Abstract
The importance of tryptophan as a precursor for neuroactive compounds has long been acknowledged. The metabolism of tryptophan along the kynurenine pathway and its involvement in mental disorders is an emerging area in psychiatry. We performed a meta-analysis to examine the differences in kynurenine metabolites in major depressive disorder (MDD), bipolar disorder (BD), and schizophrenia (SZ). Electronic databases were searched for studies that assessed metabolites involved in the kynurenine pathway (tryptophan, kynurenine, kynurenic acid, quinolinic acid, 3-hydroxykynurenine, and their associate ratios) in people with MDD, SZ, or BD, compared to controls. We computed the difference in metabolite concentrations between people with MDD, BD, or SZ, and controls, presented as Hedges’ g with 95% confidence intervals. A total of 101 studies with 10,912 participants were included. Tryptophan and kynurenine are decreased across MDD, BD, and SZ; kynurenic acid and the kynurenic acid to quinolinic acid ratio are decreased in mood disorders (i.e., MDD and BD), whereas kynurenic acid is not altered in SZ; kynurenic acid to 3-hydroxykynurenine ratio is decreased in MDD but not SZ. Kynurenic acid to kynurenine ratio is decreased in MDD and SZ, and the kynurenine to tryptophan ratio is increased in MDD and SZ. Our results suggest that there is a shift in the tryptophan metabolism from serotonin to the kynurenine pathway, across these psychiatric disorders. In addition, a differential pattern exists between mood disorders and SZ, with a preferential metabolism of kynurenine to the potentially neurotoxic quinolinic acid instead of the neuroprotective kynurenic acid in mood disorders but not in SZ.
Similar content being viewed by others
Introduction
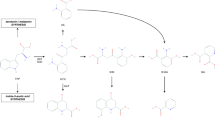
The importance of tryptophan as a precursor for neuroactive compounds has long been acknowledged. The metabolism of tryptophan along the kynurenine pathway and its possible involvement in the pathophysiology of mental disorders is an emerging area in psychiatry [1,2,3,4,5]. Alongside the biosynthesis of serotonin, an estimated 90% of tryptophan is metabolized via the kynurenine pathway [6]. Humans lack the ability to synthesize tryptophan, which has to be acquired from diet [7]. Tryptophan is initially metabolized to kynurenine via the rate-limiting enzymes tryptophan 2,3-dioxygenase (TDO, stress responsive) and indoleamine 2,3-dioxygenase (IDO1, immune responsive). Within the central nervous system (CNS), kynurenine is converted to 3-hydroxyanthranilic acid via anthranilic acid or 3-hydroxykynurenine, and subsequently into quinolinic acid in the microglia. This pathway has been linked with neurotoxicity, via free radical generation contributing to oxidative stress, and the excitotoxic effects of quinolinic acid as a glutamate N-methyl-D-aspartate (NMDA) receptor agonist [8, 9]. Conversely, in astrocytes, kynurenine is converted to kynurenic acid, which has neuroprotective potential, both via NMDA and α7 nicotinic acetylcholine receptors antagonism, as well as through its anti-inflammatory and immunosuppressive functions [7, 9, 10].
Existing preclinical and clinical data suggest that dysregulation of the metabolic fate of tryptophan via the kynurenine pathway may be implicated in a range of severe psychiatric disorders, including: major depressive disorder (MDD) [2], bipolar disorder (BD), and schizophrenia (SZ) [11]. The kynurenine pathway is also influenced by inflammation [12] wherein a pro-inflammatory state can divert tryptophan metabolism towards the kynurenine pathway and away from serotonin production [1]. It is hypothesized that mental disorders are associated with the preferential switch in kynurenine metabolism to favour production of the neurotoxic quinolinic acid metabolite in detriment of the conceptually neuroprotective kynurenic acid in mood disorders and with a switch in favor of kynurenic acid in SZ [1].
Inflammation-associated influx of pro-inflammatory molecules into the brain can amplify macrophage infiltration; these cells have an ~30-fold increased capacity to produce quinolinic acid compared to microglia [13, 14]. The accumulation of quinolinic acid may over-stimulate glutamate receptors resulting in neuronal damage [15], and has been implicated in the often persistent nature of MDD. Conversely, an increase in kynurenic acid has been implicated in BD and SZ, despite theoretical neuroprotection [16]. Some studies have shown that concentrations of kynurenic acid in the cerebrospinal fluid (CSF) are increased in BD and SZ [17,18,19]. Induction and exacerbation of schizophrenia-like symptoms by other NMDA receptor antagonists such as ketamine support the hypothesis of the influence of kynurenic acid on positive symptoms of SZ [20, 21]. Although human studies reporting associations between kynurenic acid levels and SZ behaviour are inconsistent, manipulation of kynurenic acid levels in animal studies support involvement of kynurenic acid, with increased concentrations causing significant cognitive impairment, and reductions potentially resulting in cognitive improvement [22].
Due to the growing number of human studies that have compared the level of kynurenine pathway metabolites in people with mental disorders to healthy controls, some meta-analyses have previously been conducted [22,23,24]. However, those reviews included a relatively small number of studies, focused on a narrow range of metabolites, and/or examined only one psychiatric disorder. In this systematic review and meta-analysis, we aim to build upon previous work by examining blood levels of an extended range of kynurenine metabolites and metabolite ratios (e.g., kynurenine to tryptophan ratio) across three major mental disorders: MDD, BD, and SZ. Furthermore, we sought to explore the influence of blood source and demographic factors that influence kynurenine metabolism via sub-group and meta-regression analyses.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [25]. Electronic databases (Embase, PubMed, and the Cochrane database) were systematically searched from journal inception until June 2019 to identify peer-reviewed studies that assessed kynurenine metabolites (i.e., tryptophan, kynurenine, kynurenic acid, quinolinic acid, 3-hydroxykynurenine, and their associate ratios) in people with either MDD, SZ, or BD, compared to healthy controls. The search terms related to kynurenine metabolites (e.g., kynurenine, tryptophan, indoleamine 2,3-dioxygenase) and mental disorders (e.g., depression, psychosis, schizophrenia). See Supplementary Material for full search terms used. The reference list of major reviews and prior meta-analyses on the topic were also searched for eligible studies.
Study selection
Eligible studies were those: (1) published in English; (2) observational studies including participants with MDD, BD (in any mood state) or SZ, diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) or the International Classification of Diseases (ICD) criteria, compared to healthy controls; (3) measuring tryptophan or any metabolite of the kynurenine pathway in blood (plasma or serum) including kynurenine, kynurenic acid, quinolinic acid, 3-hydroxykynurenine, as well as the ratio of these metabolites (i.e., kynurenine/tryptophan ratio, kynurenic acid/quinolinic acid ratio, and kynurenic acid/kynurenine, and kynurenic acid/3-hydroxykynurenine). Studies that reported mixed data (e.g., a combined sample with >1 of the following populations: MDD, BD, and/or SZ) where outcomes were not disaggregated by diagnosis were excluded; however, studies that combined people with SZ and schizoaffective disorders were included. Studies were also excluded if they measured genetic outcomes only, included samples with other chronic diseases (e.g., celiac disease) or substance abuse diagnoses; or did not include a healthy control comparison group; reviews, abstracts, conference papers, and study protocols were also excluded. If two or more manuscripts reported on the same or overlapping participant samples, data were only extracted for the manuscript that included the largest sample size.
Four investigators independently conducted the searches using Covidence Systematic Review Software. Authors first screened for eligibility based on titles and abstracts according to the eligibility criteria. Articles for which this was unclear were carried forward into the full-text review. The full-text of the remaining papers was independently reviewed by two authors, and eligible studies were included. Disagreements were managed by discussion to reach consensus.
Data extraction
The following data were extracted: study design, key characteristics of participants (e.g., age and sex), outcomes of interest, and key results (means, standard deviations, sample size, and/or p values) of the metabolites. Where results were reported as median and interquartile range, these were converted to mean and standard deviation using the formula proposed by Wan et al. [26] and Lou et al. [27].
