Abstract
Important questions remain about the profile of cognitive impairment in psychotic disorders across adulthood and illness stages. The age-associated profile of familial impairments also remains unclear, as well as the effect of factors, such as symptoms, functioning, and medication. Using cross-sectional data from the EU-GEI and GROUP studies, comprising 8455 participants aged 18 to 65, we examined cognitive functioning across adulthood in patients with psychotic disorders (n = 2883), and their unaffected siblings (n = 2271), compared to controls (n = 3301). An abbreviated WAIS-III measured verbal knowledge, working memory, visuospatial processing, processing speed, and IQ. Patients showed medium to large deficits across all functions (ES range = –0.45 to –0.73, p < 0.001), while siblings showed small deficits on IQ, verbal knowledge, and working memory (ES = –0.14 to –0.33, p < 0.001). Magnitude of impairment was not associated with participant age, such that the size of impairment in older and younger patients did not significantly differ. However, first-episode patients performed worse than prodromal patients (ES range = –0.88 to –0.60, p < 0.001). Adjusting for cannabis use, symptom severity, and global functioning attenuated impairments in siblings, while deficits in patients remained statistically significant, albeit reduced by half (ES range = –0.13 to –0.38, p < 0.01). Antipsychotic medication also accounted for around half of the impairment in patients (ES range = –0.21 to –0.43, p < 0.01). Deficits in verbal knowledge, and working memory may specifically index familial, i.e., shared genetic and/or shared environmental, liability for psychotic disorders. Nevertheless, potentially modifiable illness-related factors account for a significant portion of the cognitive impairment in psychotic disorders.
Similar content being viewed by others
Introduction
Cognitive impairment is a common feature of schizophrenia and related psychotic disorders. Some even argue that schizophrenia should be conceptualized as a cognitive rather than psychotic illness, with cognitive dysfunction representing the core component of the disorder [1, 2]. Indeed, the DSM-V emphasizes the importance of assessing cognitive functioning alongside the five symptom domains (delusions, hallucinations, disorganized speech, disorganized, behavior, negative symptoms) [3], and in ICD-11, cognitive symptoms are listed alongside positive, negative, depressive, manic, and psychomotor symptoms [4]. However, despite more than a century of research on cognitive functioning in schizophrenia-related disorders, important knowledge gaps remain.
First, the profile of cognitive impairment across the lifespan remains poorly characterized, and its relationship with illness stages is unclear. Evidence suggests that patients with schizophrenia-related disorders experience some degree of cognitive decline over their lifetime, with the largest decline occurring during the years prior and up to the first few years after onset [5]. After illness onset, both cross-sectional [6] and longitudinal [7] evidence suggests at least some stabilization of impairment. However, there is also evidence for decline after onset [8], a second ‘peak’ in decline during later, chronic illness stages [9], and increased risk of dementia in very-late onset schizophrenia [10]. Efforts to examine the profile of specific cognitive functions across adulthood have also yielded mixed findings [6, 8]. Delineating the profile of these functions across adulthood and illness stages may reveal critical functions and periods for detection and intervention.
Second, cognitive decline has not been fully considered as an age-associated process, rather than in relation to stage of illness (i.e., premorbid, first-episode, chronic) [5]. Similarly, most studies have examined early [7] or late adulthood [9], without being able to trace cognitive functioning across the entirety of adulthood. While evidence suggests that cognitive decline during the first two decades of life reflects a failure to keep up with developmental norms rather than loss of cognitive capacity [11, 12], studies have not yet delineated the nature of age-associated processes throughout adulthood. The importance of considering cognitive functioning beyond adolescence is highlighted by the fact that brain and cognitive development continue well into the third decade of life [13].
Third, studies have yet to examine the full age-associated profile of familial deficits. Substantial evidence suggests that relatives of patients with schizophrenia-related disorders also show some degree of cognitive impairment [14, 15], at least in part due to shared heritable genetic mechanisms. The genetic underpinnings of cognitive impairments in schizophrenia-related disorders have been demonstrated by studies showing overlap between schizophrenia polygenic risk scores (PRS) and cognitive performance [16], as well as educational attainment [17]. However, despite continued evidence for familial cognitive impairments, it remains unclear whether siblings show greater impairments in certain domains and whether the age-associated pattern of cognitive impairments resembles that of patients. A detailed evaluation of the familiality of cognitive deficits across adulthood and cognitive domains may provide important additional etiological clues about the genetic and neurobiological underpinnings of cognitive impairments in schizophrenia-related disorders.
Lastly, it remains unknown whether illness-related factors such as symptom severity, global functioning, and antipsychotic medication influence cognitive impairments differentially throughout adulthood. The age-associated influence of cannabis use on cognitive impairments is similarly unclear, despite the role cannabis may play in the emergence of psychotic symptoms [18]. The potentially moderating effect of sex on age-associated cognitive processes in psychotic disorders also remains largely unexplored. Examining these factors across adulthood may advance understanding of the etiology of cognitive impairment, as well as its clinical significance.
