Abstract
Serotonin and dopamine are putatively involved in the etiology and treatment of anxiety disorders, but positron emission tomography (PET) studies probing the two neurotransmitters in the same individuals are lacking. The aim of this multitracer PET study was to evaluate the regional expression and co-expression of the transporter proteins for serotonin (SERT) and dopamine (DAT) in patients with social anxiety disorder (SAD). Voxel-wise binding potentials (BPND) for SERT and DAT were determined in 27 patients with SAD and 43 age- and sex-matched healthy controls, using the radioligands [11C]DASB (3-amino-4-(2-dimethylaminomethylphenylsulfanyl)-benzonitrile) and [11C]PE2I (N-(3-iodopro-2E-enyl)-2beta-carbomethoxy-3beta-(4′-methylphenyl)nortropane). Results showed that, within transmitter systems, SAD patients exhibited higher SERT binding in the nucleus accumbens while DAT availability in the amygdala, hippocampus, and putamen correlated positively with symptom severity. At a more lenient statistical threshold, SERT and DAT BPND were also higher in other striatal and limbic regions in patients, and correlated with symptom severity, whereas no brain region showed higher binding in healthy controls. Moreover, SERT/DAT co-expression was significantly higher in SAD patients in the amygdala, nucleus accumbens, caudate, putamen, and posterior ventral thalamus, while lower co-expression was noted in the dorsomedial thalamus. Follow-up logistic regression analysis confirmed that SAD diagnosis was significantly predicted by the statistical interaction between SERT and DAT availability, in the amygdala, putamen, and dorsomedial thalamus. Thus, SAD was associated with mainly increased expression and co-expression of the transporters for serotonin and dopamine in fear and reward-related brain regions. Resultant monoamine dysregulation may underlie SAD symptomatology and constitute a target for treatment.
Similar content being viewed by others
Introduction
Social anxiety disorder (SAD) is a highly common psychiatric condition associated with anxious and avoidant behavior in any situation where the individual is subject to scrutiny or becomes the center of attention. This is often a lifelong problem affecting the personal as well as the professional domain [1]. The biological basis of this disorder is still largely unknown although functional neuroimaging studies of SAD have reported aberrant activation and functional connectivity of the amygdala, and other nodes of the brain’s fear network, in response to socially threatening stimuli [2].
Serotonin has long been implicated in the regulation of mood and anxiety [3, 4] and because this neurotransmitter is a major target for pharmaceuticals that are effective for SAD [5] it may be of particular etiological relevance. In earlier nuclear imaging research, patients with SAD exhibited reduced serotonin-1A receptor binding in limbic and paralimbic regions including the amygdala and dorsal raphe nuclei [6]. Moreover, a PET study from our group reported increased presynaptic serotonin synthesis in the amygdala, raphe nuclei, striatum, hippocampus, and anterior cingulate cortex (ACC) [7] and these results were essentially replicated in a separate cohort of patients and controls [8]. Interestingly, amygdala serotonin synthesis capacity correlated with social anxiety symptom severity [7] and was reduced, concomitantly with stress-related amygdala activation, after successful pharmacological treatment [9]. There are also two previous nuclear imaging studies on the serotonin transporter (SERT), both noting higher SERT binding potential (BP) in SAD patients in the thalamus [7, 10] and additionally in the raphe nuclei region, striatum, and insula [7]. The latter findings were demonstrated by use of PET and [11C]DASB (3-amino-4-(2-dimethylaminomethylphenylsulfanyl)-benzonitrile), a highly selective ligand to the SERT [11]. Based on these results, we previously suggested that SAD entails an overactive presynaptic serotonergic system [7, 8].
While mesocortical [12] and mesolimbic [13] dopaminergic neurons are also sensitive to aversive stimuli, the dopamine system has a crucial role in driving prosocial behavior, reward processing, positive affect, and approach motivation [14,15,16,17]. Because it has been reported that SAD is associated with diminished pleasure from social activity and social-motivational dysfunction, an etiologic role for dopamine has been suggested in this disorder [18]. However, only a few nuclear imaging studies have examined putative dopamine abnormalities in SAD [19]. Altered striatal [20,21,22] and extra-striatal [23] dopamine D2 binding have been evaluated but findings have been mixed. Results from studies targeting the dopamine transporter (DAT) are also inconclusive, noting either increased [10] or decreased [24] transporter availability in SAD as well as no difference between SAD patients and healthy controls [22]. Interestingly, Warwick et al. reported increased striatal DAT binding after the treatment of SAD with the SSRI escitalopram [25] suggesting serotonergic influences on dopamine signaling. It should be noted that all previous nuclear imaging studies targeting the DAT in SAD have used SPECT with ligands that are not specific for DATs. In comparison, PET images have higher resolution than SPECT and radioligands that bind highly selectively to DAT, like [11C]PE2I (N-(3-iodoprop-2E-enyl)-2b-carbomethoxy-3b-(4-methyl-phenyl)nortropane), can now be used to improve data quality [26].
