Abstract
Chronic exposure to stress is associated with increased incidence of depression, generalized anxiety, and PTSD. However, stress induces vulnerability to such disorders only in a sub-population of individuals, as others remain resilient. Inflammation has emerged as a putative mechanism for promoting stress vulnerability. Using a rodent model of social defeat, we have previously shown that rats with short-defeat latencies (SL/vulnerable rats) show increased anxiety- and depression-like behaviors, and these behaviors are mediated by inflammation in the ventral hippocampus. The other half of socially defeated rats show long-latencies to defeat (LL/resilient) and are similar to controls. Because gut microbiota are important activators of inflammatory substances, we assessed the role of the gut microbiome in mediating vulnerability to repeated social defeat stress. We analyzed the fecal microbiome of control, SL/vulnerable, and LL/resilient rats using shotgun metagenome sequencing and observed increased expression of immune-modulating microbiota, such as Clostridia, in SL/vulnerable rats. We then tested the importance of gut microbiota to the SL/vulnerable phenotype. In otherwise naive rats treated with microbiota from SL/vulnerable rats, there was higher microglial density and IL-1β expression in the vHPC, and higher depression-like behaviors relative to rats that received microbiota from LL/resilient rats, non-stressed control rats, or vehicle-treated rats. However, anxiety-like behavior during social interaction was not altered by transplant of the microbiome of SL/vulnerable rats into non-stressed rats. Taken together, the results suggest the gut microbiome contributes to the depression-like behavior and inflammatory processes in the vHPC of stress vulnerable individuals.
Similar content being viewed by others
Introduction
Chronic stress increases the risk of developing many psychiatric disorders such as anxiety, depression, and post-traumatic stress disorder [1,2,3]. While these disorders can be triggered or increased by chronic stress, not all individuals develop psychiatric disorders in response to chronic stress [4,5,6,7,8]. The mechanisms underlying vulnerability to stress are unclear, however, mounting evidence suggests that increased central inflammatory processes may be involved. Indeed, inflammatory cytokines are elevated in depressed patients [9,10,11], and numerous animal studies have shown inflammatory mechanisms underlie depressive-type behaviors [9, 10, 12]. In a recent study in rats, we showed that vulnerability to social defeat is due, in part, to increases in pro-inflammatory processes and vascular remodeling in the ventral hippocampus (vHPC) [13]. Increased vascular density following vascular remodeling can facilitate neural transmission and communication across the so-called neurovascular unit [14], thereby promoting increased activity in stress-sensitive regions. These data are consistent with studies suggesting that inflammation is a contributing factor in stress-related mood disorders [15,16,17,18].
Considerable evidence shows a link between the gut microbiome and immune system function and regulation [17, 19]. Furthermore, differences in gut microbiota have been identified in humans with a variety of psychiatric diseases, including depression, anxiety, and schizophrenia [20, 21], and several animal models of psychiatric diseases show disturbances in the gut microbiome [17]. The brain can influence microbiota composition in the gut through effects on digestive and immune molecules [19]. In turn, the gut can influence the brain via the vagal nerve and other putative mechanisms [22]. However, the specific brain regions that interact with the gut microbiome remain elusive. Based on our previous work that the stress vulnerable phenotype is characterized by a pro-inflammatory state in the vHPC, we hypothesized that the gut microbiome contributes to vulnerability to stress through inflammatory changes in the vHPC.
Materials and methods
Animals
Singly housed male Sprague–Dawley rats (275–300g) were used as experimental intruder rats. Male Long–Evans (LE) retired breeders (600–800g) were used as residents. Rats were purchased from Charles River (Wilmington, MA) and were singly housed on a 12:12-h light:dark schedule (lights on at 0700 hours) with food and water available ad libitum. All experiments were conducted, and samples were collected between 1000 and 1300 hours to minimize circadian influences on microbiome [23]. Procedures were approved by the Children’s Hospital of Philadelphia’s Institutional Animal Care and Use Committee and conformed to the NIH Guide for the Use of Laboratory Animals.
Study 1: Analysis of gut microbiota in resilient, vulnerable, and non-stressed rats
Experimental design
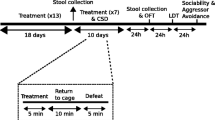
Rats were randomly assigned to either a defeat group or a control group. Fresh fecal samples were collected following handling at 24 h before the first social defeat (day 0) and 24 h after the 7th episode of social defeat (30 min/day) or 7th episode of novel cage exposure (30 min/day) in control rats (day 8). Retired male breeder LE rats were pre-selected for aggression and used as the residents. Each intruder was exposed to a new resident rat on each day, and the same residents were used for the entirety of the experiment. The experimental design is shown in Fig. 1a. Group sizes were: control; n = 8, SL n = 9, LL n = 10, and sizes were selected based on our previous work using this defeat paradigm [13, 24, 25]. All samples were collected at approximately 1000 h, frozen on dry ice and stored at −80 °C until analysis. Fecal samples were examined instead of cecum contents to allow us to assess whether pre-existing differences predict resiliency or vulnerability [26]. Fecal samples provide a reliable measure of gut microbiota composition [26,27,28]. Shotgun metagenome sequencing was used to assess quantitative differences in bacterial species composition between groups. Analysis was conducted at the PennCHOP Microbiome Program. See Supplementary Methods for information on shotgun sequencing.