Using the largest sample of patients with schizophrenia-related disorders, non-psychotic siblings, and controls to date, we examined cognitive impairments across adulthood. We used cross-sectional data on general and specific functions from 8,455 individuals aged 18 to 65 from the Genetic Risk and Outcome of Psychosis (GROUP) and EUropean network of national schizophrenia networks studying Gene-Environment Interactions (EU-GEI) studies, which comprise prodromal (i.e., converted to a schizophrenia-related disorder during the study), first-episode and established illness stages. We examined whether: (1) cognitive impairments in patients differ by age category (i.e., very early-, early- and mid-adulthood) and/or stage of illness (i.e., prodromal stage, first-episode, established stage), (2) siblings of these patients show a similar pattern of impairment, and (3) this impairment is influenced by socioeconomic status, education, sex, symptom severity, global functioning, antipsychotic medication, and cannabis use.
Methods
Sample
Data were collected in 30 centers across 13 countries (UK, Netherlands, Spain, France, Italy, Serbia, Turkey, Austria, Switzerland, Germany, Australia, Denmark, Brazil), and were part of the baseline assessment for the EU-GEI study, which ran from May 1st 2010 to April 30th 2015 [19, 20], or the GROUP study, which ran from April 2004 to December 2013 [21]. Ethical approval was granted in each center and all participants provided written informed consent. Of the combined dataset of 10,136 individuals, 685 (21.6%) patients, 259 (11.0%) siblings and 334 (10.0%) controls did not complete cognitive testing, leaving a total of 8858 individuals (3341 controls, 2347 siblings, 3,170 patients). Patients were either in the prodromal (i.e., had converted to a schizophrenia-related disorder during the study period), first-episode or established stage of illness, and were excluded if an organic cause was the primary reason for their psychotic symptoms. Control participants were excluded if they had a past or current diagnosis of any schizophrenia-related disorder. All participants had to have adequate language skills local to each center in order to undergo cognitive testing. Other exclusion and inclusion criteria for individual studies/work packages covering the different illness stages are described in the supplement.
Measures
Cognition
An abbreviated WAIS-III [22], comprising the information, arithmetic, block design, and digit symbol coding subtests, was used to measure performance in the domains of verbal knowledge, working memory visuospatial processing, and processing speed, respectively. Each WAIS subtest taps into many different abilities and the domains mentioned herein are simplified. In GROUP, all items of each subtest were administered. In EU-GEI, the digit symbol coding was administered for the standard time, along with every second item of the block design and arithmetic subtests, and every third item of the information subtest [23]. Raw scores were then multiplied by two (arithmetic and block design) or three (information). This abbreviated WAIS-III version was developed for EU-GEI and provides a reliable approximation of IQ and the four subtests [23].
Sociodemographic characteristics
Age, sex, ethnicity, years of education, and parental socioeconomic status (SES) were obtained (Table 1). In EU-GEI, parental SES (i.e., occupation level) was obtained using an amended version of the Medical Research Council Socioeconomic Schedule (MRC SDS) [24], and in GROUP using a comparable scale. Current cannabis use was ascertained in GROUP using the Composite International Diagnostic Interview (CIDI) [25] and in EUGEI using the Cannabis Experiences Questionnaire (CEQ) [26].
Clinical characteristics
Diagnoses were obtained using the Operational Criteria Checklist algorithm (OPCRIT) [27]. OPCRIT shows high interrater reliability generally [28] and in our study, after training (κ = 0.7). Illness duration and current antipsychotic medication use (yes/no) were assessed using the abbreviated Nottingham Onset Schedule (NOS) [29]. Symptom severity and general functioning were measured using the Global Assessment of Functioning (GAF) symptom and disability scales [30], respectively.
Statistical analyses
Group differences in sample characteristics were examined using χ2, t- and Mann–Whitney-U-tests. Raw scores on the digit symbol coding, block design, information and arithmetic subtests, and the sum of these subtests (i.e., raw IQ) were z transformed. Thus, β values throughout represent standardized effect sizes (ES), with values 0.2, 0.5, and 0.8 indicating small, medium, and large ESs, respectively. These z scores were used in all statistical analyses, and are plotted by age separately for each country in sFigs. 1–5.
Age-associated group differences in cognitive functioning
Multilevel linear models (MLMs) were fitted to account for the hierarchical structure of the data (i.e., with random intercepts for country, center, and family). Based on age distributions (sFig. 6) and nonlinear relationships between age and cognitive functioning (Fig. 1), we categorized individuals into approximately equal-sized age groups: 18–25, 26–39, and 40–65 years, representing very early-, early- and mid-adulthood, respectively (Table 1). Effects of interest were group main effects and the interaction between group and age category. A statistically significant group main effect would indicate a difference in cognitive performance between patients and/or siblings compared to controls. A statistically significant group-by-age interaction would indicate that group differences in cognitive performance differ by participants’ age. Sex and ethnicity were entered as covariates in all models.
Illness stage and duration
The effect of illness stage was examined using MLMs as described above, but with prodromal patients set as the reference. Illness stage was based on study (i.e., prodrome study = prodromal stage, first-episode study = first-episode, course studies = established stage), except for individuals in the course studies with an illness duration of less than 2 years (n = 314), who were considered first-episode. Illness duration (measured in years from illness onset) was entered into MLMs as a continuous effect of interest.