Statistical Analyses
The meta-analyses were conducted in Comprehensive Meta-Analysis 3.0 [28] using a random-effects model [29] to account for possible heterogeneity between studies. The following outcomes were explored in separate meta-analyses: (1) The difference in metabolite concentrations between people with MDD, BD, or SZ, and healthy controls, presented as Hedges’ g (with 95% confidence intervals (CI)). For studies that compared different clinical groups to the same group of healthy controls, an averaged effect size (ES) across all clinical groups was computed and used in the pooled analysis. (2) Potential moderators: When there were a sufficient number of studies, effect modifiers including clinical population, age, sex distribution, and blood sample type (plasma or serum) were examined using sub-group analyses and meta-regressions (when at least 3 and 10 studies were available, respectively). The influence of individual studies on the overall ES was also investigated using a leave-one-out sensitivity analyses [30].
To examine the possibility of publication bias affecting the results, the Eggers’ t-test was conducted for meta-analyses with ≥10 studies, as per the Cochrane Collaboration Handbook [31]. Similarly, funnel plots of effect sizes for each metabolite with ≥10 studies were generated. A Duval and Tweedie’s ‘trim-and-fill’ analysis was applied to the random-effects models to re-calculate the pooled effect size to account for any studies which may introduce publication bias. The significance of heterogeneity among studies was examined by the Q statistics, and the I2 was then used to assess the level that the variance among studies could be accounted for by potential systematic factors. We performed a meta-analysis only when at least three studies for a given metabolite were available.
Results
The search strategy resulted in 6519 de-duplicated studies that were screened. A total of 101 studies, comprising 128 comparisons (k), 10,912 participants (5856 with MDD, BD or SZ, and 5056 controls) were included (Fig. 1) [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126]. Sample sizes for each study had a median of 58 participants (range 15–1101). Eighty-six comparisons measured the metabolites in plasma, while 42 measured metabolites in serum. The median age of participants was 25 years (range 25–67). We reported the results according to the different disorders investigated.
Major depressive disorder
A total of 59 studies, comprising 77 comparisons with 6819 participants (n = 3896 with MDD and n = 2923 healthy controls), were included [32, 33, 35, 36, 41,42,43,44,45,46,47, 50, 51, 56,57,58,59, 61, 62, 64, 65, 67,68,69, 72,73,74,75,76,77,78,79,80,81,82,83,84,85,86, 90, 91, 93, 96, 98, 103, 104, 106, 108, 110, 111, 113, 115, 117, 121,122,123,124,125,126]. Of the comparisons that reported medication details (k = 62; 80%), most comparisons (k = 52, 84%) included participants that were either treatment naive or had been removed from medication prior to study commencement. Characteristics of the studies and details regarding the metabolites assessed in each study were reported in Table 1.
Tryptophan (g = −0.51, 95% CI = −0.63 to −0.39, p = <0.001, I2 = 76%, k = 57, n = 7252), kynurenine (g = −0.26, 95% CI = −0.35 to −0.16, p = <0.001, I2 = 44%, k = 26, n = 4790), and kynurenic acid (g = −0.37, 95% CI = −0.52 to −0.21, p = 0.001, I2 = 60%, k = 18, n = 1959) were significantly lower in people with MDD than in healthy controls. There were no significant differences in quinolinic acid (g = −0.03, 95% CI = −0.28 to 0.22, p = 0.80, I2 = 71%, k = 9, n = 991) and 3-hydroxykynurenine (g = 0.04, 95% CI = −0.11 to 0.18, p = 0.63, I2 = 0%, k = 6, n = 757) between people with MDD and healthy controls (Fig. 2a, Supplementary Figs. 1–9).
a) Major depressive disorder compared to healthy controls; b) Bipolar disorder compared to healthy controls; c) Schizophrenia compared to healthy controls. TRYP Tryptophan, KYN Kynurenine, KYNA Kynurenic Acid, 3-HK 3-Hydroxykynurenine, QUIN Quinolinic Acid, KYNA/KYN Kynurenic Acid/Kynurenine Ratio, KYN/TRYP Kynurenine/Tryptophan Ratio, KYNA/QA Kynurenic Acid/Quinolinic Acid Ratio, KYNA/3HK Kynurenic Acid/3-Hydroxykynurenine Ratio.
Ratios of kynurenic acid to kynurenine (g = −0.39, 95% CI = −0.73 to −0.04, p = 0.03, I2 = 82%, k = 6, n = 859), kynurenic acid to quinolinic acid (g = −0.54, 95% CI = −0.82 to −0.27, p = <0.001, I2 = 59%, k = 5, n = 633) and kynurenic acid to 3-hydroxykynurenine (g = −0.42, 95% CI = −0.63 to −0.21, p = <0.001, I2 = 23%, k = 4, n = 577) were significantly lower in people with MDD compared to healthy controls. There was no statistically significant difference in the kynurenine to tryptophan ratio (g = 0.10, 95% CI = −0.05 to 0.25, p = 0.18, I2 = 71%, k = 17, n = 3997) (Figs. 2a and 3).
Minus sign (−) denotes decreased levels of metabolites or ratios in the disorder compared to healthy controls; plus sign (+) denotes increased levels of metabolites or ratios in the disorder compared to healthy controls; and an X denotes a non-significant difference in the disorder compared to healthy controls. When a given disorder was not included in a given metabolite or ratio, it denotes that the given metabolite or ratio was not meta-analysed for that given disorder. When a given metabolite is coded with both minus or plus sign after an X, it denotes that a planned sub-group analysis found that metabolite altered.
In order to verify the consistency of our results, we conducted several sensitivity and sub-groups analyses. In a sensitivity analyses of sample source (plasma vs. serum), the one study that assessed kynurenic acid to kynurenine ratio in plasma reported significantly lower levels in people with MDD compared to controls, while studies (n = 5) that assessed serum samples reported no significant differences. There was no difference in effect or heterogeneity for all other metabolites. Meta-regression analyses based on age and sex (% male sex) were also not significant (Supplementary Table 1).
In the leave-one-out analyses, the removal of Umehara et al. [117] resulted in a significant difference in kynurenine to tryptophan ratio whereby people with MDD had a significantly higher ratio compared to healthy controls (g = 0.15, 95% CI = 0.007 to 0.291, p = 0.04, I2 = 66, k = 11, n = 3931). No other metabolites or ratios were influenced by removing any individual study. Funnel plots are included in the Supplementary Material (Supplementary Figs. 10–13). While Egger’s regression test for kynurenic acid (p = 0.44) and for kynurenine to tryptophan ratio (p = 0.15) had no indication of publication bias, possible publication bias was detected for tryptophan (p = <0.001) and kynurenine (p = 0.002). However, the effect sizes of these metabolites were unaffected by a random effects trim-and-fill analysis.
Bipolar disorder
A total of 14 studies, comprising 15 unique comparisons and 1202 participants (n = 497 BD, n = 705 healthy controls), were included in these meta-analyses [36, 38, 41, 60, 72, 73, 87, 89, 94, 95, 102, 105, 108, 124]. Of the comparisons that reported medication details (k = 10; 66%), five comparisons (50%) included participants that were either treatment naive or had been removed from medication prior to study commencement while the other half had at least 50% of participants receiving medication. Details of the studies and metabolites assessed can be found in Table 2.
Tryptophan (g = −0.56, 95% CI = −0.76 to −0.35, p = <0.001, I2 = 48, k = 12, n = 851), kynurenic acid (g = −0.44, 95% CI = −0.65 to −0.24, p = <0.001, I2 = 33%, k = 7, n = 615) and kynurenic acid to quinolinic acid (g = −0.44, 95% CI = −0.67 to −0.21, p = <0.0001, I2 = 62%, k = 3, n = 299) were significantly lower in people with BD compared to healthy controls. There were no statistically significant differences in quinolinic acid (g = −0.40, 95% CI = −1.01 to 0.20, p = 0.19, I2 = 88%, k = 5, n = 416), kynurenine (g = −0.12, 95% CI = −0.43 to 0.19, p = 0.46, I2 = 79%, k = 9, n = 898), 3-hydroxykynurenine (g = 0.06, 95% CI = −0.23 to 0.34, I2 = 62%, p = 0.70, k = 6, n = 572), and kynurenine to tryptophan ratio (g = 0.11, 95% CI = −0.16 to 0.38, p = 0.41, I2 = 65%, k = 7, n = 737) (Figs. 2b and 3, and Supplementary Figs. 14–20).