Differences in the fecal microbiome of SL/vulnerable and LL/resilient rats. a Experimental design used in Study 1. b Significantly lower defeat latencies (averaged over the 7 days of defeat) in SL/vulnerable compared with LL/resilient rats (SL n = 9, LL n = 10, control = 8). c There was a significant increase in alpha diversity as represented by richness 10k (richness rarified at 10,000 read counts) in SL/vulnerable rats as a result of defeat (from days 0 to 8) and on day 8 compared with non-stressed controls. There was a trend (p < 0.06) for higher alpha diversity in SL/vulnerable compared with LL/resilient rats on day 8. d PcoA plot based on Bray–Curtis distances demonstrating a significant shift in SL/vulnerable and LL/resilient microbiota on day 8 relative to their pre-stress baseline day 0 sample. e Class-level differences in relative abundance of Actinobacteria (higher abundance in SL/vulnerable rats relative to control and LL rats at day 8), Bacilli (increased in LL/resilient rats from days 0 to 8 and higher abundance relative to control on day 8), Clostridia (higher abundance in SL/vulnerable rats relative to control rats at day 8), and Bacteroidia (decreased abundance in SL/vulnerable rats at day 8). f Relative abundance at genus-level of control, SL/vulnerable, and LL/resilient rats in select microbiota genera (selected by proportion present in population). At the genus level, g the relative abundance of Firmicutes Clostridium was higher in SL/vulnerable rats relative to control rats at day 8. At the species level, h the relative abundance of Lactobacillus Reuteri was higher in SL/vulnerable and LL/resilient rats relative to control rats, and increased in SL/vulnerable rats from days 0 to 8. i Social defeat significantly increased the Firmicutes/Bacteroidetes ratio in both social defeat groups (SL/vulnerable and LL/resilient). j The ratio of Bacilli/Clostridia was significantly higher in LL/resilient rats relative to control rats, and this ratio increased in LL/resilient rats from days 0 to 8. Data represent mean + SEM. *p < 0.05. “a” denotes significant time effect (FDR-corrected p < 0.05), and “b” denotes significant day 8 group effect (FDR-corrected p < 0.05)
Social defeat paradigm and identification of SL/vulnerable and LL/resilient rats
The social defeat paradigm and determination of short-latency (SL/vulnerable) and long-latency (LL/resilient) rats was previously published [24, 29,30,31] (see Supplementary Methods). Rats exhibiting passive behavior and short-latencies (SL/vulnerable) to defeat exhibit increased anxiety- and depressive-like behaviors [13, 18, 25], whereas rats exhibiting active coping behavior and long-latencies (LL/resilient) to defeat are not different from controls in these behaviors and thus are resilient to the effects of defeat. Control rats are placed in a novel cage for 30 min/day for 7 days. The latency differences are observable by 4–5 days of defeat [24].
Study 2: Determine whether fecal transplants from vulnerable or resilient rats transfer behavioral and neural phenotypes into naive, non-stressed recipient rats
In the current study, we chose to examine the Porsolt forced swim test (FST) as a measure of depression-like behavior, and the social interaction task as a measure of anxiety-like behavior. Both of these tasks show measures of construct validity including responsiveness to anti-depressant and anxiolytic drugs, respectively [32,33,34,35]. Moreover, previous work from our lab and others have shown these behaviors to be consistently altered in SL/rats, and affected by inflammatory processes in the vHPC [13, 25].
Experimental timeline
To obtain samples for microbiota transplantation, fecal samples were collected 24 h after 7 days of social defeat from (donor) rats identified as SL/vulnerable or LL/resilient rats or from control rats. In the microbiota recipient rats, on day 0, body weights were obtained and fecal samples were collected to determine their microbiome profiles prior to transplant (experimental design in Fig. 2a). Then on days 1–5, a fecal slurry (2 ml of 5 mg/ml in phosphate buffered saline (PBS)) from non-stressed control, SL/vulnerable, or LL/resilient donors was delivered by oral gavage into the naive recipient rats once daily (microbiota recipient groups). Another group of naive rats was administered PBS by oral gavage daily. Rats were randomly assigned to one of these four conditions. Rats were not pre-treated with antibiotics because pre-treatment with antibiotics provided little impact on efficacy of fecal transplants [27] and can affect rodent behavior [20, 36]. On day 6, a fecal sample was collected and rats were tested for behavior in the social interaction test (10 min). Reductions in interaction time with a novel conspecific rat are interpreted as increased anxiety-like behavior [37, 38]. On days 7 and 8, rats were tested in the FST. Increases in time spent immobile are interpreted as increases in passive, depressive-type behavior [39, 40]. Data from our lab and from others have shown that stress vulnerable rats exhibit reductions in interaction time and increases in immobility indicating increases in anxiety-like and depressive-like behavior, respectively, whereas resilient rats are unaffected [13, 24, 25, 41, 42]. Twenty-four hours following FST, a fecal sample, brains, and blood were collected basally. Two cohorts of rats were used in this study (total group sizes are control n = 14, LL n = 13, SL n = 13, vehicle n = 15), both cohorts received gavage from the same set of donor rats. Both cohorts were used for behavioral testing. Brain measures were from one or the other of the two cohorts (depending on the measure) and the first cohort was used for microbiome sequencing (control n = 7, vehicle n = 7, SL n = 8, LL n = 7). 16-s sequencing was used to assess efficacy of fecal transplants in reshaping the gut microbiota of one cohort of non-stressed recipient rats. See Supplementary Methods for information on 16-s sequencing.