Sociodemographic and illness-related factors
We entered sociodemographic and illness-related factors (current cannabis use; symptom severity i.e., GAF symptoms; global functioning i.e., GAF disability; illness duration; parental SES; years of education; antipsychotic medication) as covariates into separate MLMs to examine whether each of these factors influenced group and group-by-age effects.
Sex-related differences
We fitted MLMs separately in males and females to examine potential sex differences in group and group-by-age effects. In order to formally test for sex-related differences, we also entered sex-by-group, and three-way interactions between sex, group, and age into MLMs on the whole sample.
Sensitivity analyses
Sensitivity analyses were conducted to determine whether cognitive patterns were similar in patients with non-affective and affective psychosis. To rule out any potential bias due to the inclusion of patients without a participating sibling (37.4% of sample), we repeated the main analyses including only patients with a participating sibling. We also analyzed controls and siblings with high GAF disability scores (80+, controls: n = 2193; siblings: n = 834) and low GAF disability scores (<80, controls: n = 400; siblings: n = 405) separately to examine potential bias from missing GAF data.
An adjusted p-value threshold of 0.005 (0.05 ÷ 10 (5 cognitive subtests × 2 statistical models for (1) main effects and (2) interaction effects)) was used in all models to account for multiple comparisons. Statistical analyses were performed in Stata 15.1 [31]. The R [32] package ggplot2 [33] was used to create graphics.
Results
Sample characteristics
Table 1 shows sample characteristics. The small number of participants below the age of 18 were excluded (n = 179). Thus, the final sample comprised 2883 patients, 2271 siblings, and 3301 controls. Of this sample, 1805 (62.6%) patients had at least one participating sibling (range = 1–5; median = 1).
Older participants showed lower scores than younger participants
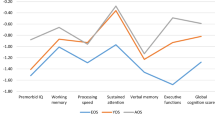
Across all groups, IQ (β = –0.42, z = –12.01, SE = 0.04, p < 0.001), block design (β = –0.45, z = –11.39, SE = 0.04, p < 0.001), and digit symbol coding (β = –0.42, z = –12.15, SE = 0.04, p < 0.001) was significantly associated with participant age, such that participants in mid-adulthood performed worse than participants in very-early-adulthood (Fig. 2, Table 2).
Patients showed substantial cognitive impairments that were not associated with participant age
Patients showed medium to large deficits across all cognitive measures (Fig. 2, Table 2). Large deficits were seen on IQ (β = –0.73, z = –20.39, SE = 0.04, p < 0.001), and digit symbol coding (β = –0.71, z = –20.30, SE = 0.03, p < 0.001). Medium deficits were observed on information (β = –0.45, z = –11.05, SE = 0.04, p < 0.001), arithmetic (β = –0.66, z = –15.87, SE = 0.04, p < 0.001), and block design (β = –0.45, z = –11.05, SE = 0.04, p < 0.001). No group-by-age interactions reached statistical significance, suggesting no differential association between cognitive performance and participant age in patients and controls. Namely, older participants scored worse than younger participants in both groups, and the magnitude of difference between older and younger participants was not significantly different between groups (Table 2).
First-episode patients performed worse than patients in other illness-stages
First-episode patients performed worse than prodromal patients on IQ (β = –0.88, z = –4.75, SE = 0.19, p < 0.001), information (β = –0.60, z = –3.06, SE = 0.19, p = 0.002), arithmetic (β = –0.61, z = –3.11, SE = 0.20, p = 0.002), block design (β = –0.82, z = –4.07, SE = 0.20, p < 0.001), and slightly worse than established stage patients on information (β = –0.16, z = –3.72, SE = 0.04, p < 0.001). Established stage patients performed worse than prodromal patients on IQ (β = –0.80, z = –4.26, SE = 0.19, p < 0.001), arithmetic (β = –0.58, z = –2.89, SE = 0.20, p = 0.004), block design (β = –0.70, z = –3.42, SE = 0.20, p = 0.001), and digit symbol coding (β = –0.66, z = 3.65, SE = 0.18, p < 0.001). All differences remained after adjusting for age. Illness duration showed no statistically significant effects on cognition.
Siblings showed small cognitive impairments that were smaller in older than younger participants
Compared to controls, siblings showed small deficits on IQ (β = –0.14, z = –3.65, SE = 0.04, p < 0.001), information (β = –0.33, z = –7.64, SE = 0.04, p < 0.001) and arithmetic (β = –0.23, z = –4.97, SE = 0.05, p < 0.001) (Fig. 2, Table 2). These deficits were smaller in mid-adulthood than in early-adulthood, reflected by significant group-by-age interactions on IQ (β = 0.16, z = 2.88, SE = 0.06, p = 0.004), information (β = 0.22, z = 3.47, SE = 0.06, p = 0.001) and block design (β = 0.20, z = 3.15, SE = 0.06, p = 0.002) (Table 2). Thus, while older participants scored worse than younger participants in both groups, the magnitude of difference between older and younger participants was smaller in siblings than controls.