In a series of sub-group analyses, studies measuring kynurenine in plasma reported significantly lower levels in people with BD (g = −0.34, 95% CI = −0.62 to −0.061, p = 0.02), while those measuring kynurenine in serum reported no significant difference between people with BD and healthy controls (g = −0.01, 95% CI = −0.41 to 0.39, p = 0.96; Supplementary Table 2).
Meta-regression analyses showed that mean age was positively associated with higher levels of kynurenic acid (p = 0.04) and kynurenine to tryptophan ratio (p < 0.0001), as well as lower levels of tryptophan (p = 0.03), in people with BD compared to healthy controls. Sub-group analysis by biological source (plasma or serum) greatly decreased heterogeneity in studies that measured kynurenine in plasma (I2 = 0%) and tryptophan when measured in serum (I2 = 0%). Planned sub-group analysis based on bipolar phase (depressed, euthymic, manic) showed some differences according to mood state. Tryptophan and kynurenic acid were decreased in acute states (i.e., mania and depression) but not in euthymia; kynurenine and quinolinic acid were decreased only during a depressive state; 3-hydroxykynurenine and kynurenine to tryptophan ratio were increased only in euthymia. However, the small number of studies included in each of these sub-group analyses should be noted. Regarding sex, the % of male sex in the studies was associated with a lower ratio of kynurenine to tryptophan ratio in people with BD compared to healthy controls. No other significant results were reported and due to the limited number of studies, sub-group analyses were not conducted for all other metabolites (Supplementary Table 2).
In the leave–one-out analyses, the removal of the study by Reininghaus et al. [102], which was the only study that reported increased levels of kynurenine in BD, resulted in significant difference in kynurenine between people with BD and healthy controls, with a pooled ES showing decreased kynurenine levels in BD compared to controls (g = −0.23, 95% CI = −0.38 to −0.071, p = 0.04). No other metabolites or ratios were influenced by removing any other individual study. Egger’s regression test for tryptophan did not detect possible publication bias (Supplementary Fig. 21). Trim-and-fill analysis resulted in no change to the original effect size (adjusted effect size = −0.79, original effect size = −0.73).
Schizophrenia
Thirty studies, comprising 36 unique comparisons and 2891 participants (n = 1463 with SZ, n = 1428 healthy controls) were included in the meta-analyses [34, 36, 37, 39, 40, 48, 49, 52,53,54,55, 63, 66, 70, 71, 88, 92, 97, 99,100,101, 107, 109, 112, 114, 116, 118,119,120, 124]. Similar to MDD, of the comparisons that reported medication details (k = 33; 92%), most comparisons (k = 19, 58%) included participants that were either treatment naive or had been removed from medication prior to study commencement. Characteristics of these studies are in Table 3.
Tryptophan (g = −0.24, 95% CI = −0.46 to −0.01, p = 0.04, I2 = 85%, k = 25, n = 2803) was significantly lower in people with SZ compared to healthy controls. There were no significant differences in kynurenine (g = −0.06, 95% CI = −0.31 to 0.20, p = 0.65, I2 = 76%, k = 13, n = 1312), kynurenic acid (g = 0.06, 95% CI = −0.36 to 0.48, p = 0.78, I2 = 87%, k = 10, n = 884), 3-hydroxykynurenine (g = −0.18, 95% CI = −0.91 to 0.56, p = 0.64, I2 = 93%, k = 5, n = 502), quinolinic acid (g = 1.00, 95% CI = −0.43 to 2.44, p = 0.17, I2 = 95%, k = 4, n = 349), kynurenic acid to 3-hydroxykynurenine ratio (g = −0.53, 95% CI = −1.11 to 0.05, I2 = 81%, p = 0.07, k = 3, n = 310), kynurenic acid to kynurenine ratio (g = −0.28, 95% CI = −0.65 to 0.08, p = 0.13, I2 = 30%, k = 3, n = 189), and kynurenine to tryptophan ratio (g = −0.05, 95% CI = −0.60 to 0.49, p = 0.85, I2 = 85%, k = 6, n = 407) (Figs. 2c and Fig. 3, and Supplementary Figs. 22–29).
A leave-one-out analysis significantly affected the results for kynurenic acid to kynurenine ratio, turning the results significant, with a lower ratio, when the study by Barry et al. was excluded (g = −0.49, 95% CI = −0.85 to −0.12, p = 0.01) [34]; also decreased heterogeneity was found when this study was excluded (I2 = 0). This particular study had a low risk of bias but included only participants whose disorder were stable. Tryptophan was particularly susceptible to a leave-one-out analysis with the removal of 15 separate studies resulting in a lack of significant difference between people with SZ and controls. No other metabolite was affected by the removal of any individual study.
In a set of sub-group analyses, studies that assessed kynurenine to tryptophan ratio in plasma reported higher levels in people with SZ than healthy controls (g = 0.36, 95% CI = 0.004 to 0.626, p = 0.05) and decreased heterogeneity (I2 = decreased from 85% to 22%), while there was no significant difference in those that measured this ratio in serum; similar results were seen for kynurenine, where kynurenine levels were decreased in plasma but not in serum (Supplementary Table 3). Meta-regression analyses on these same metabolites using mean age and sex showed no statistical significance. Due to the limited number of studies, sub-group analyses were not conducted for all metabolites (Supplementary material 2). Egger’s regression test for tryptophan (p = 0.43) and kynurenine (p = 0.09) indicated no sign of publication bias (Supplementary Figs. 30 and 31). Trim-and-fill analysis did not change the original effect sizes.
Discussion
This is the largest and most comprehensive meta-analysis to investigate the role of the kynurenine pathway across major mental disorders. We report several significant differences in metabolites of the kynurenine pathway between people with MDD, BD, or SZ, and healthy controls. These differences are evident both in the main analyses, as well as in the sub-group analyses assessing metabolites in plasma, a blood source that yields more accurate results than serum [127, 128]. In particular, the results of these meta-analyses suggest that tryptophan and kynurenine are lower across all conditions studied. While kynurenic acid and the kynurenic acid to quinolinic acid ratio appear decreased in mood disorders (i.e., MDD and BD), kynurenic acid is not altered in SZ. Kynurenic acid to 3-hydroxykynurenine ratio is decreased in MDD but not in SZ. Kynurenic acid to kynurenine ratio was decreased in both MDD and SZ, and the kynurenine to tryptophan ratio was increased, again, in both MDD and SZ. However, when considering only the main analyses in SZ, only tryptophan was altered. In addition, there are no significant differences in quinolinic acid or 3-hydroxykynurenine in any included meta-analyses; this may be due to the relatively few studies included in these particular meta-analyses and also due to low sensitivity of laboratorial assays in assessing these particular metabolites. Most significant effect sizes are only small to moderate in magnitude.
Tryptophan, the main precursor of the kynurenine pathway, is decreased across all mental disorders considered. This likely reflects its relative importance in these disorders, particularly in the context of serotonin bioavailability. Decreased bioavailability of tryptophan is at least partially responsible for the depleted levels of serotonin found in MDD. This decrease in tryptophan is probably also, again, at least partially, responsible for the decreased bioavailability of kynurenine that we found across MDD, BD, and SZ. Furthermore, we also saw an increase in the kynurenine to tryptophan ratio in MDD and SZ. This suggests that the decrease in serotonin bioavailability, traditionally considered as one of the bases of the monoamine hypothesis, is secondary not only to a decreased pool of tryptophan but also to a shift in the tryptophan metabolism away from serotonin towards kynurenine in mood disorders and SZ.