Biopsychological theories of personality have proposed that approach-avoidance conflicts in social situations, a prominent feature of SAD symptomatology, reflect the balance between serotonin and dopamine signaling in neural pathways underlying fear and reward [27,28,29,30]. Consistent with these theoretical models, pharmacological and anatomical studies support that the serotonin and dopamine systems have reciprocal functional influences on each other [31,32,33,34]. At the anatomical level, serotonergic cell bodies in the raphae nuclei project to the striatum where their axon terminals are in close proximity to dopamine cells [35] and, in rats, there is evidence of a direct serotonergic inhibitory input from the median raphe nucleus to the dopaminergic substantia nigra neurons [36]. However, it is not known if serotonin-dopamine interactions are involved in the pathophysiology of anxiety disorders like SAD, and nuclear imaging studies directly addressing this topic are therefore needed. Transporter functions may be particularly relevant targets for such studies [37]. For example, SERT availability predicts amygdala reactivity in healthy volunteers [38] and polymorphisms in the genes encoding SERT and DAT, influence amygdala responsiveness in patients with SAD [39,40,41].
The principal aim of the present multitracer PET study was to examine the intra-regional co-expression of serotonin and DATs in patients with SAD, as compared with healthy controls, by estimating the statistical interaction effect of SERT/DAT binding in fear- and reward-relevant brain regions. We used PET with [11C]DASB and [11C]PE2I as radiotracers to determine if there is a different brain SERT/DAT-balance in the two groups. In addition, differences between SAD patients and controls were evaluated within the two monoamine transporters separately. We here sought to replicate our earlier results on increased SERT availability in SAD [7] in a new and larger sample of patients and controls. Due to the contradictory results of earlier SPECT studies on DAT, we were also interested in putative SAD-related aberrations in DAT binding when using a more sensitive method, i.e., [11C]PE2I PET.
Materials and methods
Participants
Twenty-nine patients with SAD and forty-three healthy controls (HC) underwent [11C]DASB and [11C]PE2I PET imaging. Two patients with SAD were excluded from all analyses due to magnetic resonance imaging (MRI) contraindications and withdrawing from the study before completed MRI, respectively, leaving 27 patients (17 men, 10 women; mean ± SD age, 31.10 ± 10.32 years), and 43 HC (23 men, 20 women; 32.81 ± 11.56 years) in the analyses. In addition, one male HC was excluded from all SERT-related analyses because of no DASB signal in large areas of the selected regions-of-interest (ROIs). The mean duration of illness was estimated at 19.6 years. All participants were right-handed except three participants in the control group. To our knowledge, there is no evidence that handedness affects SERT/DAT distribution. The groups did not differ from each other in age (t = 0.655, P = .51) or sex distribution (χ2 = 0.608, P = 0.60). Participants were recruited through advertisements in newspapers, public billboards, and the internet. Exclusion criteria were age <18 or >65 years, earlier PET-scan, contraindications for MRI, pregnancy, menopause, substance abuse or dependency, any ongoing severe somatic disease or serious psychiatric disorder (e.g., major depressive disorder, suicidality or psychosis), any ongoing treatment for psychiatric disorders or treatment that was terminated <3 months ago.
All participants were screened for participation using an extensive online form and those who did not meet the exclusion criteria were administered an excerpt from the Structured Clinical Diagnostic Interview for the DSM-IV [42] and the full Mini-International Neuropsychiatric Interview [43] via telephone to ensure that all patients in the SAD group fulfilled the DSM-IV criteria for SAD as primary diagnosis, and that none in the control group had a psychiatric diagnosis. Social anxiety symptom severity was measured with the self-report version of the Liebowitz Social Anxiety Scale (LSAS-SR) [44], with higher scores indicating greater severity (range 0–144). SAD patients (mean ± SD: 84.96 ± 20.37) and HC (7.93 ± 7.46) differed significantly on this scale (t(66) = 22.13, P < 0.001). All participants provided written informed consent and the study was approved by the Regional Ethical Review Board in Uppsala as well as the Uppsala University Radiation Safety Committee.
Imaging procedure
Positron emission tomography
A Siemens ECAT EXACT HR+ (Siemens/CTI) was used to acquire the PET images with 63 contiguous planes of data and slice thickness of 2.46 mm resulting in a total axial field of view of 155 mm. Participants fasted for at least 3 h and refrained from alcohol, nicotine, and caffeine for at least 12 h before the scan. Participants were positioned supine in the scanner with their head gently fixated and a venous catheter for tracer injections was inserted in the participants’ arm. A 10-min transmission scan for attenuation correction was performed using three retractable germanium (68Ge) rotating line sources.
The participants were injected with on average 334.43 ± 22.75 MBq of the [11C]PE2I tracer through an intravenous bolus and 22 frames of data were acquired over 80 min of data (4 × 60 s, 2 × 120 s, 4 × 180 s, 12 × 300 s). Following a 45–60 min waiting period to allow for sufficient radio decay, acquisition commenced for [11C]DASB using an identical injection procedure and an average activity of 329.93 ± 29.70 MBq. In total, 22 frames of data were acquired over 60 min (1 × 60 s, 4 × 30 s, 3 × 60 s, 4 × 120 s, 2 × 180 s, 8 × 300 s).