Effects of fecal transplants on reshaping the gut microbiome. a Experimental design. b PCoA plot showing weighted UniFrac distances, which demonstrate significantly changed microbiota in naive rats receiving fecal transplants from SL/vulnerable and LL/resilient rats from days 0 to 5 but no significant changes in gut microbial communities in rats receiving microbiota from control non-stressed rats or in rats receiving gavage of vehicle (PBS). c Sourcetracker analysis assessing the total microbiota composition of the day 6 fecal sample relative to the day 0 sample. The proportion of day 0 fecal sample composition remaining at day 6 in rats that received microbiota from SL/vulnerable rats or LL/resilient rats was approximately 10 and 20%, respectively. Thus, rats treated with microbiota from SL/vulnerable or LL/resilient rats had significantly altered microbiota from their pre-treatment levels (F3, 23 = 26.04, p = 0.0001; n = 6–7 per group). *p < 0.05
Immunoassays
Immunohistochemical analyses of ionized calcium-binding adapter molecule (Iba1) and von Willebrand factor (VWF; marker for blood vessels) were conducted in sections of the vHPC as previously described [13] (details in Supplementary Methods). Iba1 is a typical marker for neuroinflammation [43,44,45,46,47,48,49]. S100β (Abnova cat. no. KA0037), IL-1β (Abcam cat. no. 100768), and IL-10 (Novex cat. no. KRC0101) content in the vHPC and/or in plasma were measured by enzyme-linked immunosorbent assay (ELISA). Plasma S100β is a soluble astrocytic protein that reliably reflects increased blood-brain barrier (BBB) permeability [44, 50,51,52,53,54,55,56]. IL-1β is a pro-inflammatory cytokine that increases Iba1 expression and promotes neuroinflammation [43, 57]. IL-10 is a well-established anti-inflammatory cytokine [58]. A Luminex assay was used to assess inflammatory cytokines in rats that received fecal microbiome transplants (Millipore, cat. no. RECYTMAG-65K-13).
Statistical analyses
For statistical comparisons of two groups, the Student’s t-test was used. For comparisons of more than two groups, an analysis of variance (ANOVA) was used followed by Bonferroni post hoc tests. An α level of 0.05 (two-tailed) was set for significance. More detail is provided in the figure legends. Values over 2 standard deviations from the mean were removed. Statistical analyses were done in SPSS version 17, R or Graphpad Prism 7. For all behavioral analyses, experimenters were blind to group assignment.
Results
Study 1: Analysis of gut microbiota in resilient, vulnerable, or non-stressed rats
Characterization of SL/vulnerable and LL/resilient rats
Mean latencies to social defeat were significantly lower in SL/vulnerable rats compared with LL/resilient (Fig. 1b), as defined in our previously published data [13, 24, 25].
Stress vulnerability is associated with increased alpha diversity
Alpha diversity assesses differences in within-subjects diversity. It was not different between groups on day 0, indicating no pre-existing differences. Alpha diversity increased in SL/vulnerable rats from days 0 to 8 (p = 0.003; Fig. 1c), but did not increase in control or LL/resilient rats. On day 8, alpha diversity was higher in SL/vulnerable rats relative to control rats (p = 0.001) and tended to be higher in SL/vulnerable relative to LL/resilient rats on day 8 (p = 0.06). These data suggest that SL/vulnerable rats show increased diversity in their gut microbiome following social defeat. LL/resilient and control rats were not significantly different from one another.
Social defeat increases beta diversity
Beta diversity was examined to determine how the whole microbial community differs between samples. There were no significant differences in beta diversity as assessed by Bray–Curtis distances at day 0 (Fig. 1d). Bray–Curtis distances increased in both SL/vulnerable and LL/resilient rats from days 0 to 8 (p = 0.02). At day 8, Bray–Curtis distances significantly shifted in both SL/vulnerable and LL/resilient rats relative to control rats (p = 0.022). These data suggest that there are no pre-existing differences amongst groups, but with repeated defeat, both SL/vulnerable and LL/resilient rats showed significantly different expression of bacteria at the community level. We also examined beta diversity in the LE resident rats (not shown). This was significantly different in the resident rats relative to control, SL/vulnerable and LL/resilient rats at day 0. An Adonis test on day 8 showed that SL/vulnerable (p = 0.005) and LL/resilient rats (p = 0.004) both had significantly different Bray–Curtis distances relative to the resident rats indicating that the microbiota of defeated rats did not become similar to that of the resident rats. These data suggest that the shift in bacterial communities in defeated rats was not due to exposure to the microbiota of the resident rats.
Class-level differences
From days 0 to 8, four classes (out of five that met criterion for analysis; see Supplementary Methods for shotgun metagenome sequencing for criterion) were significantly changed in SL/vulnerable rats (Fig. 1e): there were significant increases in Bacilli (p = 0.01), a trend for increased Clostridia (p = 0.1), and a significant decrease in Bacteroidia (p = 0.007). In LL/resilient rats, there was a trend for increased Bacilli (p = 0.1). Although no group differences were observed on day 0, on day 8, Actinobacteria were significantly higher in SL/vulnerable rats relative to control rats (p = 0.04) and LL/resilient rats (p = 0.05), Bacilli was significantly higher in SL/vulnerable rats relative to control rats (p = 0.0004), Clostridia were significantly higher in SL/vulnerable rats relative to control rats, (p = 0.05), and Bacteroidia were significantly decreased in SL/vulnerable rats relative to control rats at day 8 (p = 0.004). No significant differences were observed between LL/resilient and control rats on day 8 at the class level.