While a decrease in the bioavailability of tryptophan and a shift of tryptophan metabolism from serotonin to kynurenine appears to occur in both mood disorders (MDD and BD) and in SZ, downstream kynurenine, our data suggest that biological pathways may differ. In mood disorders, kynurenic acid is lower, and the ratios of kynurenic acid to kynurenine, and of kynurenic acid to 3-hydroxykynurenine are decreased compared to healthy subjects, suggesting an imbalance between the putatively neuroprotective kynurenic acid and the neurotoxic quinolinic acid, with a preponderance of quinolinic acid. Quinolinic acid, however, is not increased in mood disorders, thus it is not possible to say if this imbalance is due to a decrease in kynurenic acid or to a decrease in kynurenic acid in tandem with an increase in quinolinic acid. Yet, in contrast to mood disorders, we found no convincing evidence of imbalance between kynurenic and quinolinic acids in SZ; both metabolites are not altered in the periphery, and the kynurenic acid to 3-hydroxykynurenine and the kynurenic acid to kynurenine ratios are also not altered.
Our results differ to some extent from prior meta-analyses [22,23,24]. Due to an increased number of studies included and sub-group analyzes, we were able to identify several altered metabolites and ratios. Arnone et al. [23] reported decreased kynurenine levels in MDD; however, they reported no significant difference in tryptophan, kynurenine to tryptophan ratio, the kynurenic acid to quinolinic acid ratio and no difference in any metabolite in BD. Ogyu et al. [24], similarly to our data, reported decreased kynurenine and kynurenic acid in MDD, and also reported quinolinic acid decreased only in a sub-group analysis with only drug-free participants. Finally, consistent with our study, Plitman et al. [22] found no difference in blood kynurenic acid in SZ; however, they also reported kynurenic acid increased in the CNS.
The role of peripheral kynurenine pathway metabolites on central brain function is not fully understood. Tryptophan and kynurenine metabolites can cross the blood-brain barrier; however, the relevance of other metabolites within peripheral circulation requires further investigation. Recently, a study in people with depression reported that plasma kynurenine metabolites are correlated with kynurenine metabolites in cerebrospinal fluid and that a high plasma kynurenine to tryptophan ratio was strongly associated with depression severity [129]. Another study reported that plasma kynurenine to tryptophan ratio was inversely correlated with glutamate levels in the frontal white matter of people with SZ, suggesting that peripheral markers of kynurenine may be related to central mechanisms [40].
Similar to previously conducted meta-analyses [22,23,24], there was a generally high level of statistical heterogeneity in our meta-analyses; the heterogeneity in most meta-analyses decreased when we considered only plasma as the blood source. As a large number of studies included in this review did not include information regarding other potentially related factors, such as symptom severity, medication dose, smoking status, these factors were not included in our meta-analyses. We found that age and sex influenced a number of metabolites in BD but not in MDD or SZ. Furthermore, the high heterogeneity displayed in this meta-analysis suggests that kynurenine pathway metabolism is not homogenous in people with these mental disorders. To guide precision medicine efforts, the investigation of potential sub-populations that are more susceptible to alterations in the kynurenine pathway are warranted [130, 131].
Strengths and limitations
Our study analyzed several components of the kynurenine pathway across MDD, BD, and SZ; by comparing the metabolites alterations across disorders, we were able to not only identify particular alterations in metabolites, but also to derive a pattern for the kynurenine pathway in mood disorders and SZ sufficient to identify a possible divergent pattern between conditions. We also relied on a large sample size (101 studies with 10,281 subjects). Our positive results are unlikely to be substantially influenced by publication bias, given that the funnel plots were mostly symmetrical, and the trim-and-fill procedure did not point to any missing study necessary to impute in order to ‘correct’ the ES. In addition, through a series of sensitivity and sub-group analyses, we were able to rule out the possibility that the results were biased due to a unique outlier. This approach also allowed us to investigate and rule out any single study as the sole source of the high heterogeneity found in virtually all analyses.
Notwithstanding its strengths, our study has some limitations; we included only studies that assessed the kynurenine pathway in peripheral blood; as discussed, we do not know how well peripheral levels reflect brain levels, or activity, of these metabolites. Also, for BD, we grouped all studies together, regardless of mood state; our sub-group analyzes according to mood state suggest that alterations can be greater during acute episodes but the number of studies for each metabolite in each mood state was small and these results are exploratory. Another point is the possible role of diet in modulating tryptophan metabolites. Emerging studies suggest that dietary components can influence tryptophan and kynurenine metabolites [132,133,134]. The included studies did not describe diet composition of the participants; however, previous studies demonstrate that people with mental illness report dietary patterns that are different to healthy controls [135]. It is possible that the participants with a psychiatric disorder had a different diet composition compared to controls and this might have influenced the results [136]. Finally, the metabolites were assessed using a variety of laboratory techniques. Due to the high variability and inconsistent reporting of relevant methods, we did not perform sub-group analyzes according to technique.
Conclusions
This is the first meta-analysis to examine the differences in kynurenine metabolites and to ascertain the kynurenine pathway alteration across MDD, BD, and SZ. In particular, tryptophan and kynurenine are decreased across MDD, BD, and SZ; kynurenic acid and the kynurenic acid to quinolinic acid ratio are decreased in mood disorders (i.e., MDD and BD), whereas kynurenic acid is not altered in SZ; kynurenic acid to 3-hydroxykynurenine ratio is decreased in MDD but not SZ. Kynurenic acid to kynurenine ratio is decreased in both MDD and SZ, and the kynurenine to tryptophan ratio is increased, again, in both MDD and SZ. Taken together, our results suggest that there is a shift in the tryptophan metabolism from serotonin to the kynurenine pathway, across these psychiatric disorders. In addition, a differential pattern exists between mood disorders and SZ, with a preferential metabolism of kynurenine to the potentially neurotoxic quinolinic acid instead of the neuroprotective kynurenic acid in mood disorders but not in SZ. Further studies are required to examine the individual characteristics that modulate kynurenine metabolism in these disorders and to investigate potential treatment options that target the kynurenine pathway in an individualized way as preconized by precision psychiatry [130, 131].
References
Myint AM. Kynurenines: from the perspective of major psychiatric disorders. FEBS J. 2012;279:1375–85.
Pu J, Liu Y, Zhang H, Tian L, Gui S, Yu Y, et al. An integrated meta-analysis of peripheral blood metabolites and biological functions in major depressive disorder. Mol Psychiatry. 2020. https://doi.org/10.1038/s41380-020-0645-4. [Epub ahead of print].
Höglund E, Øverli Ø, Winberg S. Tryptophan metabolic pathways and brain serotonergic activity: a comparative review. Front Endocrinol. 2019;10:158.
Fernstrom JD. A perspective on the safety of supplemental tryptophan based on its metabolic fates. J Nutr. 2016;146:2601S–2608S.
Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med. 2006;8:1–27.
Schwarcz R, Bruno JP, Muchowski PJ, Wu H-Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–77.
Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357:eaaf9794. https://doi.org/10.1126/science.aaf9794.
Stone TW, Forrest CM, Darlington LG. Kynurenine pathway inhibition as a therapeutic strategy for neuroprotection. FEBS J. 2012;279:1386–97.
Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Disco. 2002;1:609–20.
Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology. 2017;112(Pt B):399–412.
Lovelace MD, Varney B, Sundaram G, Lennon MJ, Lim CK, Jacobs K, et al. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology. 2017;112(Pt B):373–88.
Campbell BM, Charych E, Lee AW, Möller T. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci. 2014;8:12.
Espey MG, Chernyshev ON, Reinhard JF Jr, Namboodiri MA, Colton CA. Activated human microglia produce the excitotoxin quinolinic acid. Neuroreport. 1997;8:431–4.
Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229.
Tavares RG, Tasca CI, Santos CE, Alves LB, Porciuncula LO, Emanuelli T, et al. Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem Int. 2002;40:621–7.
Erhardt S, Schwieler L, Imbeault S, Engberg G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology. 2017;112(Pt B):297–306.
Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2012;38:426–32.