Magnetic resonance imaging
The participants’ PET images were co-registered to their individual T1-weighted MR image to make ROI analyses possible. Thus, participants underwent an anatomical T1-weighted MR scan (echo time = 50 ms; repetition time = 500 ms; Field of view = 240 × 240 mm2; voxel size = 0.8 × 1.0 × 2.0 mm3; 170 contiguous slices) on a Philips Achieva 3.0 T whole body MR-scanner (Philips Medical Systems, Best, The Netherlands) with an 8-channel head-coil. Five SAD and twenty-four HC participants were scanned with a 32-channel head coil due to scanner upgrade.
Data preprocessing
With regard to PET data, ordered subset expectation maximization with six iterations and eight subsets and a 4 mm Hanning post filter with appropriate corrections were used to reconstruct dynamic images. The dynamic PET images were realigned to adjust for inter-frame movement using VOIager software 4.0.7 (GE Healthcare, Uppsala, Sweden). Voxel-wise parametric images of non-displaceable BP (BPND) were calculated for both radioligands with the cerebellum as reference region. Reference Logan [45] was used for [11C]DASB (time interval 30–60 min), where BPND was estimated as the distribution volume ratio (DVR)-1 (DVR-1 = BP). Receptor parametric mapping [46], a basis function implementation of the simplified reference tissue model, was used for [11C]PE2I [47]. Cerebellar gray matter was selected as reference region for both radioligands because it has none to negligible levels of SERT and DAT. BPND-images were automatically outlined on each participant’s anatomical T1-weighted image using the PVElab software [48].
The [11C]DASB BPND and [11C]PE2I BPND images were co-registered to the anatomical T1-weighted MR image using Statistical Parametric Mapping 8 (SPM8; (Wellcome Department of Cognitive Neurology, University College London, www.fil.ion.ucl.ac.uk) implemented in Matlab (Mathworks Inc., Nantucket, MA, USA). The T1-image was then segmented and normalized to the Montreal Neurological Institute standard space and the transformation parameters applied to the [11C]DASB and [11C]PE2I BPND images, resulting in parametric images with 2 mm isotropic voxels. Images were then smoothed using a 12 mm Gaussian kernel.
Statistical analysis
ROIs were selected based on expected radioligand uptake and earlier neuroimaging research in SAD and anxiety disorders [6, 7, 10, 22,23,24, 49, 50]. The a priori defined ROIs for both tracers were the amygdala, hippocampus, caudate nucleus, putamen, nucleus accumbens (NAcc), pallidum, and thalamus. The [11C]PE2I uptake is largely limited to these regions. For [11C]DASB the ACC, insula cortex, and raphe nuclei were added as additional ROIs. All anatomical regions except NAcc and raphe nuclei were defined using the automated anatomical labeling (AAL) library from the Wake Forest University Pickatlas [51]. The AAL library does not contain any definitions of NAcc or raphe nuclei and therefore the Hammersmith atlas [52] and PVElab were used for these regions, respectively. NAcc was included to enable the evaluation of differential effects in the ventral versus dorsal striatum.
Patient and control group BPND distributions were tested for normality and heterogeneity and were deemed to be well in range for using parametric analyses.
To examine group differences (SAD vs. HC) in BP, two-sample t tests were performed for SERT and DAT BPND separately in SPM8 with age, sex, and scanner version as covariates. The analyses were corrected for familywise error (FWE) within the ROIs using random field theory and the statistical threshold was set at PFWE < 0.05.
Group differences in regional co-expression were assessed by comparing SERT-DAT partial Pearson’s product-moment correlations (R) between groups, with age, sex, and MR-scanner version partialled out. Correlations between SERT and DAT BPND were calculated at the voxel level for the SAD group and HC group separately, as a measure of regional co-expression using the same methodology as in a previous multitracer PET study [49]. Correlation coefficients were then Fisher transformed to Z-values which were subsequently used in voxel-wise group comparisons with the statistical threshold set to P < 0.05 [49]. Analyses were performed in MatlabR2018a.
To evaluate the relation between SAD symptom severity within SAD patients and BP, a multiple regression was performed in SPM8 with LSAS-SR score as a predictor for SERT and DAT BPND separately with age and sex as covariates and the statistical threshold set at PFWE < 0.05. To examine the effect of SERT-DAT regional co-expression on symptom severity within the patient group in our a priori defined ROIs, BPs for SERT, DAT, and their statistical interaction were entered as predictors into voxel-wise regression models with symptom severity (LSAS-SR) as outcome variable in Matlab with the threshold set to P < 0.05.
Results
Serotonin transporter availability
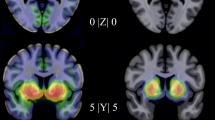
Mean BPND values for each ROI are listed in Supplementary Table 1. SAD patients as compared with HC had significantly higher SERT availability ([11C]DASB BPND) in the left NAcc (Table 1, Fig. 1c) and, at uncorrected level, in all of the investigated ROIs except the raphae and pallidum (Supplementary Table 2, Supplementary Fig. 1). SERT availability did not correlate with symptom severity (LSAS-SR) within patients at the a priori p-level, while there were several positive correlations within our ROIs at P < 0.05 uncorrected (Supplementary Table 2).