Sociodemographic and illness-related factors influenced cognitive impairments
Figure 3 shows differences in effect sizes of group main effects for models adjusting for each covariate, compared to the unadjusted model. Adjusting for parental SES statistically attenuated deficits in verbal knowledge, and working memory in siblings, but effect sizes remained small (sTable 1, sFig. 7). Adjusting for current cannabis use (sTables 2, sFig. 8) and years of education (sTables 3, sFig. 9) attenuated the small IQ deficit in siblings. Adjusting for symptom severity (GAF symptoms) (sTable 4, sFig. 10) and global functioning (GAF disability) (sTable 5, sFig. 11) attenuated the IQ and arithmetic deficits in siblings, and the block design impairment in patients. Information, arithmetic, and symbol coding deficits in patients remained statistically significant, but were reduced by more than half when adjusting for these factors (sTables 4 and 5). Interestingly, when entering both global functioning and symptom severity into the same model, only functioning remained statistically associated with cognition (z = 9.84, SE = 0.001, p < 0.001), while symptoms were not (z = 1.59, SE = 0.001, p = 0.11). This finding suggests that the association between functioning and cognition may account for most of the association between symptoms and cognition. Finally, adjusting for antipsychotic medication attenuated the information and block design impairments in patients, and reduced the magnitude of deficits in IQ, arithmetic, and digit symbol coding by about half (sTable 6, sFig. 12). sTable 7 shows correlations between sociodemographic and illness-related factors and all cognitive measures.
Cognitive impairments were comparable in male and female patients but not male and female siblings
Compared to controls, both male and female patients showed medium to large impairments across all cognitive measures (sTables 8 and 9). Accordingly, sex-by-group interactions did not reveal any sex-related differences in patients on any cognitive measure, such that cognitive impairments were comparable in male and female patients. On the other hand, male siblings showed a smaller deficit on information (β = –0.21, z = –3.34, SE = 0.06, p = 0.001), than female siblings (β = –0.40, z = –6.72, SE = 0.06, p < 0.001), and the deficit on arithmetic did not reach significance in male siblings (β = –0.12, z = –1.83, SE = 0.07, p = 0.07), but did in female siblings (β = –0.30, z = –4.94, SE = 0.06, p < 0.001) (sTables 8 and 9, sFigs. 13 and 14). Accordingly, significant sex-by-group interactions on information (β = –0.11, z = –2.46, SE = 0.05, p = 0.014) and arithmetic (β = –0.13, z = –0.05, SE = 2.66, p = 0.008), confirmed that female siblings showed greater deficits on these domains than male siblings.
Group-by-age interactions no longer reached significance in male siblings, but female siblings showed a significant group-by-age interaction on information between early- and mid-adulthood (β = 0.27, z = 3.25, SE = 0.08, p = 0.001), suggesting a differential association between cognitive performance and participant age in female siblings and controls (sTables 8 and 9). Namely, while older participants scored worse than younger participants across groups, the magnitude of difference between older and younger participants was smaller in female siblings than controls. However, none of the sex-by-group-by-age interactions reached statistical significance, suggesting negligible sex-related differences in age-associated effects on cognitive functioning.
Sensitivity analyses
Sensitivity analyses comparing patients with non-affective and affective psychosis, patients with and without participating siblings, and participants with high and low GAF scores revealed similar patterns overall (sResults).
Discussion
In this large, cross-sectional patient-sibling-control study, patients with psychotic disorders showed large, widespread cognitive impairments, which were not associated with participant age. However, first-episode patients showed larger deficits than prodromal patients, and a slightly larger verbal knowledge deficit compared to established stage patients. Siblings showed small deficits on IQ, and measures of verbal knowledge and working memory, which were attenuated when adjusting for cannabis use, symptom severity, global functioning, and education. These findings add to current knowledge in several important ways.
First, effects of participant age on the magnitude of cognitive impairments were minimal. Examining cognitive raw scores throughout adulthood revealed that older patients showed lower scores than younger patients, but also that the same was true of controls, such that the magnitude of impairment remained stable. However, magnitude of impairment did differ by illness stage, with first-episode patients showing much larger deficits than prodromal patients. In line with previous evidence [34], impairments in the oldest siblings were smaller than impairments in the youngest siblings, which may be because older siblings have passed the critical period for psychosis-onset, while younger siblings have not and may still be at risk for psychotic disorders. Alternatively, controls may experience greater age-associated cognitive decline later in adulthood, while siblings and patients, who already experienced decline earlier in adulthood, may show relative stabilization or even normalization [6]. Our results should also be considered in the context of the well-documented Flynn effect [35], whereby cognitive performance and IQ in any fixed age group improves over time due to improvements in education, nutrition, etc. Thus, while our finding of lower cognitive scores in older participants compared to younger participants may reflect age-associated cognitive decline [36, 37], the Flynn effect may also account for this finding. Conversely, recent data suggest a reversal of the Flynn effect [38,39,40], which may partly explain our finding of smaller impairments in older siblings compared to younger siblings. Moreover, while we made considerable efforts to recruit a well-matched representative sample using quota sampling methods, we cannot rule out selection bias. For example, participation in research studies is associated with better cognitive functioning and this bias may be more pronounced in later adulthood. Similarly, since lower IQ is associated with earlier mortality [41], older individuals with more pronounced cognitive impairment may be less likely to participate. Future longitudinal studies that are able to prospectively follow individuals throughout adulthood are needed to determine the profile and underlying mechanisms of age-associated cognitive processes in psychotic disorders. Overall, our findings support previous evidence that most of the cognitive deficit associated with psychotic disorders is already apparent at illness onset [7].