Olsson SK, Samuelsson M, Saetre P, Lindstrom L, Jonsson EG, Nordin C, et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of patients with bipolar disorder. J Psychiatry Neurosci. 2010;35:195–9.
Olsson SK, Sellgren C, Engberg G, Landen M, Erhardt S. Cerebrospinal fluid kynurenic acid is associated with manic and psychotic features in patients with bipolar I disorder. Bipolar Disord. 2012;14:719–26.
Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455–67.
de Lucena D, Fernandes BS, Kunz M, Fries GR, Stertz L, Aguiar B, et al. Lack of association between serum brain-derived neurotrophic factor levels and improvement of schizophrenia symptoms in a double-blind, randomized, placebo-controlled trial of memantine as adjunctive therapy to clozapine. J Clin psychiatry. 2010;71:91.
Plitman E, Iwata Y, Caravaggio F, Nakajima S, Chung JK, Gerretsen P, et al. Kynurenic acid in schizophrenia: a systematic review and meta-analysis. Schizophrenia Bull. 2017;43:764–77.
Arnone D, Saraykar S, Salem H, Teixeira AL, Dantzer R, Selvaraj S. Role of Kynurenine pathway and its metabolites in mood disorders: a systematic review and meta-analysis of clinical studies. Neurosci Biobehav Rev. 2018;92:477–85.
Ogyu K, Kubo K, Noda Y, Iwata Y, Tsugawa S, Omura Y, et al. Kynurenine pathway in depression: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:16–25.
Moher D, Liberati A, Tetzlaff J, Altman DG. The PG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat methods Med Res. 2018;27:1785–805.
Borenstein MHLV, Higgins JPT, Rothstein HR, Comprehensive meta-analysis version 3. Biostat 104 Englewood, NJ, 2011.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin trials. 1986;7:177–88.
Mander A, Clayton D. Assessing the influence of a single study in meta-analysis. Stata Tech Bull Repr. 1999;8:108–10.
Higgins JPT, GSe. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. The Cochrane Collaboration: Available at: http://www.cochrane-handbook.org. Accessed 28 May 2014.
Anderson I, Parry-Billings M, Newsholme E, Poortmans J, Cowen P. Decreased plasma tryptophan concentration in major depression: relationship to melancholia and weight loss. J Affect Disord. 1990;20:185–91.
Baranyi A, Amouzadeh-Ghadikolai O, Von Lewinski D, Breitenecker RJ, Stojakovic T, März W, et al. Beta-trace protein as a new non-invasive immunological marker for quinolinic acid-induced impaired blood-brain barrier integrity. Sci Rep. 2017;7:43642.
Barry S, Clarke G, Scully P, Dinan T. Kynurenine pathway in psychosis: evidence of increased tryptophan degradation. J Psychopharmacol. 2009;23:287–94.
Bhagwagar Z, Hafizi S, Cowen P. Cortisol modulation of 5-HT-mediated growth hormone release in recovered depressed patients. J Affect Disord. 2002;72:249–55.
Brundin L, Sellgren C, Lim C, Grit J, Pålsson E, Landen M, et al. An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Transl psychiatry. 2016;6:e865.
Cao B, Wang D, Brietzke E, McIntyre RS, Pan Z, Cha D, et al. Characterizing amino-acid biosignatures amongst individuals with schizophrenia: a case–control study. Amino acids. 2018;50:1013–23.
Castillo MFR, Murata S, Schwarz M, Schütze G, Moll N, Martin B, et al. Celecoxib augmentation of escitalopram in treatment-resistant bipolar depression and the effects on quinolinic acid. Neurol, Psychiatry Brain Res. 2019;32:22–9.
Chiappelli J, Notarangelo FM, Pocivavsek A, Thomas MA, Rowland LM, Schwarcz R, et al. Influence of plasma cytokines on kynurenine and kynurenic acid in schizophrenia. Neuropsychopharmacology. 2018;43:1675–80.
Chiappelli J, Postolache TT, Kochunov P, Rowland LM, Wijtenburg SA, Shukla DK, et al. Tryptophan metabolism and white matter integrity in schizophrenia. Neuropsychopharmacology. 2016;41:2587–95.
Chiaroni P, Azorin J-M, Bovier P, Widmer J, Jeanningros R, Barré A, et al. A multivariate analysis of red blood cell membrane transports and plasma levels of L-tyrosine and L-tryptophan in depressed patients before treatment and after clinical improvement. Neuropsychobiology. 1990;23:1–7.
Cho HJ, Savitz J, Dantzer R, Teague TK, Drevets WC, Irwin MR. Sleep disturbance and kynurenine metabolism in depression. J Psychosom Res. 2017;99:1–7.
Coppen A, Eccleston E, Peet M. Total and free tryptophan concentration in the plasma of depressive patients. Lancet. 1973;302:60–63.
Cowen P, Parry-Billings M, Newsholme E. Decreased plasma tryptophan levels in major depression. J Affect Disord. 1989;16:27–31.
Czermak C, Hauger R, Drevets WC, Luckenbaugh DA, Geraci M, Charney DS, et al. Plasma NPY concentrations during tryptophan and sham depletion in medication-free patients with remitted depression. J Affect Disord. 2008;110:277–81.
Dahl J, Andreassen OA, Verkerk R, Malt UF, Sandvik L, Brundin L, et al. Ongoing episode of major depressive disorder is not associated with elevated plasma levels of kynurenine pathway markers. Psychoneuroendocrinology. 2015;56:12–22.
DeMyer MK, Shea PA, Hendrie HC, Yoshimura NN. Plasma tryptophan and five other amino acids in depressed and normal subjects. Arch Gen psychiatry. 1981;38:642–6.
Domingo EF, Krause RR. Plasma tryptophan tolerance curves in drug free normal controls, schizophrenic patients and prisoner volunteers. J Psychiatr Res. 1974;10:247–61.
Domingues DS, Crevelin EJ, de Moraes LAB, Cecilio Hallak JE, de Souza Crippa JA, Costa Queiroz ME. Simultaneous determination of amino acids and neurotransmitters in plasma samples from schizophrenic patients by hydrophilic interaction liquid chromatography with tandem mass spectrometry. J Sep Sci. 2015;38:780–7.
Doolin K, Allers KA, Pleiner S, Liesener A, Farrell C, Tozzi L, et al. Altered tryptophan catabolite concentrations in major depressive disorder and associated changes in hippocampal subfield volumes. Psychoneuroendocrinology. 2018;95:8–17.
Ebesunun M, Eruvulobi H, Olagunju T, Owoeye O. Elevated plasma homocysteine in association with decreased vitamin B12, folate, serotonin, lipids and lipoproteins in depressed patients. Afr J Psychiatry. 2012;15:25–29.
Fazio F, Lionetto L, Curto M, Iacovelli L, Cavallari M, Zappulla C, et al. Xanthurenic acid activates mGlu2/3 metabotropic glutamate receptors and is a potential trait marker for schizophrenia. Sci Rep. 2015;5:17799.
Fekkes D, Bode WT, Zijlstra FJ, Pepplinkhuizen L. Eicosanoid and amino acid metabolism in transient acute psychoses with psychedelic symptoms. Prostaglandins, Leukotrienes Essent Fat acids. 1996;54:261–4.
Freedman DX, Belendiuk K, Belendiuk GW, Crayton JW. Blood tryptophan metabolism in chronic schizophrenics. Arch Gen Psychiatry. 1981;38:655–9.
Fukushima T, Iizuka H, Yokota A, Suzuki T, Ohno C, Kono Y, et al. Quantitative analyses of schizophrenia-associated metabolites in serum: serum D-lactate levels are negatively correlated with gamma-glutamylcysteine in medicated schizophrenia patients. PloS One. 2014;9:e101652. https://doi.org/10.1371/journal.pone.0101652.
Guicheney P, Leger D, Barrat J, Trevoux R, LIGNIÈRES BD, Roques P, et al. Platelet serotonin content and plasma tryptophan in peri‐and postmenopausal women: variations with plasma oestrogen levels and depressive symptoms. Eur J Clin Investig. 1988;18:297–304.