The left panel shows mean serotonin transporter (SERT) binding potential (BPND) in (a) social anxiety disorder (SAD) patients and (b) healthy controls. c A cluster with significantly enhanced SERT binding potential (BPND) was found in the SAD group in the nucleus accumbens but (d) no clusters where symptom severity was significantly related to SERT BPND were detected at corrected p-levels. The right panel shows corresponding mean dopamine transporter (DAT) BPND in (e) SAD patients and (f) healthy controls. g No clusters where SAD differed significantly from controls on DAT BPND were detected at corrected p-levels but (h) clusters with a significant positive correlation between symptom severity, as measured with the Liebowitz social anxiety scale (LSAS-SR), and DAT BPND were found in the amygdala, hippocampus, pallidum, and putamen. Coordinates are in Montreal Neurological Institute space. The colorbar indicates binding potentials for the two top rows. Parametric images are overlaid on a standard MRI image.
Dopamine transporter availability
There were no significant between-group differences in DAT binding levels ([11C]PE2I BPND) in any of the specified ROI:s at the a priori statistical threshold. At the more liberal p < 0.05 uncorrected level, there was higher DAT availability in the patient group in the left amygdala, and bilateral hippocampus and striatum (Supplementary Table 2, Supplementary Fig. 1). In the SAD group, symptom severity (LSAS-SR) correlated positively with DAT availability in the right amygdala, left hippocampus and in a cluster in the right putamen extending into pallidum, see Table 1 and Fig. 1h. A trend in the same direction was evident in several of the investigated ROIs including the left amygdala (PFWE = 0.081), left putamen (PFWE = 0.055), left pallidum (PFWE = 0.065), and bilateral NAcc (PFWE = 0.098)—see Supplementary Fig. 1.
Regional co-expression of serotonin and dopamine transporters
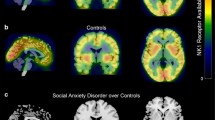
SERT-DAT regional co-expression was almost exclusively positive within both groups, and no significant negative co-expressions were found in any region (Fig. 2a, b). Group comparisons showed significantly higher SERT-DAT co-expression, as reflected by higher positive transporter correlations, in the SAD group relative to HC in the left amygdala, and the right-sided caudate, putamen, NAcc and posterior ventral thalamus—see Table 2 and Fig. 2c. A lower co-expression in the dorsomedial thalamus was found in the SAD group (<HC). A follow-up logistic regression analysis of these clusters showed that SAD diagnosis was significantly predicted by the interaction between transporters in the amygdala (P = 0.032, Z = −2.15), putamen (P = 0.036, Z = −2.09), and dorsomedial thalamus (P = 0.013, Z = 2.49). In addition, inclusion of transporter interaction terms in the model increased the model fit, compared with main effects of transporters, as reflected by McFadden R2 values (interaction/main effects: amygdala = 0.25/0.18; putamen = 0.24/0.19; thalamus = 0.25/0.15). Within the patient group, SERT × DAT interactions did not predict social anxiety symptom severity (LSAS-SR).
Regional co-expression of serotonin (SERT; [11C]DASB BPND) and dopamine (DAT; [11C]PE2I BPND) transporters, indexed by positive voxel-wise Pearson’s product-moment correlation coefficients, for (a) patients with social anxiety disorder (SAD), and (b) healthy controls. c Clusters of significantly higher regional co-expression in the SAD group in the amygdala, caudate, putamen, nucleus accumbens, and thalamus as compared with the healthy controls. The colorbar indicates Pearson’s correlation coefficients for the two top rows. Parametric images are overlaid on a standard MRI image.
Discussion
We used multitracer PET, with validated and highly specific radioligands, to evaluate differences in regional expression and co-expression of serotonin and dopamine transporters between patients with SAD and healthy controls. There were indices of upregulated transporter expression within each transmitter system, and significantly higher SERT/DAT co-expression, in SAD patients in brain regions involved in fear and reward processing. Significant statistical interactions between the transporters, indicating increased SERT/DAT co-expression in SAD relative to HC, were prominent in the amygdala, a structure heavily implicated in fear and anxiety [53, 54] strongly influenced both by serotonin [55] and dopamine [56]. This was also noted in the putamen which is involved in reinforcement learning [57] and may be structurally enlarged in SAD [58]. The thalamus was the only brain region exhibiting a mixed direction pattern since a higher positive SERT-DAT correlation in patients than controls was noted in the posterior ventral region while the reverse was true in the dorsomedial thalamus where the transporter interaction term also significantly predicted SAD diagnosis. In addition, higher positive SERT-DAT correlations in SAD (>HC) were noted in the right caudate nucleus in the dorsal striatum as well as the NAcc in the ventral striatum. The NAcc has been found to be important both for aversion and reward behaviors [59] and, together with the amygdala, it is considered to be part of a ventral system of emotion regulation [60, 61].
