093.
Jean-Richard-Dit-Bressel P, Clifford CWG, McNally GP. Analyzing event-related transients: confidence intervals, permutation tests, and consecutive thresholds. Front Mol Neurosci. 2020;13:14.
Acknowledgements
These studies were supported by National Institutes of Health grants MH077681, MH105824 and DA033945 from the National Institutes of Health and a NARSAD Distinguished Investigator grant from the Brain and Behavior Research Foundation. This work was funded in part by the State of Connecticut, Department of Mental Health and Addiction Services, but this publication does not express the views of the Department of Mental Health and Addiction Services or the State of Connecticut. We thank the National Institutes of Health Drug Supply Program for providing CNO.
Author information
Authors and Affiliations
Contributions
YSM designed, carried out and contributed to all experiments, analyzed data, and wrote the manuscript. TNM, AI, CNM, DCT, MB conducted DREADD experiments, histology, and analyses; LV, RCB conducted optogenetics experiments, histology, and analyses; IME and CO conducted fiber photometry experiments, histology, and analyses; WZ carried out electrophysiological recordings and analyses. MRP designed the study, analyzed outcomes, and wrote the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mineur, Y.S., Mose, T.N., Vanopdenbosch, L. et al. Hippocampal acetylcholine modulates stress-related behaviors independent of specific cholinergic inputs. Mol Psychiatry 27, 1829–1838 (2022). https://doi.org/10.1038/s41380-021-01404-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01404-7
- Springer Nature Limited
This article is cited by
-
Distinct synaptic mechanisms drive the behavioral response to acute stress and rapid correction by ketamine
Neuropsychopharmacology (2024)
-
Basal forebrain cholinergic signalling: development, connectivity and roles in cognition
Nature Reviews Neuroscience (2023)
-
Stress-induced changes of the cholinergic circuitry promote retrieval-based generalization of aversive memories
Molecular Psychiatry (2022)
-
The interaction between hippocampal cholinergic and nitrergic neurotransmission coordinates NMDA-dependent behavior and autonomic changes induced by contextual fear retrieval
Psychopharmacology (2022)