Hayward G, Goodwin GM, Cowen PJ, Harmer CJ. Low-dose tryptophan depletion in recovered depressed patients induces changes in cognitive processing without depressive symptoms. Biol Psychiatry. 2005;57:517–24.
Healy D, Carney P, Leonard B. Monoamine-related markers of depression: changes following treatment. J Psychiatr Res. 1982;17:251–60.
Hennings A, Schwarz MJ, Riemer S, Stapf TM, Selberdinger VB, Rief W. Exercise affects symptom severity but not biological measures in depression and somatization–results on IL-6, neopterin, tryptophan, kynurenine and 5-HIAA. Psychiatry Res. 2013;210:925–33.
Hoekstra R, Fekkes D, Loonen A, Pepplinkhuizen L, Tuinier S, Verhoeven W. Bipolar mania and plasma amino acids: increased levels of glycine. Eur Neuropsychopharmacol. 2006;16:71–7.
Hoekstra R, van den Broek WW, Fekkes D, Bruijn JA, Mulder PG, Pepplinkhuizen L. Effect of electroconvulsive therapy on biopterin and large neutral amino acids in severe, medication-resistant depression. Psychiatry Res. 2001;103:115–23.
Hu L-J, Li X-F, Hu J-Q, Ni X-J, Lu H-Y, Wang J-J, et al. A simple HPLC–MS/MS method for determination of tryptophan, kynurenine and kynurenic acid in human serum and its potential for monitoring antidepressant therapy. J Anal Toxicol. 2017;41:37–44.
Joaquim HP, Costa AC, Gattaz WF, Talib LL. Kynurenine is correlated with IL-1β in plasma of schizophrenia patients. J Neural Transm. 2018;125:869–73.
Joseph MS, Brewerton TD, Reus VI, Stebbins GT. Plasma-tryptophan/neutral amino acid ratio and dexamethasone suppression in depression. Psychiatry Res. 1984;11:185–92.
Karege F, Widmer J, Bovier P, Gaillard J-M. Platelet serotonin and plasma tryptophan in depressed patients: effect of drug treatment and clinical outcome. Neuropsychopharmacology. 1994;10:207–14.
Kim Y-K, Myint A-M, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Cytokine changes and tryptophan metabolites in medication-naive and medication-free schizophrenic patients. Neuropsychobiology. 2009;59:123–9.
Krause D, Kirnich VB, Stapf TM, Hennings A, Riemer S, Riedel M, et al. Values of cytokines and tryptophan metabolites over a 12 weeks time course in patients with depression and somatoform disorder. Clin Psychopharmacol Neurosci. 2019;17:34.
Krause D, Myint A-M, Schuett C, Musil R, Dehning S, Cerovecki A, et al. High kynurenine (a tryptophan metabolite) predicts remission in patients with major depression to add-on treatment with celecoxib. Front Psychiatry. 2017;8:16.
Kuwano N, Kato TA, Setoyama D, Sato-Kasai M, Shimokawa N, Hayakawa K, et al. Tryptophan-kynurenine and lipid related metabolites as blood biomarkers for first-episode drug-naïve patients with major depressive disorder: an exploratory pilot case-control study. J Affect Disord. 2018;231:74–82.
Lee M, Jayathilake K, Dai J, Meltzer HY. Decreased plasma tryptophan and tryptophan/large neutral amino acid ratio in patients with neuroleptic-resistant schizophrenia: relationship to plasma cortisol concentration. Psychiatry Res. 2011;185:328–33.
Leppik L, Kriisa K, Koido K, Koch K, Kajalaid K, Haring L, et al. Profiling of amino acids and their derivatives biogenic amines before and after antipsychotic treatment in first-episode psychosis. Front Psychiatry. 2018;9:155.
Liu H, Ding L, Zhang H, Mellor D, Wu H, Zhao D, et al. The metabolic factor kynurenic acid of kynurenine pathway predicts major depressive disorder. Front psychiatry. 2018;9:552.
Lucca A, Lucini V, Piatti E, Ronchi P, Smeraldi E. Plasma tryptophan levels and plasma tryptophan/neutral amino acids ratio in patients with mood disorder, patients with obsessive-compulsive disorder, and normal subjects. Psychiatry Res. 1992;44:85–91.
Maes M, De Backer G, Suy E, Minner B. Increased plasma serine concentrations in depression. Neuropsychobiology. 1995;31:10–5.
Maes M, Galecki P, Verkerk R, Rief W. Somatization, but not depression, is characterized by disorders in the tryptophan catabolite (TRYCAT) pathway, indicating increased indoleamine 2, 3-dioxygenase and lowered kynurenine aminotransferase activity. Neuroendocrinol Lett. 2011;32:264–73.
Maes M, Jacobs MP, Suy E, Minner B, Leclercq C, Christiaens F, et al. Suppressant effects of dexamethasone on the availability of plasma L-tryptophan and tyrosine in healthy controls and in depressed patients. Acta Psychiatr Scandinavica. 1990;81:19–23.
Maes M, Meltzer HY, Scharpè S, Bosmans E, Suy E, De Meester I, et al. Relationships between lower plasma L-tryptophan levels and immune-inflammatory variables in depression. Psychiatry Res. 1993;49:151–65.
Maes M, Verkerk R, Vandoolaeghe E, Van Hunsel F, Neels H, Wauters A, et al. Serotonin-immune interactions in major depression: lower serum tryptophan as a marker of an immune-inflammatory response. Eur Arch Psychiatry Clin Neurosci. 1997;247:154–61.
Maes M, Wauters A, Verkerk R, Demedts P, Neels H, Van Gastel A, et al. Lower serum L-tryptophan availability in depression as a marker of a more generalized disorder in protein metabolism. Neuropsychopharmacology. 1996;15:243–51.
Mauri MC, Ferrara A, Boscati L, Bravin S, Zamberlan F, Alecci M, et al. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology. 1998;37:124–9.
Meier TB, Drevets WC, Wurfel BE, Ford BN, Morris HM, Victor TA, et al. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain, Behav, Immun. 2016;53:39–48.
Moaddel R, Shardell M, Khadeer M, Lovett J, Kadriu B, Ravichandran S, et al. Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. Psychopharmacology. 2018;235:3017–30.
Møller S, Kirk L, Honore P. Relationship between plasma ratio of tryptophan to competing amino acids and the response to L-tryptophan treatment in endogenously depressed patients. J Affect Disord. 1980;2:47–59.
Møller SE, Group DUA. Plasma amino acid profiles in relation to clinical response to moclobemide in patients with major depression. J Affect Disord. 1993;27:225–31.
Moreno FA, Gelenberg AJ, Heninger GR, Potter RL, McKnight KM, Allen J, et al. Tryptophan depletion and depressive vulnerability. Biol psychiatry. 1999;46:498–505.
Moreno J, Gaspar E, López-Bello G, Juárez E, Alcazar-Leyva S, González-Trujano E, et al. Increase in nitric oxide levels and mitochondrial membrane potential in platelets of untreated patients with major depression. Psychiatry Res. 2013;209:447–52.
Mukherjee D, Krishnamurthy VB, Millett CE, Reider A, Can A, Groer M, et al. Total sleep time and kynurenine metabolism associated with mood symptom severity in bipolar disorder. Bipolar Disord. 2018;20:27–34.
Myint A, Schwarz MJ, Verkerk R, Mueller H, Zach J, Scharpe S, et al. Reversal of imbalance between kynurenic acid and 3-hydroxykynurenine by antipsychotics in medication-naive and medication-free schizophrenic patients. Brain, Behav, Immun. 2011;25:1576–81.
Myint AM, Kim YK, Verkerk R, Park SH, Scharpe S, Steinbusch HW, et al. Tryptophan breakdown pathway in bipolar mania. J Affect Disord. 2007;102:65–72.
Myint A-M, Kim YK, Verkerk R, Scharpé S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord. 2007;98:143–51.
Ogawa S, Koga N, Hattori K, Matsuo J, Ota M, Hori H, et al. Plasma amino acid profile in major depressive disorder: Analyses in two independent case-control sample sets. J Psychiatr Res. 2018;96:23–32.