While our results on transporters are interesting in the light of pharmacologic and anatomical studies demonstrating functional reciprocity between serotonin and dopamine [31,32,33,34, 62, 63], the functionality of the altered SERT/DAT co-expression in SAD is not known. Transporter interactions may, however, impact the equilibrium between neural activations in fear and reward networks of the brain with resultant proneness for approach-avoidance conflicts at the behavioral level. Local coinciding increases of dopamine and SERT availability could thus play a causal or modulatory role in SAD, however we cannot determine if altered monoamine functions result in or from SAD.
Looking at the membrane transporter proteins separately, the increased SERT availability in patients with SAD is in line with previous reports from our [7] and other groups [10]. We recently reported increased striatal SERT availability in patients with SAD, and we here extend these findings by specifically targeting and reporting higher SERT availability in the NAcc, in the patient group. The NAcc has a high density of serotonergic neurons in its shell [64], and it is an important region for hedonic reward processing [17, 61]. Communication between the amygdala and NAcc has also been found to modulate reward-seeking behavior in rodents, where amygdaloid innervation of the NAcc reinforced additional reward-seeking mediated by dopamine D1-type receptor signaling [65]. At a lenient statistical threshold, SERT availability was increased in the SAD group in all ROIs except the pallidum and raphae, along with positive correlations with symptom severity in the amygdala, hippocampus, putamen, pallidum, and caudate nucleus (Supplementary Table 2). Because these results did not survive correction for multiple comparisons, they should be interpreted with caution. Consistently, however, other [11C]DASB PET studies have also noted positive associations between anxiety-related temperamental traits and higher SERT binding in the amygdalo-hippocampal region in rhesus monkeys [66] and the thalamus in humans [67].
The current data additionally support that DAT availability moderates SAD symptomatology as we noted significant positive correlations between symptom severity in SAD patients and DAT binding in the amygdala, hippocampus, putamen, and pallidum. However, a group difference (SAD > HC) in DAT availability was only observed in the amygdala, hippocampus, and striatum at the uncorrected p-level. Similarly, in a SPECT study on Parkinsonian patients, Moriyama et al. [68] noted no group differences in DAT BP values between SAD and non-SAD participants although SAD symptom severity correlated positively with increased DAT density in the bilateral putamen and left caudate. On the other hand, van der Wee et al. [10] noted higher striatal DAT binding in SAD patients than controls, but no correlation with behavioral measures. There are also SPECT studies of SAD reporting either null findings [22] or findings pointing in the opposite direction, i.e., lowered DAT density in patients [24]. However, in the present trial, using a larger sample and a more sensitive PET methodology with high transporter specificity, there were no brain regions demonstrating higher DAT or SERT binding in controls (>SAD) even at liberal statistical levels.
Previous neuroimaging data on increased serotonin synthesis capacity in the amygdala and limbic areas [7, 8], downregulation of serotonin-1A autoreceptors [6], and increased SERT availability [7, 10] in SAD could reflect an elevated presynaptic serotonergic activity in this disorder, consistent with animal and human data showing anxiogenic effects of serotonin in the amygdala [38, 69, 70]. Our data, demonstrating increased striatal SERT availability together with increased DAT expression correlating with social anxiety, could indicate a generally overactive presynaptic monoaminergic system, possibly due to an increased number of monoamine nerve terminals, in SAD. However, in contrast to serotonin, there are reasons to suspect dopamine hypoactivity in SAD. PET studies of Parkinson’s disease have demonstrated that greater DAT levels are associated with lower dopamine turnover and lower synaptic dopamine concentrations that cannot be explained solely by dopaminergic terminal loss [71]. Studies of DAT knockout mice consistently show that decreased DAT levels correspond to increased dopamine turnover and increased synaptic dopamine levels [72]. DAT knockout mice also appear less anxious in the elevated plus maze and other anxiety-relevant paradigms [73]. Hence, it is plausible that upregulation of the DAT leads to the increased clearance of dopamine from the synaptic space, lowered dopamine concentration and lowered dopamine turnover, possibly contributing to reduced motivational drive for, and pleasure from, social interactions. Indirect evidence for this notion has also been provided by fMRI activation studies noting reduced activation of the NAcc during social reward anticipation in individuals with SAD relative to healthy controls [18, 74]. It should be noted that the previous nuclear imaging studies of striatal DAT in SAD are inconclusive [10, 22, 24] but, to our knowledge, our study is the first one addressing this topic using PET. Additional multitracer PET studies, e.g., using measures of dopamine synthesis capacity and release, are needed to clarify if SAD is associated with presynaptic dopaminergic over- or under-activity.
A limitation of the current study is that, while the sample size is comparatively large for a PET study, lack of power should be taken into consideration. This is one possible reason why a steady pattern of group differences along with correlations between transporter availability and symptom severity, was not always observed. Another reason could be heterogeneity within the SAD sample as there may be considerable individual differences in personality traits, for example in neuroticism facets like impulsivity, or extraversion facets like excitement seeking. Monoamine transporter parameters could be more strongly related to some of these traits than others [75]. A related caveat is that we had no scales for behavioral approach specifically in our study, and because of the uptake profile of the [11C]PE2I radiotracer, we could not study DAT availability in all relevant nodes of the brain’s reward pathway such as the orbitofrontal and ventromedial prefrontal cortices. Also, the present PET data on transporter levels do not provide mechanistic insights into how serotonergic and dopaminergic signaling is altered during situational demands faced by socially anxious individuals. This question could be addressed with fMRI activation studies together with PET. A feasible way for further insight into SERT-DAT interactions could also be to study concomitant changes in both systems after effective treatment.