Genus-level differences
There were 13 significant time effects between days 0 and 8 with seven genera significantly changed in SL/vulnerable effects between days 0 and 8, and six significantly changed genera in LL/resilient rats between days 0 and 8. There were 18 significant group effects at day 8 and none at day 0. Fourteen of these significant comparisons were between SL/vulnerable and control rats, and four were between LL/resilient and control rats (select genera are summarized in Fig. 1f). The most robust change in SL/vulnerable (p = 0.008) and LL/resilient rats (p = 0.02) was the reduction of Bacteroidetes Bacteroides (Fig. 1f). Firmicutes Lactobacillus increased in both SL/vulnerable (p = 0.008) and LL/resilient rats (p = 0.04) from days 0 to 8. The mean relative abundances for Firmicutes Lactobacillus increased from 0.006 at day 0 to 0.05 in SL/vulnerable rats, and increased from 0.006 to 0.08 in LL/resilient rats. As expected from class-level analysis, many of the increased class-level bacteria identified in the SL/vulnerable rats were associated with Clostridia, such as Firmicutes Clostridium (p = 0.02; Fig. 1g) and Firmicutes Clostridiales (p = 0.06; not shown). The group means, standard errors, and significant effects are in Supplementary Tables 1 and 2.
Species-level differences
There were 24 significantly changed species in SL/vulnerable rats relative to control rats, and 16 significantly changed species in LL/resilient rats relative to control rats. There were no significantly changed species between SL/vulnerable and LL/resilient rats. For time effects, 17 species changed from days 0 to 8 in SL/vulnerable rats, and 17 species changed from days 0 to 8 in LL/resilient rats as well. Lactobacillus reuteri, which in probiotic form was shown to have anti-depressant-like effects in rodent models [22], increased LL/resilient rats (Fig. 1h; average relative abundance = 0.06; p = 0.0004) and increased to a lesser extent in SL/vulnerable rats (average relative abundance = 0.03; p = 0.0001). The group means, standard errors, and significant effects are in Supplementary Tables 3 and 4. Collectively, the results of the shotgun metagenome analysis showed that stress had a greater impact on reshaping the gut microbiota of SL/vulnerable rats than LL/resilient rats.
Increased ratio of Bacilli to Clostridia
A low ratio of Firmicutes to Bacteroidetes (F/B ratio) is considered a normal, healthy state of the intestinal microbiome [17, 19]. In the current study, the F/B ratio was increased in both SL/vulnerable and LL/resilient rats from days 0 to 8 (p < 0.05) and was higher in SL/vulnerable and LL/resilient rats relative to control rats on day 8 (p < 0.05; Fig. 1i). We also examined the ratio Bacilli to Clostridia (B/C ratio) because Bacilli contain several anti-inflammatory bacterial species such as Lactobacillus rahmnosus, Lactobacillus acidophilus, Lactobacillus reuteri [59,60,61], while increased Clostridia are often associated with an inflammatory phenotype and contain several highly pathogenic species [62,63,64]. The B/C ratio was significantly increased from days 0 to 8 in LL/resilient rats (p = 0.02; Fig. 1h) and was higher in LL/resilient rats relative to control rats on day 8 (p = 0.004), whereas there was no significant change in SL/vulnerable rats. These data suggest the B/C ratio is a novel index of stress effects, and an increase in the B/C ratio may indicate protection against the inflammatory effects of stress.
Study 2: Determine whether fecal transplants from vulnerable or resilient donor rats transfer behavioral phenotypes of the donors into non-stressed recipient rats
Oral gavage of fecal microbiota effectively reshaped microbiota of non-stressed recipients
At day 0 (24 h prior to first oral gavage; Fig. 2a shows experimental design), there were no community differences (assessed by Bray–Curtis distance) in the gut microbiota of rats assigned to any condition, as expected. On day 6 (after 5 days of gavage), there were significant shifts in Bray–Curtis distance of bacterial communities in rats receiving microbiota from SL/vulnerable rats or LL/resilient rats (p = 0.005; Fig. 2b). Sourcetracker analyses confirmed these findings: fecal communities of rats that received oral gavage of vehicle (PBS) or microbiota from non-stressed controls did not change from days 0 to 6, whereas the microbiome communities from naive rats treated with SL/vulnerable and LL/resilient microbiota changed significantly from their day 0 levels (Fig. 2c). We assessed whether there was a change in microbiome from days 6 to 9 (after behavioral testing). The only significant differences in microbiota between days 6 and 9 were in rats treated with samples from control rats (p = 0.04). There was no significant difference in weight gain over the course of the experiment across groups, indicating no adverse effects of fecal transplants compared with gavage of PBS (Fig. 3a). Taken together, these data show that oral gavage delivery of microbiota from SL/vulnerable or LL/resilient rats effectively altered the microbiota of otherwise naive non-stressed rats.
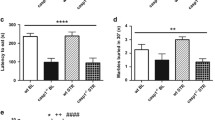
Effects of fecal transplants on promoting donor behavioral phenotypes in transplant recipients. a No change in bodyweight gain between groups across the study. Following 5 days of oral gavages (and no social defeat stress), there was b no effect of any treatment on behavior during the social interaction test (n = 11–14 per group). In the forced swim test, c latency to immobility was significantly lower in rats treated with SL/vulnerable microbiota relative to other groups (n = 9–15 per group, F3, 37 = 8.66, p = 0.0002). d Total immobility was significantly higher in rats treated with SL/vulnerable microbiota relative to rats treated with LL/resilient or control microbiota (F3, 37 = 4.85, p = 0.0061). e Time spent swimming was significantly lower in rats treated with SL/vulnerable microbiota rats relative to vehicle treatment (F3, 37 = 3.78, p = 0.01). f There were no significant differences in climbing behavior. Data represent mean+/− SEM. *p < 0.05
Fecal transplants from SL/vulnerable rats to naive, non-stressed rats promote some behavioral indices of stress vulnerability
There were no differences in time spent interacting in the social interaction test between the recipient groups (Figure 3b). These data suggests no differences in anxiety-like behavior during the social interaction test across the recipient groups (Fig. 3b). This finding was intriguing as previous work showed decreased social interaction in SL/vulnerable rats [13, 25]. In the FST, rats treated with microbiota from SL/vulnerable rats had decreased latency to immobility (Fig. 3c) and increased time spent immobile (Fig. 3d) suggesting increased passive, depression-like behaviors [40]. Rats treated with SL/vulnerable microbiota spent significantly less time swimming (Fig. 3e). Finally, SL/vulnerable rats spent less time climbing but this effect did not reach statistical significance (Fig. 3f).