Oxenkrug G, van der Hart M, Roeser J, Summergrad P. Anthranilic acid: a potential biomarker and treatment target for schizophrenia. Ann Psychiatry Ment Health. 2016;4:1059.
Pan J-X, Xia J-J, Deng F-L, Liang W-W, Wu J, Yin B-M, et al. Diagnosis of major depressive disorder based on changes in multiple plasma neurotransmitters: a targeted metabolomics study. Transl Psychiatry. 2018;8:1–10.
Platzer M, Dalkner N, Fellendorf FT, Birner A, Bengesser SA, Queissner R, et al. Tryptophan breakdown and cognition in bipolar disorder. Psychoneuroendocrinology. 2017;81:144–50.
Poletti S, Myint AM, Schüetze G, Bollettini I, Mazza E, Grillitsch D, et al. Kynurenine pathway and white matter microstructure in bipolar disorder. Eur Arch psychiatry Clin Neurosci. 2018;268:157–68.
Porter RJ, Gallagher P, Watson S, Smith MS, Young AH. Elevated prolactin responses to L-tryptophan infusion in medication-free depressed patients. Psychopharmacology. 2003;169:77–83.
Potkin SG, Cannon-Spoor HE, DeLisi LE, Neckers LM, Wyatt RJ. Plasma phenylalanine, tyrosine, and tryptophan in schizophrenia. Arch Gen Psychiatry. 1983;40:749–52.
Quintana J. Platelet serotonin and plasma tryptophan decreases in endogenous depression. Clinical, therapeutic, and biological correlations. J Affect Disord. 1992;24:55–62.
Rao ML, Gross G, Strebel B, Bräunig P, Huber G, Klosterkötter J. Serum amino acids, central monoamines, and hormones in drug-naive, drug-free, and neuroleptic-treated schizophrenic patients and healthy subjects. Psychiatry Res. 1990;34:243–57.
Rao ML, Gross G, Strebel B, Halaris A, Huber G, Bräunig P, et al. Circadian rhythm of tryptophan, serotonin, melatonin, and pituitary hormones in schizophrenia. Biol Psychiatry. 1994;35:151–63.
Ravikumar A, Deepadevi K, Arun P, Manojkumar V, Kurup P. Tryptophan and tyrosine catabolic pattern in neuropsychiatric disorders. Neurol India. 2000;48:231.
Reininghaus EZ, McIntyre RS, Reininghaus B, Geisler S, Bengesser SA, Lackner N, et al. Tryptophan breakdown is increased in euthymic overweight individuals with bipolar disorder: a preliminary report. Bipolar Disord. 2014;16:432–40.
Rief W, Pilger F, Ihle D, Verkerk R, Scharpe S, Maes M. Psychobiological aspects of somatoform disorders: contributions of monoaminergic transmitter systems. Neuropsychobiology. 2004;49:24–9.
Roiser JP, Levy J, Fromm SJ, Nugent AC, Talagala SL, Hasler G, et al. The effects of tryptophan depletion on neural responses to emotional words in remitted depression. Biol Psychiatry. 2009;66:441–50.
Savitz J, Dantzer R, Meier TB, Wurfel BE, Victor TA, McIntosh SA, et al. Activation of the kynurenine pathway is associated with striatal volume in major depressive disorder. Psychoneuroendocrinology. 2015;62:54–8.
Schwieler L, Samuelsson M, Frye MA, Bhat M, Schuppe-Koistinen I, Jungholm O, et al. Electroconvulsive therapy suppresses the neurotoxic branch of the kynurenine pathway in treatment-resistant depressed patients. J Neuroinflammation. 2016;13:51.
Sellgren CM, Gracias J, Jungholm O, Perlis RH, Engberg G, Schwieler L, et al. Peripheral and central levels of kynurenic acid in bipolar disorder subjects and healthy controls. Transl Psychiatry. 2019;9:1–9.
Shaw DM, Tidmarsh SF, Karajgi BM. Tryptophan, affective disorder and stress: an hypothesis. J Affect Disord. 1980;2:321–5.
Shovestul BJ, Glassman M, Rowland LM, McMahon RP, Liu F, Kelly DL. Pilot study examining the relationship of childhood trauma, perceived stress, and medication use to serum kynurenic acid and kynurenine levels in schizophrenia. Schizophrenia Res. 2017;185:200.
Song C, Lin A, Bonaccorso S, Heide C, Verkerk R, Kenis G, et al. The inflammatory response system and the availability of plasma tryptophan in patients with primary sleep disorders and major depression. J Affect Disord. 1998;49:211–9.
Sorgdrager F, Doornbos B, Penninx B, de Jonge P, Kema IP. The association between the hypothalamic pituitary adrenal axis and tryptophan metabolism in persons with recurrent major depressive disorder and healthy controls. J Affect Disord. 2017;222:32–9.
Sperner-Unterweger B, Miller C, Holzner B, Laich A, Widner B, Fleischhacker WW, et al. Immunologic alterations in schizophrenia: neopterin, L-kynurenine, tryptophan and T-cell subsets in the acute stage of illness. Pteridines. 2002;13:9–14.
Sublette ME, Galfalvy HC, Fuchs D, Lapidus M, Grunebaum MF, Oquendo MA, et al. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain, Behav, Immun. 2011;25:1272–8.
Szymona K, Zdzisińska B, Karakuła-Juchnowicz H, Kocki T, Kandefer-Szerszeń M, Flis M, et al. Correlations of kynurenic acid, 3-hydroxykynurenine, sIL-2R, IFN-α, and IL-4 with clinical symptoms during acute relapse of schizophrenia. Neurotox Res. 2017;32:17–26.
Teraishi T, Hori H, Sasayama D, Matsuo J, Ogawa S, Ota M, et al. 13 C-tryptophan breath test detects increased catabolic turnover of tryptophan along the kynurenine pathway in patients with major depressive disorder. Sci Rep. 2015;5:15994.
Tortorella A, Monteleone P, Fabrazzo M, Viggiano A, De Luca B, Maj M. Plasma concentrations of amino acids in chronic schizophrenics treated with clozapine. Neuropsychobiology. 2001;44:167–71.
Umehara H, Numata S, Watanabe S-y, Hatakeyama Y, Kinoshita M, Tomioka Y, et al. Altered KYN/TRP, Gln/Glu, and Met/methionine sulfoxide ratios in the blood plasma of medication-free patients with major depressive disorder. Sci Rep. 2017;7:1–8.
van de Kerkhof NW, Fekkes D, van der Heijden FM, Hoogendijk WJ, Stöber G, Egger JI, et al. Cycloid psychoses in the psychosis spectrum: evidence for biochemical differences with schizophrenia. Neuropsychiatr Dis Treat. 2016;12:1927.
Van der Heijden F, Fekkes D, Tuinier S, Sijben A, Kahn R, Verhoeven W. Amino acids in schizophrenia: evidence for lower tryptophan availability during treatment with atypical antipsychotics? J Neural Transm. 2005;112:577–85.
Wei J, Xu H, Ramchand C, Hemmings GP. Low concentrations of serum tyrosine in neuroleptic-free schizophrenics with an early onset. Schizophrenia Res. 1995;14:257–60.
Wood K, Harwood J, Coppen A. The effect of antidepressant drugs on plasma kynurenine in depressed patients. Psychopharmacology. 1978;59:263–6.
Wu Y, Mai N, Zhong X, Wen Y, Zhou Y, Li H, et al. Kynurenine pathway changes in late-life depression with memory deficit. Psychiatry Res. 2018;269:45–49.
Wu Y, Zhong X, Mai N, Wen Y, Shang D, Hu L, et al. Kynurenine pathway changes in late-life depression. J Affect Disord. 2018;235:76–81.
Wurfel B, Drevets W, Bliss S, McMillin J, Suzuki H, Ford B, et al. Serum kynurenic acid is reduced in affective psychosis. Transl psychiatry. 2017;7:e1115.