Conclusion
We demonstrate increased expression and co-expression of the transporters for serotonin and dopamine in SAD, relative to healthy controls, in fear- and reward-relevant brain regions. Presynaptic serotonergic and dopaminergic activity may be important biological factors underlying excessive social anxiety, putatively affecting aversive as well as appetitive motivation. These findings may cast further light on the pathogenesis of SAD and prove useful for the development of future anxiolytic treatments targeting the interaction between the serotonin and dopamine systems.
References
Stein MB, Stein DJ. Social anxiety disorder. Lancet 2008;371:1115–25.
Brühl AB, Delsignore A, Komossa K, Weidt S. Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev. 2014;47:260–80.
Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–66.
Gordon JA, Hen R. The serotonergic system and anxiety. Neuromolecular Med 2004;5:27–40.
Mayo-Wilson E, Dias S, Mavranezouli I, Kew KMA, Clark DM, Ades A, et al. Psychological and pharmacological interventions for social anxiety disorder in adults: a systematic review and network meta-analysis. Lancet Psychiatry 2014;1:368–76.
Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK, et al. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61:1091–89.
Frick A, Åhs F, Engman J, Jonasson M, Alaie I, Björkstrand J. et al. Serotonin synthesis and reuptake in social anxiety disorder a positron emission tomography study. JAMA Psychiatry. 2015;72:794–802.
Furmark T, Marteinsdottir I, Frick A, Heurling K, Tillfors M, Appel L, et al. Serotonin synthesis rate and the tryptophan hydroxylase-2: G-703T polymorphism in social anxiety disorder. J Psychopharmacol 2016;30:1028–35.
Frick A, Åhs F, Appel L, Jonasson M, Wahlstedt K, Bani M, et al. Reduced serotonin synthesis and regional cerebral blood flow after anxiolytic treatment of social anxiety disorder. Eur Neuropsychopharmacol 2016;26:1775–83.
van der Wee NJ, van Veen JF, Stevens H, van Vliet IM, van Rijk PP, Westenberg HG. Increased serotonin and dopamine transporter binding in psychotropic medication-naive patients with generalized social anxiety disorder shown by 123I-beta-(4-iodophenyl)-tropane SPECT. J Nucl Med. 2008;49:757–63.
Houle S, Ginovart N, Hussey D, Meyer JH, Wilson AA. Imaging the serotonin transporter with positron emission tomography: initial human studies with [11C]DAPP and [11C]DASB. Eur J Nucl Med. 2000;27:1719–22.
Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–62.
de Jong JW, Afjei SA, Pollak Dorocic I, Peck JR, Liu C, Kim C, et al. A neural circuit mechanism for encoding aversive stimuli in the mesolimbic dopamine system. Neuron. 2019;101:133–51.
Smillie LD, Wacker J. Dopaminergic foundations of personality and individual differences. Front Hum Neurosci 2014;8:874.
Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology. 2008;199:457–80.
Wacker J, Smillie LD. Trait extraversion and dopamine function. Soc Personal Psychol Compass 2015;9:225–38.
Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86:646–64.
Richey JA, Ghane M, Valdespino A, Coffman MC, Strege MV, White SW, et al. Spatiotemporal dissociation of brain activity underlying threat and reward in social anxiety disorder. Soc Cogn Affect Neurosci. 2017;12:81–94.
Doruyter A, Dupont P, Stein DJ, Lochner C, Warwick JM. Nuclear neuroimaging in social anxiety disorder: a review. J Nucl Med. 2018;59:1794–800.
Schneier FR, Liebowitz MR, Abi-dargham A, Zea-ponce Y, Lin S-H, Laruelle M. Low dopamine D 2 receptor binding potential in social phobia. Am J Psychiatry. 2000;157:457–9.
Schneier FR, Martinez D, Abi-Dargham A, Zea-Ponce Yolanda, Simpson HB, Liebowitz MR, et al. Striatal dopamine D2 receptor availability in OCD with and without comorbid social anxiety disorder: preliminary findings. Depress Anxiety. 2008;25:1–7.
Schneier FR, Abi-dargham A, Martinez D, Slifstein M, Whang DR, Liebowitz MR, et al. Dopamine transporters, D2 receptors, and dopamine release in generalized social anxiety disorder. Depress Anxiety. 2009;8:1–8.
Plavén-Sigray P, Hedman E, Victorsson P, Matheson GJ, Forsberg A, Djurfeldt DR, et al. Extrastriatal dopamine D2-receptor availability in social anxiety disorder. Eur Neuropsychopharmacol. 2017;27:462–9.
Tiihonen J, Kuikka J, Bergström K, Lepola U, Koponen H, Leinonen E. Dopamine reuptake site densities in patients with social phobia. Am J Psychiatry. 1997;154:239–42.