Second, including a large sample of siblings with similar genetic and environmental predispositions as patients, but without the potentially confounding effects of illness-related factors, provides important insights into the familiality of cognitive impairments [14]. Specifically, while patients showed medium to large deficits across all measures, siblings showed small deficits on IQ, verbal knowledge, and working memory, but not on processing speed and visuospatial processing. This latter finding contrasts with a meta-analytic finding of large processing speed deficits in first-degree relatives [42], likely because most studies in this meta-analysis combined data from parents and siblings, while we only examined siblings, who are younger. Nevertheless, our findings suggest that verbal knowledge and working memory deficits may specifically index familial, i.e., shared genetic and/or environmental, liability for psychotic disorders. Accordingly, a recent meta-analysis of cognition in first-degree relatives of patients with schizophrenia also reported the largest deficits in IQ and verbal measures [15]. The notion of verbal impairments as a familial marker for psychotic illness is in line with evidence that verbal deficits emerge early [11, 12]. Nonverbal impairments, on the other hand, emerge over time [12], increase throughout the early illness stage [11], but remain stable thereafter [8]. Thus, shared genetic and/or environmental factors may lead to deficits in verbal abilities in individuals at familial risk for psychosis, while additional risk factors, possibly interacting with these verbal deficits, may lead to emerging nonverbal deficits and psychotic illness. It is important to note that we examined only two cognitive tests requiring verbal skills, and shared genetic liability may depend on the subtest measured. For example, genomic loci that jointly influence schizophrenia and verbal-numerical reasoning have been identified [43], but a recent study showed no association between schizophrenia PRSs and a verbal reading test [44].
Third, adjusting for both symptom severity and global functioning attenuated the IQ and working memory deficits in siblings, and reduced cognitive deficits in patients by half. Yet our findings also suggest that the association between symptoms and cognition is confounded by functioning. Thus, while the two GAF subscales are highly correlated (r = 0.69) and adjusting for each subscale reduced deficits by similar magnitudes, it may be important to disentangle the effects of these factors. These findings are line with evidence of the significant impact of cognition on functional outcomes [45], as well as the lack of a strong association between cognition and symptom severity [46]. Interestingly, siblings outperformed controls in visuospatial ability and processing speed after adjustment for symptoms and functioning, suggesting a potentially protective mechanism. Deficits in working memory, visuospatial, and processing speed abilities may therefore be ameliorated by improving functioning levels. Impairments in both patients and siblings were also reduced, albeit to a lesser extent, when adjusting for education. Interestingly, this reduction was slightly more pronounced on the verbal knowledge and working memory tests, suggesting that these deficits may partly reflect an impairment in the ability to learn in standard educational settings. While the relationship between cognitive impairment and factors such as educational attainment and functioning are difficult to discern due to reverse causality, future studies that are able to disentangle whether they act as mediators, moderators, or lie on the causal pathway between cognition and psychosis will provide important insights. The finding that female siblings showed larger deficits than male siblings is also intriguing, and highlights the need for further examination of sex-specific genetic risk factors [47]. Finally, adjusting for current cannabis use had a negligible impact on patient impairments, but attenuated IQ deficits in siblings. These findings are in line with evidence of a minimal association between cannabis use and cognitive functioning in psychotic disorders [48], as well as more severe symptomatology in sibling cannabis users [49].
This study has some limitations. First, our findings require replication in longitudinal samples since we used cross-sectional data. We also cannot rule out age-associated effects on cognition in early life or late adulthood since our youngest and oldest participants were 18 and 65, respectively. Moreover, while the large age range is a strength, cohort effects should be considered. Nevertheless, the current findings are in line with longitudinal results from the same sample [50]. Second, one limitation inherent to large cohorts is the tradeoff between breadth and depth. While we examined a number of covariates, future studies that are able to examine antipsychotic dosage, type and adherence, comorbidities, such as anxiety and depression, and positive and negative psychotic symptoms are needed. Comorbidity is the rule rather than the exception in psychotic disorders [51], making it difficult to disentangle the effects of psychotic versus other psychiatric symptoms. The reduction in power due to missing data when adjusting for covariates also warrants consideration, and effect sizes should be considered alongside statistical significance. Third, while our sensitivity analyses eliminate certain sources of bias, others cannot be ruled out. Individuals with better functioning may be more likely to participate in research studies, although the reverse is also possible. Fourth, our findings regarding specific cognitive domains require replication using larger test batteries that are able to cover each domain in greater detail. Relatedly, abbreviated tests, such as those administered in EUGEI, may both over-estimate (reduced fatigue) and underestimate (less attenuated learning) cognitive functioning, especially in individuals of low ability [23]. However, our data show normal distribution of IQ and subtest scaled scores across all groups (see sTable 10).