Fecal transplants from SL/vulnerable rats to naive non-stressed rats promote pro-inflammatory phenotypes in the vHPC
There was an increase in the number and area of Iba1-positive cells in vHPC of rats treated with microbiota from SL/vulnerable rats (Fig. 4a–c) indicating increases in microglial activation. There were no significant differences in vascular remodeling as assessed by VWF staining between any groups (Fig. 1d, e). Expression of the pro-inflammatory cytokine IL-1β was significantly increased in rats that received microbiota from SL/vulnerable rats (Fig. 4f). The anti-inflammatory cytokine IL-10 [58] was significantly increased in rats that received microbiota from SL/vulnerable and LL/resilient rats (Fig. 4g). The BBB permeability marker S100β was significantly increased in plasma of rats that received microbiota from SL/vulnerable rats (Fig. 4h) consistent with previous data showing increased inflammation-induced BBB permeability in SL/vulnerable rats [13]. Corticosterone was significantly increased in the plasma of rats that received microbiota from SL/vulnerable rats (Fig. 4i).
Ventral hippocampus (vHPC) and plasma markers of inflammation are increased in non-stressed rats receiving SL/vulnerable gut microbiome transplants. a Representative immunohistochemical staining of Iba1 in the vHPC. b Average number of Iba1 immunoreactive cells and c average area of Iba1 immunoreactivity increased in vHPC of rats that received microbiota from SL/vulnerable rats relative to the other groups (n = 5–7 per group, F3, 17 = 4.86, p = 0.01). No significant differences in d number of VWF immunoreactive cells or e area of VWF immunoreactivity (n = 5–7 per group). f IL-1β protein was increased in the vHPC of rats that received microbiota from SL/vulnerable rats relative to other groups (n = 4–6 per group, F3, 19 = 5.84, p = 0.005). g IL-10 protein was increased in the vHPC of rats treated with SL/vulnerable or LL/resilient rats microbiota (n = 5–6 per group). h The blood–brain barrier permeability marker S100β was increased in plasma of rats treated with microbiota from SL/vulnerable rats relative to vehicle, control, and LL/resilient treatment groups (F3, 28 = 6.86, p = 0.002; n = 7–9 per group). i Plasma corticosterone, taken when rats were killed 24 h after FST, was increased in rats treated with SL/vulnerable microbiota (F3, 22 = 7.50, p = 0.002; n = 5–6). Data represent mean + SEM. *p < 0.05
No change in plasma levels of inflammatory cytokines
Results of Luminex assays showed that inflammatory cytokine protein concentrations did not change in any condition (Supplementary Table 5). In several cases, the concentrations of cytokines were below the detection limit of the assay. Out of 44 samples tested, there were only 16 subjects with detectable levels of IL-1β, and 4 subjects with detectable levels of IL-6. We confirmed these low levels using ELISAs. Low plasma concentrations of inflammatory cytokines suggest that peripheral inflammation was not increased in rats treated with fecal samples from rats in any condition.
Discussion
It is becoming increasingly recognized that the gut microbiome can play a role in stress-related mood disorders. Here, we specifically studied the contribution of the gut microbiome to the stress resilience/vulnerable phenotype in rats. We first determined that there were no pre-existing differences in the composition of gut microbiota between rats that subsequently become vulnerable or resilient to stress. Although stress exposure induced significant changes in microbiota in both SL/vulnerable compared with LL/resilient rats, only SL/vulnerable rats showed significant chances in alpha diversity as reflected by increased numbers of changed microbiota at class, genus, and species levels. These findings suggest that physiological processes induced by stress, processes that may not pre-exist in the gut, create conditions that allow for the growth of different bacterial communities in SL/vulnerable and LL/resilient rats. It is unclear what those processes are but one possibility is glucocorticoids. The synthetic glucocorticoid dexamethasone can increase Clostridia and other Firmicutes classes [65], which were increased in SL/vulnerable rats. These finding are intriguing as SL/vulnerable rats show delayed corticosterone habituation relative to LL/resilient following repeated social defeat [24]. In turn, Clostridia-related genera can potentially regulate corticosterone production [66]. These findings are consistent with the elevated basal glucocorticoids observed in rats that received microbiota from SL/vulnerable rats in the current study. Alternatively or in addition, the gut could be influenced by central stress-initiated processes through vagal inputs, through factors produced by the brain that access the gut through the bloodstream or factors produced in the periphery that regulate the gut [19, 22, 67]. Together, the results suggest that the gut microbiome is shaped by stress experience differently in animals that become vulnerable vs. resilient to the effects of stress.
We then examined the specific contribution of the gut microbiome to the behavioral and neural phenotypes characteristic of vulnerable and resilient rats by transferring the microbiota from SL/vulnerable and LL/resilient rats into otherwise stress-naive rats. Microbiome transplants from SL/vulnerable rats were sufficient to recapitulate specific aspects of stress vulnerability, including increasing pro-inflammatory processes such as microglial density and IL-1β expression in the vHPC, and increasing depression-like behaviors. Intriguingly, we did not observe increased anxiety-like behaviors or increased vascular remodeling in the vHPC of rats that received microbiota from SL/vulnerable rats, which we have previously identified in vulnerable rats [13]. This suggests that stress experience itself is required to produce changes in vascular remodeling, likely subsequent to stress-induced changes in neural activity, and that these contribute to increases in anxiety-related behaviors in vulnerable rats. Together, these findings suggest that the complex neural and behavioral phenotypes of vulnerable rats can be dissociated: induction of inflammatory processes in the hippocampus and depressive-like behaviors are regulated by the gut microbiome of stressed animals whereas vascular remodeling and anxiety-like behaviors are not, and likely require neural activity initiated by exposure to stress.