Warwick JM, Carey PD, Cassimjee N, Lochner S, Hemmings H, Smook-Moolman H, et al. Dopamine transporter binding in social anxiety disorder: the effect of treatment with escitalopram. Metab Brain Dis. 2012;27:151–8.
Appel L, Jonasson M, Danfors T, Nyholm D, Askmark H, Lubberink M, et al. Use of 11C-PE2I PET in differential diagnosis of parkinsonian disorders. J Nucl Med. 2015;56:234–42.
Gray JA, McNaughton N. The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. New York: Oxford University Press; 2000.
Cloninger RC. A unified diosocial theory of personality and its role in the development of anxiety states. Psychiatr Dev. 1986;4:167–226.
Kimbrel NA. A model of the development and maintenance of generalized social phobia. Clin Psychol Rev. 2008;28:592–612.
Corr PJ, McNaughton N. Neuroscience and approach/avoidance personality traits: A two stage (valuation-motivation) approach. Neurosci Biobehav Rev. 2012;36:2339–54.
Dewey SL, Smith GS, Logan J, Alexoff D, Ding YS, King P, et al. Serotonergic modulation of striatal dopamine measured with positron emission tomography (PET) and in viva microdialysis. J Neurosci. 1995;75:921–9.
Smith GS, Ma Y, Dhawan V, Chaly T, Eidelberg D. Selective serotonin reuptake inhibitor (SSRI) modulation of striatal dopamine measured with [11C]-raclopride and positron emission tomography. Synapse. 2009;63:1–6.
Daws LC. Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharm Ther. 2009;121:89–99.
Nakamura K. The role of the dorsal raphé nucleus in reward-seeking behavior. Front Integr Neurosci. 2013;7:1–18.
Esposito E, Di Matteo V, Di Giovanni G. Serotonin-dopamine interaction: an overview. Prog Brain Res. 2008;172:3–6.
Dray A, Gonye TJ, Oakley NR, Tanner T. Evidence for the existence of a raphe projection to the substantia nigra in rat. Brain Res. 1976;113:45–57.
Forstner AJ, Rambau S, Friedrich N, Ludwig KU, Böhmer AC, Mangold E, et al. Further evidence for genetic variation at the serotonin transporter gene SLC6A4 contributing toward anxiety. Psychiatr Genet. 2017;27:96–102.
Rhodes RA, Murthy NV, Dresner MA, Selvaraj S, Stavrakakis N, Babar S, et al. Human 5-HT transporter availability predicts amygdala reactivity in vivo. J Neurosci. 2007;27:9233–7.
Furmark T, Tillfors M, Garpenstrand H, Marteinsdottir I, Långström B, Oreland L, et al. Serotonin transporter polymorphism related to amygdala excitability and symptom severity in patients with social phobia. Neurosci Lett. 2004;362:189–92.
Furmark T, Henningsson S, Appel L, Åhs F, Linnman C, Pissiota A, et al. Genotype over diagnosis in amygdala responsiveness: affective processing in social anxiety disorder. J Psychiatry Neurosci. 2009;34:30–40.
Bergman O, Åhs F, Furmark T, Appel L, Linnman C, Faria V, et al. Association between amygdala reactivity and a dopamine transporter gene polymorphism. Transl Psychiatry 2014;4:e420.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Text Revision (DSM-IV-TR). 4th ed. Arlington: American Psychiatric Association; 2000.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33.
Rytwinski NK, Fresco DM, Heimberg RG, Coles ME, Liebowitz LR, Cissel S, et al. Screening for social anxiety disorder with the self-report version of the Liebowitz Social Anxiety Scale. Depress Anxiety. 2009;26:34–8.
Logan J, Fowler JS, Volkow ND, Wang G-J, Ding Y-S, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–40.
Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–87.
Jonasson M, Appel L, Engman J, Frick A, Nyholm D, Askmark H, et al. Validation of parametric methods for [11C]PE2I positron emission tomography. Neuroimage. 2013;74:172–78.
Svarer C, Madsen K, Hasselbalch SG, Pinborg LH, Haugbøl S, Frøkjær VG, et al. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage. 2005;24:969–79.
Frick A, Åhs F, Palmquist ÅM, Pissiota A, Wallenquist U, Fernandez M, et al. Overlapping expression of serotonin transporters and neurokinin-1 receptors in posttraumatic stress disorder: a multi-tracer PET study. Mol Psychiatry. 2016;21:1400–7.
Plavén-Sigray P, Gustavsson P, Farde L, Borg J, Stenkrona P, Nyberg L, et al. Dopamine D1 receptor availability is related to social behavior: a positron emission tomography study. Neuroimage. 2014;102:590–5.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9.
Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224–47.
Öhman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30:953–8.
Adhikari A, Lerner TN, Finkelstein J, Pak S, Jennings JH, Davidson TJ, et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 2015;527:179–85.
Hensler JG. Serotonergic modulation of the limbic system. Neurosci Biobehav Rev. 2006;30:203–14.