In conclusion, using a large, cross-sectional sample of patients with psychotic disorders, their siblings and controls, we found that patients showed substantial and widespread cognitive impairments, while siblings showed smaller verbal knowledge and working memory impairments. Moreover, effects of age and illness stage (beyond the first episode) on these impairments were minimal, while illness-related factors accounted for much of the impairment in siblings, and around half of the patient deficit. Thus, our findings suggest that most of the cognitive impairment associated with psychotic disorders is already apparent at illness onset, highlighting the importance of early cognitive remediation intervention efforts. Therapeutic efforts targeting illness-related factors, such as symptoms and functioning, which account for a significant portion of the cognitive impairment, could also have substantial benefits. Finally, deficits in verbal knowledge and working memory may specifically index familial liability and could be useful targets for studies aimed at elucidating the heritable neurobiological mechanisms underlying psychotic disorders.
References
Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psy. 2013;70:1107–12.
Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Critical Rev Neurobiol. 2000;14:1–21.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). Washington, D.C.: American Psychiatric Association; 2013.
World Health Organization. International classification of diseases, 11th revision (ICD-11). ICD-11 is here Geneva: World Health Organization; 2018.
Becker H, Nieman D, Wiltink S, Dingemans P, Van de Fliert J, Velthorst E, et al. Neurocognitive functioning before and after the first psychotic episode: does psychosis result in cognitive deterioration? Psych Med. 2010;40:1599–606.
Mollon J, Mathias SR, Knowles EE, Rodrigue A, Koenis MM, Pearlson GD, et al. Cognitive impairment from early to middle adulthood in patients with affective and nonaffective psychotic disorders. Psychol Med. 2019;50:48–57.
Bozikas VP, Andreou C. Longitudinal studies of cognition in first episode psychosis: a systematic review of the literature. Aust NZealand J Psych. 2011;45:93–108.
Zanelli J, Mollon J, Sandin S, Morgan C, Dazzan P, Pilecka I, et al. Cognitive change in schizophrenia and other psychoses in the decade following the first episode. Am J Psychiatry. 2019;176:811–9.
Harvey PD. What is the evidence for changes in cognition and functioning over the lifespan in patients with schizophrenia? J Clin Psychiatry. 2014;75:34–38.
Kodesh A, Goldberg Y, Rotstein A, Weinstein G, Reichenberg A, Sandin S, et al. Risk of dementia and death in very-late-onset schizophrenia-like psychosis: a national cohort study. Schizophrenia Res. 2020;223:220–6.
Mollon J, David AS, Zammit S, Lewis G, Reichenberg A. Course of cognitive development from infancy to early adulthood in the psychosis spectrum. JAMA Psychiatry. 2018;75:270–9.
Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psyc. 2010;167:160–9.
Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–55.
Snitz BE, MacDonald III AW, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2005;32:179–94.
Agnew-Blais J, Seidman LJ. Neurocognition in youth and young adults under age 30 at familial risk for schizophrenia: a quantitative and qualitative review. Cogn Neuropsychiatry. 2013;18:44–82.
van Os J, van der Steen Y, Islam MA, Guloksuz S, Rutten BP, Simons CJ, et al. Evidence that polygenic risk for psychotic disorder is expressed in the domain of neurodevelopment, emotion regulation and attribution of salience. Psychol Med. 2017;47:2421–37.
Sorensen HJ, Debost JC, Agerbo E, Benros ME, McGrath JJ, Mortensen PB, et al. Polygenic risk scores, school achievement, and risk for schizophrenia: a danish population-based study. Biol Psychiatry. 2018;84:684–91.
Kuepper R, van Os J, Lieb R, Wittchen H-U, Höfler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. Bmj. 2011;342:d738.
EU-GEI. Identifying gene-environment interactions in schizophrenia: contemporary challenges for integrated, large-scale investigations. Schizophrenia Bulletin. 2014;40:729–36.
Gayer-Anderson C, Jongsma HE, Di Forti M, Quattrone D, Velthorst E, de Haan L, et al. The EUropean Network of National Schizophrenia networks studying gene–environment interactions (EU-GEI): incidence and first-episode case–control programme. Social Psych Psych Epidemiol. 2020:55:645–57.
Korver N, Quee PJ, Boos HB, Simons CJ, de Haan L, Investigators G. Genetic Risk and Outcome of Psychosis (GROUP), a multi site longitudinal cohort study focused on gene–environment interaction: objectives, sample characteristics, recruitment and assessment methods. Int J Methods Psych Res. 2012;21:205–21.
Blyler CR, Gold JM, Iannone VN, Buchanan RW. Short form of the WAIS-III for use with patients with schizophrenia. Schizophrenia Res. 2000;46:209–15.
Velthorst E, Levine SZ, Henquet C, de Haan L, van Os J, Myin-Germeys I, et al. To cut a short test even shorter: reliability and validity of a brief assessment of intellectual ability in schizophrenia—a control-case family study. Cog Neuropsych. 2013;18:574–93.
Mallet J. MRC sociodemographic schedule. Section of Social Psychiatry, Institute of Psychiatry; 1997.
WHO. Composite international diagnostic interview. Geneva, Switzerland: World Health Organization; 1990.
Di Forti M, Morgan C, Dazzan P, Pariante C, Mondelli V, Marques TR, et al. High-potency cannabis and the risk of psychosis. Br J Psych. 2009;195:488–91.
McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness: development and reliability of the OPCRIT system. Arch Gen Psych. 1991;48:764–70.
Craddock N, Asherson P, Owen MJ, Williams J, Mcguffin P, Farmer AE. Concurrent validity of the OPCRIT diagnostic system: comparison of OPCRIT diagnoses with consensus best-estimate lifetime diagnoses. Br J Psych. 1996;169:58–63.
Singh SP, Cooper JE, Fisher HL, Tarrant CJ, Lloyd T, Banjo J, et al. Determining the chronology and components of psychosis onset: the Nottingham Onset Schedule (NOS). Schizophrenia Res. 2005;80:117–30.
Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale: reliability and validity of the Global Assessment of Functioning (GAF). Br J Psych. 1995;166:654–9.
StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp; 2017. https://www.stata.com/features/documentation/. Accessed on 1 March 2018.
R-Core-Team. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. http://www.R-project.org.
Wickham H. ggplot2: Elegant graphics for data analysis. New York: Springer-Verlag; 2016.
Zalesky A, Pantelis C, Cropley V, Fornito A, Cocchi L, McAdams H, et al. Delayed development of brain connectivity in adolescents with schizophrenia and their unaffected siblings. JAMA Psychiatry. 2015;72:900–8.
Flynn JR. What is intelligence?: Beyond the Flynn effect. Cambridge, UK: Cambridge University Press; 2007.
Salthouse TA. When does age-related cognitive decline begin? Neurobiol Aging. 2009;30:507–14.
Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, et al. Age-associated cognitive decline. Br Med Bull. 2009;92:135–52.
Dutton E, van der Linden D, Lynn R. The negative Flynn Effect: a systematic literature review. Intelligence. 2016;59:163–9.
Teasdale TW, Owen DR. A long-term rise and recent decline in intelligence test performance: the Flynn Effect in reverse. Pers Ind Diff. 2005;39:837–43.
Bratsberg B, Rogeberg O. Flynn effect and its reversal are both environmentally caused. Proc Nat Acad Sci. 2018;115:6674–8.
Sachs GA, Carter R, Holtz LR, Smith F, Stump TE, Tu W, et al. Cognitive impairment: an independent predictor of excess mortality: a cohort study. Ann Intern Med. 2011;155:300–8.
Dickinson D. Digit symbol coding and general cognitive ability in schizophrenia: worth another look? Br J Psychiatry. 2008;193:354–6.
Smeland OB, Frei O, Kauppi K, Hill WD, Li W, Wang Y, et al. Identification of genetic loci jointly influencing schizophrenia risk and the cognitive traits of verbal-numerical reasoning, reaction time, and general cognitive function. JAMA Psychiatry. 2017;74:1065–75.
Shafee R, Nanda P, Padmanabhan JL, Tandon N, Alliey-Rodriguez N, Kalapurakkel S, et al. Polygenic risk for schizophrenia and measured domains of cognition in individuals with psychosis and controls. Transl Psychiatry. 2018;8:78.
Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–88.
Gold JM. Cognitive deficits as treatment targets in schizophrenia. Schizophr Res. 2004;72:21–28.
Goldstein JM, Cherkerzian S, Tsuang MT, Petryshen TL. Sex differences in the genetic risk for schizophrenia: History of the evidence for sex‐specific and sex‐dependent effects. Am J Med Gen Part B: Neuropsych Genetics. 2013;162:698–710.
Loberg EM, Hugdahl K. Cannabis use and cognition in schizophrenia. Front Hum Neurosci. 2009;3:53.
van Winkel R, Investigators G. Further evidence that cannabis moderates familial correlation of psychosis-related experiences. PLoS One. 2015;10:e0137625.
Van Haren NEM, Van Dam DS, Stellato RK. Genetic risk and outcome of psychosis (group) investigators. Change in IQ in schizophrenia patients and their siblings: a controlled longitudinal study. Psychol Med. 2019;49:2573–81.
Knowles EEM, Mathias SR, Pearlson GD, Barrett J, Mollon J, Denbow D, et al. Clinical correlates of subsyndromal depression in African American individuals with psychosis: The relationship with positive symptoms and comorbid substance dependence. Schizophr Res. 2019;206:333–46.