Although we did not detect changes in anxiety-like behavior using the social interaction task, it is possible that inclusion of another behavioral test for anxiety-like behavior would have yielded different results. We chose the social interaction task because treatment with anxiolytic drugs increases interaction times [33, 34], suggesting this task has strong construct validity as a measure of anxiety-like behavior in rodents. Furthermore, based on extensive work from our lab and others, reductions in interaction time are a robust and well replicated effect in SL/vulnerable rats [13, 25]. Finally, reductions in interaction time are strongly associated with brain inflammatory measures that we examined in the current study [13].
Rats treated with microbiota from SL/vulnerable rats had increased pro-inflammatory processes in the vHPC. Greater area and increased cell count of Iba1-positive microglia are indicative of increased cellular proliferation of microglia and morphological changes in microglia towards a pro-inflammatory phenotype [43,44,45,46,47,48,49, 68,69,70]. These effects can be directly induced by IL-1β [13, 43,44,45,46,47,48,49], which was also increased in rats treated with SL/vulnerable microbiota. We also observed increased expression of the BBB permeability marker S100β in rats that received microbiota from SL/vulnerable rats. Previous data, including our own, have shown that elevated plasma S100β is strongly reflective of increased BBB permeability [13, 44, 50,51,52,53,54,55,56]. BBB permeability is regulated by inflammatory processes in the brain and is used routinely as an index for brain inflammation [65, 71,72,73,74,75]. Taken together, these data suggest that changes in the gut microbiota of SL/vulnerable rats, possibly towards higher levels of Clostridia and potentially Actinobacteria [76], created a milieu of inflammatory intermediates that promoted brain inflammation, potentially increasing depression-like behaviors characteristic of stress vulnerable animals.
Rats that received microbiota from SL/vulnerable or LL/resilient rats both showed increased expression of the anti-inflammatory cytokine IL-10. However, increased pro-inflammatory processes, including elevated IL-1β in the vHPC, were only observed in rats that received microbiota from SL/vulnerable rats. This finding suggests rats treated with microbiota from LL/resilient rats may have had increased pro-inflammatory processes, but via activation of appropriate anti-inflammatory mechanisms downstream of IL-10, these rats were able to buffer inflammation in the vHPC. We did not find differences in cytokine expression in plasma in any condition. Most samples were below detectability limits of the assays. This could be because blood was taken 4 days after end of transplant gavage and after behavioral testing. It is intriguing to find that the gut changes in this study were associated with changes in brain inflammation in absence of peripheral changes. It is possible that peripheral inflammation is not changed or that its changed early on but brain inflammatory processes are activated and stay elevated longer, past the stress and thus are measurable at the timepoints assessed here. In the current study, we focused on the vHPC because our previous work showed that inflammation in the vHPC, rather than the dorsal hippocampus, was an important mediator of vulnerability [13]. It is possible that other brain regions were affected by microbiome transplants and may exhibit changes in neuroinflammatory processes and influence behavior.
We discovered patterns in LL/resilient rats that may improve our understanding of the contribution of the gut microbiome to stress resilience. The increase in the ratio of Bacilli to Clostridia in LL/resilient rats is especially intriguing as there is beneficial Lactobacillus in this class. Lactobacillus reuteri, which has beneficial mood effects [22, 77], had higher relative abundances in LL/resilient rats than in SL/vulnerable rats. These data suggest Lactobacillus are generally increased by social defeat stress, but to a greater extent in LL/resilient rats. Therefore, Lactobacillus in LL/resilient rats may provide a protective mechanism against the adverse effects of increased Clostridia, or perhaps occupy the ecological niche that would otherwise be occupied by Clostridia or Actinobacteria.
Collectively, the results of this study suggest that the gut microbiome is shaped by stress experience differently in vulnerable compared with resilient animals. Stress, regardless of vulnerability or resilience, altered the proliferation of some bacterial species in all rats. In addition, unique bacterial species were promoted in SL/vulnerable vs. LL/resilient rats, particularly the immune-modulating class Clostridia. The gut microbiota of vulnerable rats was sufficient to drive inflammatory processes in the vHPC such as increased microglial activity and increased IL-1β in the vHPC, as well as increased BBB permeability. Importantly, the gut microbiota of SL/vulnerable rats was sufficient to produce the depressive-like phenotype. However, gut-driven processes did not increase vascular remodeling or promote anxiety-like behavior during social interaction that we previously observed in stress vulnerable rats [13, 31]. These data suggest stress effects in the brain are required for vascular remodeling and anxiety-like behavior during social interaction, whereas the gut microbiome is a driver of important pro-inflammatory processes in the vHPC and depressive-like behaviors in rats vulnerable to stress. Confirmation of these findings using a broader behavioral battery is important. Future work will be aimed at determining whether inflammatory processes in the vHPC are necessary for depressive-like behavior promoted by gut microbiota. In conclusion, this study advances our understanding of the unique contribution of the gut microbiome in stressed animals and identified inflammatory processes in the vHPC associated with depressive-like behaviors that are shaped specifically by the gut microbiome.
References
Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319.
Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–91.
van Praag HM. Can stress cause depression? Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:891–907.