Salgado-Pineda P, Delaveau P, Blin O, Nieoullon A. Dopaminergic contribution to the regulation of emotional perception. Clin Neuropharmacol. 1979;28:228–37.
Schultz W. Reward functions of the basal ganglia. J Neural Transm. 2016;123:679–93.
Bas-Hoogendam JM, van Steenbergen H, Pannekoek NJ, Fouche JP, Lochner C, Hattingh CJ, et al. Voxel-based morphometry multi-center mega-analysis of brain structure in social anxiety disorder. NeuroImage Clin. 2017;16:678–88.
Carlezon WA, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56:122–32.
Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50.
Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–52.
Moran RJ, Kishida KT, Lohrenz T, Saez I, Laxton AW, Witcher MR, et al. The protective action encoding of serotonin transients in the human brain. Neuropsychopharmacology. 2018;43:1425–35.
Browne CJ, Abela AR, Chu D, Li Z, Ji X, Lambe EK, et al. Dorsal raphe serotonin neurons inhibit operant responding for reward via inputs to the ventral tegmental area but not the nucleus accumbens: evidence from studies combining optogenetic stimulation and serotonin reuptake inhibition. Neuropsychopharmacology. 2018;44:793–804.
Shirayama Y, Chaki S. Neurochemistry of the nucleus accumbens and its relevance to depression and antidepressant action in rodents. Curr Neuropharmacol. 2006;4:277–91.
Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–80.
Oler JA, Fox AS, Shelton SE, Christian BT, Dhanabalan M, Oakes TR, et al. Serotonin transporter availability in the amygdala and bed nucleus of the stria terminalis predicts anxious temperament and brain glucose metabolic activity. J Neurosci. 2009;29:9961–6.
Takano A, Arakawa R, Hayashi M, Takahashi H, Ito H, Suhara T. Relationship between neuroticism personality trait and serotonin transporter binding. Biol Psychiatry. 2007;62:588–92.
Moriyama TS, Felicio AC, Chagas MHN, Tardelli VS, Ferraz HB, Tumas V, et al. Increased dopamine transporter density in Parkinson’s disease patients with social anxiety disorder. J Neurol Sci. 2011;310:53–7.
Fisher PM, Meltzer CC, Ziolko SK, Price JC, Hariri AR. Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nat Neurosci. 2006;9:1362–3.
Näslund J, Studer E, Pettersson R, Hagsäter M, Nilsson S, Nissbrandt H, et al. Differences in anxiety-like behaviour within a batch of Wistar rats are associated with differences in serotonergic transmission, enhanced by acute SSRI administration and abolished by serotonin depletion. Int J Neuropsychopharmacol. 2015;18:pyv018.
Sossi V, De La Fuente-Fernández R, Schulzer M, Troiano AR, Ruth TJ, Stoessl AJ. Dopamine transporter relation to dopamine turnover in Parkinson’s disease: a positron emission tomography study. Ann Neurol. 2007;62:468–74.
Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, et al. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci. 2001;98:1982–87.
Carpenter AC, Saborido TP, Stanwood GD. Development of hyperactivity and anxiety responses in dopamine transporter-deficient mice. Dev Neurosci 2012;34:250–7.
Richey JA, Rittenberg A, Hughes L, Damiano CR, Sabatino A, Miller S, et al. Common and distinct neural features of social and non-social reward processing in autism and social anxiety disorder. Soc Cogn Affect Neurosci. 2014;9:367–77.
Farde L, Plavén-Sigray P, Borg J, Cervenka S. Brain neuroreceptor density and personality traits: towards dimensional biomarkers for psychiatric disorders. Philos Trans R Soc B Biol Sci. 2018;373:20170156.
Acknowledgements
Supported by the Swedish Research Council and Riksbankens Jubileumsfond—the Swedish Foundation for Humanities and Social Sciences (TF). AF is supported by The Kjell and Märta Beijer Foundation. We thank Jörgen Rosén, Fredrik Åhs, Johannes Björkstrand, Thomas Ågren, Hanna Wallberg, Henrik Annerstedt, Nimo Farah, Jonas Engman, Per Carlbring, Gerhard Andersson, Margareta Reis, and Elna-Marie Larsson for their assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hjorth, O.R., Frick, A., Gingnell, M. et al. Expression and co-expression of serotonin and dopamine transporters in social anxiety disorder: a multitracer positron emission tomography study. Mol Psychiatry 26, 3970–3979 (2021). https://doi.org/10.1038/s41380-019-0618-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-019-0618-7
- Springer Nature Limited
This article is cited by
-
Oxytocin receptor gene polymorphism (rs53576) and depressive symptoms: a systematic review and meta-analysis
Current Psychology (2024)
-
Relationships between serotonin availability and frontolimbic response to fearful and threatening faces
Scientific Reports (2023)
-
Dopaminergic and cholinergic modulation of the amygdala is altered in female mice with oestrogen receptor β deprivation
Scientific Reports (2023)
-
Distinct serotonergic pathways to the amygdala underlie separate behavioral features of anxiety
Nature Neuroscience (2022)
-
Application of positron emission tomography in psychiatry—methodological developments and future directions
Translational Psychiatry (2022)