Young KD, Drevets WC, Dantzer R, Teague TK, Bodurka J, Savitz J. Kynurenine pathway metabolites are associated with hippocampal activity during autobiographical memory recall in patients with depression. Brain, Behav, Immun. 2016;56:335–42.
Zhou Y, Zheng W, Liu W, Wang C, Zhan Y, Li H, et al. Cross-sectional relationship between kynurenine pathway metabolites and cognitive function in major depressive disorder. Psychoneuroendocrinology. 2019;101:72–9.
Andreazza AC, Laksono I, Fernandes BS, Toben C, Lewczuk P, Riederer P, et al. Guidelines for the standardized collection of blood-based biomarkers in psychiatry: Steps for laboratory validity–a consensus of the Biomarkers Task Force from the WFSBP. World J Biol Psychiatry. 2019;20:340–51.
Fernandes B, Steiner J, Berk M, Molendijk M, Gonzalez-Pinto A, Turck C, et al. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol psychiatry. 2015;20:1108–19.
Haroon E, Welle JR, Woolwine BJ, Goldsmith DR, Baer W, Patel T, et al. Associations among peripheral and central kynurenine pathway metabolites and inflammation in depression. Neuropsychopharmacology. 2020;45:998–1007. https://doi.org/10.1038/s41386-020-0607-1.
Fernandes BS, Williams LM, Steiner J, Leboyer M, Carvalho AF, Berk M. The new field of ‘precision psychiatry’. BMC Med. 2017;15:80.
Fernandes BS, Borgwardt S, Carvalho AF, Steiner J. Back to the future: on the road towards precision psychiatry. Front Psychiatry. 2020;11:112. https://doi.org/10.3389/fpsyt.2020.00112.
Gostner JM, Becker K, Croft KD, Woodman RJ, Puddey IB, Fuchs D, et al. Regular consumption of black tea increases circulating kynurenine concentrations: a randomized controlled trial. BBA Clin. 2015;3:31–5.
Gualdoni GA, Fuchs D, Zlabinger GJ, Gostner JM. Resveratrol intake enhances indoleamine-2, 3-dioxygenase activity in humans. Pharmacol Rep. 2016;68:1065–8.
Rudzki L, Ostrowska L, Pawlak D, Małus A, Pawlak K, Waszkiewicz N, et al. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2019;100:213–22.
Teasdale SB, Ward PB, Samaras K, Firth J, Stubbs B, Tripodi E, et al. Dietary intake of people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry. 2019;214:251–9.
Zhu C, Sawrey-Kubicek L, Beals E, Rhodes CH, Houts HE, Sacchi R, et al. Human gut microbiome composition and tryptophan metabolites were changed differently by fast food and Mediterranean diet in four days: A pilot study. Nutr Res. 2020.
Carl GF, Brogan MP, Young BK. Is plasma serine a marker for psychosis? Biol Psychiatry. 1992;31:1130–5.
Domino EF, Krause RR. Free and bound serum tryptophan in drug-free normal controls and chronic schizophrenic patients. Biol Psychiatry. 1974;8:265–79.
Acknowledgements
WM is currently funded by an Alfred Deakin Postdoctoral Research Fellowship and a Multiple Sclerosis Research Australia early-career fellowship. WM has previously received funding from the Cancer Council Queensland and university grants/fellowships from La Trobe University, Deakin University, University of Queensland, and Bond University. WM has received industry funding and has attended events funded by Cobram Estate Pty. Ltd. WM has received travel funding from Nutrition Society of Australia. WM has received consultancy funding from Nutrition Research Australia. WM has received speakers honoraria from The Cancer Council Queensland and the Princess Alexandra Research Foundation. BS is supported by a Clinical Lectureship (ICA-CL-2017-03-001) jointly funded by Health Education England (HEE) and the National Institute for Health Research (NIHR). BS is partly funded by the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust. BS also holds active grants with the Medical Research Council and Guys and St Thomas Charity (GSTT). JC has received research support from Deakin University. AJW is supported by a Deakin University Dean’s Research Postdoctoral Fellowship, and has received research support previously from the Trisno Family Gift, and Deakin University. MB is supported by a NHMRC Senior Principal Research Fellowship (1059660 and 1156072). MB has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Rotary Health, A2 milk company, Meat and Livestock Board, Woolworths, Avant and the Harry Windsor Foundation, has been a speaker for Astra Zeneca, Lundbeck, Merck, Pfizer, and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Lundbeck Merck, Pfizer and Servier—all unrelated to this work. The views expressed are those of the author(s) and not necessarily those of the (partner organization), the NHS, the NIHR, the Department of Health and Social Care, the MRC, NHMRC, or GSTT. AO is supported by a Future Leader Fellowship (#101160) from the Heart Foundation Australia and Wilson Foundation. She has received research funding from National Health & Medical Research Council, Australian Research Council, University of Melbourne, Deakin University, Sanofi, Meat and Livestock Australia and Woolworths Limited and Honoraria from Novartis. The Food & Mood Centre has received funding from the Fernwood Foundation, the A2 Milk Company and Be Fit Foods – unrelated to this paper. ML is funded by a Deakin University PhD Scholarship and has received research support from BeFit Foods. SGC has received a grant for external rotation during psychiatry training period, from Fundación de Psiquiatría y Salud Mental. SGC has received CME-related honoraria, or consulting fees from Janssen-Cilag, Italfarmaco, Angelini and Lundbeck all unrelated to this work. APC Microbiome Ireland is funded by Science Foundation Ireland (SFI), through the Irish Government’s National Development Plan (grant number SFI/12/RC/2273 P2). GC is supported by the Health Research Board (HRB) (grant no ILP-POR-2017-013). AJM is funded by an Australian Rotary Health/Ten Island Tassie Tag Along Tour Funding Partner PhD Scholarship. TR has received grants, fellowships and research support from University of the Sunshine Coast, Australian Postgraduate Awards, Fernwood Foundation and Be Fit Food. TR received consultancy, honoraria and travel funds from Oxford University Press, the University of Melbourne, the University of Sydney, Bond University, University of Southern Queensland, Dietitians Association of Australia, Nutrition Society of Australia, The Royal Australian and New Zealand College of Psychiatrists, Academy of Nutrition and Dietetics, Black Dog Institute, Australian Rotary Health, Australian Disease Management Association, Department of Health and Human Services, Primary Health Networks, Barwon Health, West Gippsland Healthcare Group, Central West Gippsland Primary Care Partnership, Parkdale College, Positive Schools, City of Greater Geelong and Global Age.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
BSF, APD, AFC, BS, JC, MS, ML, SGC, PTT, WM, AJM, TR, AR, AJW, PYL, MB, AON, FJ, and JCS have no conflict of interest regarding this manuscript. GC from APC Microbiome Ireland has conducted studies in collaboration with several companies, including GSK, Pfizer, Cremo, Suntory, Wyeth, Mead Johnson, Nutricia, 4D Pharma, and DuPont. GC has been an invited speaker at meetings organized by Janssen and is receipt of research funding from Pharmavite. GC is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this report.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Marx, W., McGuinness, A.J., Rocks, T. et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: a meta-analysis of 101 studies. Mol Psychiatry 26, 4158–4178 (2021). https://doi.org/10.1038/s41380-020-00951-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-00951-9
- Springer Nature Limited
This article is cited by
-
Dynamics of human serotonin synthesis differentially link to reward anticipation and feedback
Molecular Psychiatry (2024)
-
Sex- and suicide-specific alterations in the kynurenine pathway in the anterior cingulate cortex in major depression
Neuropsychopharmacology (2024)
-
Elevated serum kynurenic acid in individuals with first-episode psychosis and insufficient response to antipsychotics
Schizophrenia (2024)
-
Cannabinoid Modulation of Monoamine Levels in Mouse Brain: Unveiling Neurochemical Dynamics through an Innovative High-performance Liquid Chromatography-Fluorescence Detection Bioanalysis
Journal of Analysis and Testing (2024)
-
Frontostriatal circuitry and the tryptophan kynurenine pathway in major psychiatric disorders
Psychopharmacology (2024)