Bowen MT, Dass SA, Booth J, Suraev A, Vyas A, McGregor IS. Active coping toward predatory stress is associated with lower corticosterone and progesterone plasma levels and decreased methylation in the medial amygdala vasopressin system. Horm Behav. 2014;66:561–6.
Fleshner M, Maier SF, Lyons DM, Raskind MA. The neurobiology of the stress-resistant brain. Stress. 2011;14:498–502.
Koolhaas JM. Coping style and immunity in animals: making sense of individual variation. Brain Behav Immun. 2008;22:662–7.
Ono Y, Lin HC, Tzen KY, Chen HH, Yang PF, Lai WS, et al. Active coping with stress suppresses glucose metabolism in the rat hypothalamus. Stress. 2012;15:207–17.
Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–95.
Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229.
Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13:467–75.
Asnis GM, De La Garza R 2nd. Interferon-induced depression: strategies in treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:808–18.
Ma L, Demin KA, Kolesnikova TO, Khatsko SL, Zhu X, Yuan X, et al. Animal inflammation-based models of depression and their application to drug discovery. Expert Opin Drug Discov. 2017;12:995–1009.
Pearson-Leary J, Eacret D, Chen R, Takano H, Nicholas B, Bhatnagar S. Inflammation and vascular remodeling in the ventral hippocampus contributes to vulnerability to stress. Transl Psychiatry. 2017;7:e1160.
Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–85.
Felger JC, Haroon E, Miller AH. Risk and resilience: animal models shed light on the pivotal role of inflammation in individual differences in stress-induced depression. Biol Psychiatry. 2015;78:7–9.
Piletz JE, Halaris A, Iqbal O, Hoppensteadt D, Fareed J, Zhu H, et al. Pro-inflammatory biomakers in depression: treatment with venlafaxine. World J Biol Psychiatry. 2009;10:313–23.
Wong ML, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016;21:797–805.
Wood SK, Wood CS, Lombard CM, Lee CS, Zhang XY, Finnell JE, et al. Inflammatory factors mediate vulnerability to a social stress-induced depressive-like phenotype in passive coping rats. Biol Psychiatry. 2015;78:38–48.
Ja Foster, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–12.
Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21:738–48.
Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46–52.
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–5.
Liang X, FitzGerald GA. Timing the microbes: the circadian rhythm of the gut microbiome. J Biol Rhythms. 2017;32:505–515.
Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–805.
Chen RJKG, Sengupta A, Heydendael W, Nicholas B, Beltrami S, Luz S, et al. MicroRNAs as biomarkers of resilience or vulnerability to stress. Neuroscience. 2015;305:36–48.
Stanley D, Geier MS, Chen H, Hughes RJ, Moore RJ. Comparison of fecal and cecal microbiotas reveals qualitative similarities but quantitative differences. BMC Microbiol. 2015;15:51.
Manichanh C, Reeder J, Gibert P, Varela E, Llopis M, Antolin M, et al. Reshaping the gut microbiome with bacterial transplantation and antibiotic intake. Genome Res. 2010;20:1411–9.
Thomas V, Clark J, Dore J. Fecal microbiota analysis: an overview of sample collection methods and sequencing strategies. Future Microbiol. 2015;10:1485–504.
Wood SK. Individual differences in the neurobiology of social stress: implications for depression-cardiovascular disease comorbidity. Curr Neuropharmacol. 2014;12:205–11.
Wood SK, Bhatnagar S. Resilience to the effects of social stress: evidence from clinical and preclinical studies on the role of coping strategies. Neurobiol Stress. 2015;1:164–73.
Wood SK, Wood CS, Lombard CM, Lee CS, Zhang XY, Finnell JE, et al. Inflammatory factors mediate vulnerability to a social stress-induced depressive-like phenotype in passive coping rats. Biol Psychiatry. 2015;78:38–48.
Meeker HC, Chadman KK, Heaney AT, Carp RI. Assessment of social interaction and anxiety-like behavior in senescence-accelerated-prone and -resistant mice. Physiol Behav. 2013;118:97–102.
File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24.
de Angelis L, File SE. Acute and chronic effects of three benzodiazepines in the social interaction anxiety test in mice. Psychopharmacol (Berl). 1979;64:127–9.
Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacol (Berl). 2005;177:245–55.
Lurie I, Yang YX, Haynes K, Mamtani R, Boursi B. Antibiotic exposure and the risk for depression, anxiety, or psychosis: a nested case-control study. J Clin Psychiatry. 2015;76:1522–8.
Lopez P, Halary S, Bapteste E. Highly divergent ancient gene families in metagenomic samples are compatible with additional divisions of life. Biol Direct. 2015;10:64.
Gonzalez LE, Andrews N, File SE. 5-HT1A and benzodiazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test, but not in the elevated plus-maze. Brain Res. 1996;732:145–53.
Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacol (Berl). 1995;121:66–72.
Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–69.
Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2011;152:629–38.
Han A, Yeo H, Park MJ, Kim SH, Choi HJ, Hong CW, et al. IL-4/10 prevents stress vulnerability following imipramine discontinuation. J Neuroinflamm. 2015;12:197.
Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64:300–16.
Jeong HK, Ji K, Min K, Joe EH. Brain inflammation and microglia: facts and misconceptions. Exp Neurobiol. 2013;22:59–67.
Le Blon D, Hoornaert C, Daans J, Santermans E, Hens N, Goossens H, et al. Distinct spatial distribution of microglia and macrophages following mesenchymal stem cell implantation in mouse brain. Immunol Cell Biol. 2014;92:650–8.
Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, et al. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun. 2010;24:1058–68.
Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–8.
Klein R, Roggendorf W. Increased microglia proliferation separates pilocytic astrocytomas from diffuse astrocytomas: a double labeling study. Acta Neuropathol. 2001;101:245–8.