Acknowledgements
The European Community’s Seventh Framework Programme under grant agreement No. HEALTH-F2-2010-241909 (EU-GEI). The Geestkracht programme of the Dutch Health Research Council (Zon-Mw)(GROUP). Dr. Velthorst is supported by The Seaver Foundation. Dr. Pantelis was supported by a NHMRC Senior Principal Research Fellowship (ID: 1105825), a NHMRC Program Grant (ID: 1150083). The Melbourne arm of the study was supported by a grant from the Australian National Health & Medical Research Council (NHMRC-EU grant ID: 567215). The French cohort was supported by the French Ministry grant (PHRC ICAAR - AOM07-118). The Spanish sample was supported by the Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III (SAM16PE07CP1, PI16/02012, PI19/024), Madrid Regional Government (B2017/BMD-3740 AGES-CM-2), Fundación Familia Alonso and Fundación Alicia Koplowitz. The Brazilian sample was supported by FAPESP-Brazil (grant 2012/05178-0). Additional support was provided by a Medical Research Council Fellowship to Dr. Kempton (grant MR/J008915/1). Dr. Kirkbride was supported by the National Institute for Health Research University College London Hospital Biomedical Research Centre. Dr. Nelson was supported by an NHMRC Senior Research Fellowship (1137687). We would like to thank the EU-GEI WP2 Group not mentioned in main author list: Kathryn Hubbard, Stephanie Beards, Simona A. Stilo, Pedro Cuadrado, José Juan Rodríguez Solano, David Fraguas, Álvaro Andreu-Bernabeu, Gonzalo López, Bibiana Cabrera, Juan Nacher, Javier Costas, Mario Matteis [8], Marta Rapado-Castro, Emiliano González, Covadonga M. Díaz-Caneja [8], Emilio Sánchez, Manuel Durán-Cutilla, Nathalie Franke, Fabian Termorshuizen, Daniella van Dam, Elles Messchaart, Marion Leboyer [4], Franck Schürhoff, Stéphane Jamain, Grégoire Baudin, Aziz Ferchiou, Baptiste Pignon, Jean-Romain Richard, Thomas Charpeaud, Anne-Marie Tronche, Flora Frijda, Giovanna Marrazzo, Crocettarachele Sartorio, Fabio Seminerio, Camila Marcelino Loureiro, Rosana Shuhama, Mirella Ruggeri, Chiara Bonetto, Doriana Cristofalo, Domenico Berardi, Marco Seri, Elena Bonora, Anastasios Nougus, Giuseppe D’Andrea, Laura Ferraro, Giada Tripoli, Ulrich Reininghaus, Enrique García Bernardo, Laura Roldán, Esther Lorente-Rovira, Ma Soledad Olmeda, Daniele La Barbera, Cristina M. Del-Ben, Lucia Sideli. Study funders contributed to the salaries of the research workers employed, but did not participate in the study design, data analyses, data interpretation, or writing of the manuscript. All authors had full access to the study data and had final responsibility for the decision to submit for publication.
EU-GEI High Risk Study
Maria Calem5, Stefania Tognin5, Gemma Modinos5, Sara Pisani5, Tamar C. Kraan3, Daniella S. van Dam3, Nadine Burger53, G. Paul Amminger58, Athena Politis58, Joanne Goodall58, Stefan Borgwardt77, Erich Studerus77, Ary Gadelha55, Elisa Brietzke78, Graccielle Asevedo55, Elson Asevedo55, Andre Zugman55, Tecelli Domínguez-Martínez79, Manel Monsonet80, Paula Cristóbal-Narváez80, Anna Racioppi56, Thomas R. Kwapil81, Mathilde Kazes61, Claire Daban61, Julie Bourgin61, Olivier Gay61, Célia Mam-Lam-Fook61, Dorte Nordholm82, Lasse Rander82, Kristine Krakauer82, Louise Birkedal Glenthøj82, Birte Glenthøj83, Dominika Gebhard62, Julia Arnhold84, Joachim Klosterkötter62, Iris Lasser63, Bernadette Winklbaur63
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Dr. Arango. has been a consultant to or has received honoraria or grants from Acadia, Angelini, Gedeon Richter, Janssen Cilag, Lundbeck, Minerva, Otsuka, Roche, Sage, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion and Takeda. Dr. Pantelis has received honoraria for talks at educational meetings and has served on an advisory board for Lundbeck, Australia Pty Ltd. Dr. Bernardo has been a consultant for, received grant/research support and honoraria from, and been on the speakers/advisory board of ABBiotics, Adamed, Angelini, Casen Recordati, Eli Lilly, Ferrer, Forum Pharmaceuticals, Janssen-Cilag, Lundbeck, Menarini, Otsuka, Takeda and Somatics. MO Krebs received financial support from Janssen, Otsuka Lundbeck alliance, EIsai for educational activities. All other authors report no financial relationships with commercial interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the EU-GEI High Risk Study are listed below Acknowledgements.
Supplementary information
41380_2020_969_MOESM1_ESM.pdf
Cognitive Functioning throughout Adulthood and Illness Stages in Individuals with Psychotic Disorders and their Unaffected Siblings - Supplementary Materials
Rights and permissions
About this article
Cite this article
Velthorst, E., Mollon, J., Murray, R.M. et al. Cognitive functioning throughout adulthood and illness stages in individuals with psychotic disorders and their unaffected siblings. Mol Psychiatry 26, 4529–4543 (2021). https://doi.org/10.1038/s41380-020-00969-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-00969-z
- Springer Nature Limited
This article is cited by
-
Kognitive Beeinträchtigung in Zusammenhang mit Schizophrenie (CIAS): Diagnostik und Therapie
psychopraxis. neuropraxis (2024)
-
Cognitive impairment in schizophrenia: aetiology, pathophysiology, and treatment
Molecular Psychiatry (2023)
-
The relationship between genetic liability, childhood maltreatment, and IQ: findings from the EU-GEI multicentric case–control study
Social Psychiatry and Psychiatric Epidemiology (2023)
-
Extrapyramidal symptoms predict cognitive performance after first-episode psychosis
Schizophrenia (2022)