Piskunov A, Stepanichev M, Tishkina A, Novikova M, Levshina I, Gulyaeva N. Chronic combined stress induces selective and long-lasting inflammatory response evoked by changes in corticosterone accumulation and signaling in rat hippocampus. Metab Brain Dis. 2016;31:445–54.
Watson P, Shirreffs SM, Maughan RJ. Blood-brain barrier integrity may be threatened by exercise in a warm environment. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1689–94.
Kawata K, Liu CY, Merkel SF, Ramirez SH, Tierney RT, Langford D. Blood biomarkers for brain injury: what are we measuring? Neurosci Biobehav Rev. 2016;68:460–73.
Blyth BJ, Farhavar A, Gee C, Hawthorn B, He H, Nayak A, et al. Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. J Neurotrauma. 2009;26:1497–507.
Bargerstock E, Puvenna V, Iffland P, Falcone T, Hossain M, Vetter S, et al. Is peripheral immunity regulated by blood-brain barrier permeability changes? PLoS ONE. 2014;9:e101477.
Xanthos DN, Sandkuhler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci. 2014;15:43–53.
Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–47.
Venkatesan C, Chrzaszcz M, Choi N, Wainwright MS. Chronic upregulation of activated microglia immunoreactive for galectin-3/Mac-2 and nerve growth factor following diffuse axonal injury. J Neuroinflamm. 2010;7:32.
Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78:151–6.
Lalani I, Bhol K, Ahmed AR. Interleukin-10: biology, role in inflammation and autoimmunity. Ann Allergy Asthma Immunol. 1997;79:469–83.
Lee J, Yang W, Hostetler A, Schultz N, Suckow MA, Stewart KL, et al. Characterization of the anti-inflammatory Lactobacillus reuteri BM36301 and its probiotic benefits on aged mice. BMC Microbiol. 2016;16:69.
Archer AC, Muthukumar SP, Halami PM. Anti-inflammatory potential of probiotic Lactobacillus spp. on carrageenan induced paw edema in Wistar rats. Int J Biol Macromol. 2015;81:530–7.
Li H, Zhang L, Chen L, Zhu Q, Wang W, Qiao J. Lactobacillus acidophilus alleviates the inflammatory response to enterotoxigenic Escherichia coli K88 via inhibition of the NF-kappaB and p38 mitogen-activated protein kinase signaling pathways in piglets. BMC Microbiol. 2016;16:273.
Maukonen J, Satokari R, Matto J, Soderlund H, Mattila-Sandholm T, Saarela M. Prevalence and temporal stability of selected clostridial groups in irritable bowel syndrome in relation to predominant faecal bacteria. J Med Microbiol. 2006;55(Pt 5):625–33.
Rao K, Erb-Downward JR, Walk ST, Micic D, Falkowski N, Santhosh K, et al. The systemic inflammatory response to Clostridium difficile infection. PLoS ONE. 2014;9:e92578.
Yu H, Chen K, Sun Y, Carter M, Garey KW, Savidge TC, et al. Cytokines are markers of the Clostridium difficile-induced inflammatory response and predict disease severity. Clin Vaccin Immunol. 2017;24:e00037–17.
Huang EY, Inoue T, Va Leone, Dalal S, Touw K, Wang Y, et al. Using corticosteroids to reshape the gut microbiome: implications for inflammatory bowel diseases. Inflamm Bowel Dis. 2015;21:963–72.
Mudd AT, Berding K, Wang M, Donovan SM, Dilger RN. Serum cortisol mediates the relationship between fecal Ruminococcus and brain N-acetylaspartate in the young pig. Gut Microbes. 2017; 8:589–600.
Bharwani A, Mian MF, Foster JA, Surette MG, Bienenstock J, Forsythe P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology. 2016;63:217–27.
Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. J Neuroinflamm. 2004;1:14.
Graeber MB, Li W, Rodriguez ML. Role of microglia in CNS inflammation. FEBS Lett. 2011;585:3798–805.
Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflamm. 2015;12:114.
Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–6.
Furusawa Y, Obata Y, Fukuda S, Ta Endo, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50.
Lopetuso LR, Scaldaferri F, Petito V, Gasbarrini A. Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog. 2013;5:1.
Macfabe DF. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Health Dis. 2012;23:1–24.
Umesaki Y, Setoyama H, Matsumoto S, Imaoka A, Itoh K. Differential roles of segmented filamentous bacteria and clostridia in development of the intestinal immune system. Infect Immun. 1999;67:3504–11.
Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21:1–11.
Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry. 2017;82:472–87.
Acknowledgements
This work was supported by the Defense Advanced Research Projects Agency (DARPA) and the U.S. Army Research Office under grant number W911NF1010093 to SB. We would like to thank Victoria Siu, Zoe Temple, and Ria Chhabra for assistance with data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pearson-Leary, J., Zhao, C., Bittinger, K. et al. The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Mol Psychiatry 25, 1068–1079 (2020). https://doi.org/10.1038/s41380-019-0380-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-019-0380-x
- Springer Nature Limited
This article is cited by
-
Gut microbial CAZymes markers for depression
Translational Psychiatry (2024)
-
Differential recruitment of brain circuits during fear extinction in non-stressed compared to stress resilient animals
Scientific Reports (2024)
-
Microbiota-Gut-Brain Axis in Psychiatry: Focus on Depressive Disorders
Current Epidemiology Reports (2024)
-
Dectin-1 signaling on colonic γδ T cells promotes psychosocial stress responses
Nature Immunology (2023)
-
ASMT determines gut microbiota and increases neurobehavioral adaptability to exercise in female mice
Communications Biology (2023)