Abstract
The role of salvage high-dose chemotherapy and autologous stem cell transplantation (sHDCT/ASCT) for relapsed and/or refractory multiple myeloma (RRMM) in the era of continuous novel agent treatment has not been defined. This randomized, open-label, phase III, multicenter trial randomized patients with 1st–3rd relapse of multiple myeloma (MM) to a transplant arm (n = 139) consisting of 3 Rd (lenalidomide 25 mg, day 1–21; dexamethasone 40 mg, day 1, 8, 15, and 22; 4-week cycles) reinduction cycles, sHDCT (melphalan 200 mg/m2), ASCT, and lenalidomide maintenance (10 mg/day) or to a control arm (n = 138) of continuous Rd. Median PFS was 20.7 months in the transplant and 18.8 months in the control arm (HR 0.87; 95% CI 0.65–1.16; p = 0.34). Median OS was not reached in the transplant and 62.7 months in the control arm (HR 0.81; 95% CI 0.52–1.28; p = 0.37). Forty-one patients (29%) did not receive the assigned sHDCT/ASCT mainly due to early disease progression, adverse events, and withdrawal of consent. Multivariate landmark analyses from the time of sHDCT showed superior PFS and OS (p = 0.0087/0.0057) in patients who received sHDCT/ASCT. Incorporation of sHDCT/ASCT into relapse treatment with Rd was feasible in 71% of patients and did not significantly prolong PFS and OS on ITT analysis while patients who received sHDCT/ASCT may have benefitted.
Similar content being viewed by others
Introduction
HDCT followed by ASCT is standard of care in frontline treatment of eligible patients with MM [1, 2]. Initial randomized trials demonstrated superiority over conventional dose chemotherapy in terms of both PFS and overall survival (OS) [3, 4]. In the meantime, the introduction of novel agents has substantially improved prognosis. Still the PFS benefit of HDCT/ASCT for newly diagnosed MM has been confirmed when compared to effective novel agent consolidation regimens including the immunomodulatory drug (IMiD) lenalidomide and/or the proteasome inhibitor bortezomib [5, 6].
Almost all MM patients eventually suffer disease relapse. While no universal standard treatment has been established for RRMM, therapeutic options and survival have increased during the last decade. Continuous Rd until disease progression had become a treatment standard at the time of conception of this trial.
Although widely used in clinical practice, the role of sHDCT/ASCT in the era of continuous novel agent treatment has not been defined. Several, predominantly retrospective, and/or uncontrolled analyses [7] have established feasibility and efficacy. A single prospective randomized controlled trial of sHDCT/ASCT has been published (NCRI Myeloma X Relapse) [8]. Cook et al. demonstrated superior PFS and OS with melphalan 200 mg/m2 followed by ASCT as compared to conventional dose cyclophosphamide (400 mg/m2 weekly) for 12 weeks and also confirmed safety of the procedure [9]. However, in the light of much more effective novel agent regimens, cyclophosphamide consolidation is not considered a relevant comparator anymore.
The ReLApsE trial was therefore designed to investigate the role of sHDCT/ASCT compared to continuous novel agent treatment until disease progression. Salvage HDCT/ASCT was integrated into a lenalidomide-based backbone of Rd induction and lenalidomide maintenance and was compared to standard continuous Rd.
Methods
Trial design
This randomized, controlled, open-label, multicenter phase III trial was done at 16 trial sites (university and community hospitals) in Germany. The trial was approved by the national regulatory authority as well as the ethics committees of the University of Heidelberg and all participating trial sites. The trial was conducted according to the Declaration of Helsinki and ICH-GCP guidelines. A summary of the trial protocol has been published previously [10]. All authors had access to the primary clinical trial data.
Patients
Patients at 1st–3rd relapse of MM according to IMWG criteria [11], aged 18–75 years and with remission of ≥12 months in case of frontline HDCT/ASCT were recruited. Further inclusion criteria were a WHO performance score (PS) ≤ 2, availability of cryopreserved stem cells for participants aged ≥71 years, laboratory findings within defined ranges at inclusion (absolute neutrophil count [ANC] ≥ 1/nl, platelet count ≥75/nl, creatinine clearance ≥30 ml/min; total bilirubin ≤2× the upper limit of normal (ULN), and alanine aminotransferase ≤3× ULN except for MM-related elevations). Exclusion criteria included prior sHDCT/ASCT for relapsed disease, lenalidomide refractoriness or disease progression within 6 months after the end of lenalidomide-based treatment, prior allogeneic transplantation, nonsecretory MM not amenable to radiographic monitoring, plasma cell leukemia and severe cardiac, pulmonary, neurologic, psychiatric, or infectious comorbidities. All patients provided written informed consent.
Procedures
Patients were randomized 1:1 between the transplant and the control arm with stratification according to trial site and frontline HDCT/ASCT (yes vs. no). Randomization was carried out centrally at the GMMG trial office in Heidelberg, Germany, using the Randi2 open source software package (http://dschrimpf.github.io/randi3/). Patients as well as investigators were aware of treatment allocation at all times.
In the transplant arm, patients received reinduction treatment with three cycles of Rd (lenalidomide 25 mg, day 1–21; dexamethasone 40 mg, day 1, 8, 15, and 22; 4-week cycles). Eligible patients (available stem cells, WHO PS ≤ 2, absence of severe comorbidities) proceeded to sHDCT (melphalan 100 mg/m2 on days −3 and −2) followed by ASCT (≥2 × 106 CD34+ cells/kg on day 0). Lenalidomide maintenance (10 mg daily; 3 month cycles) was initiated no later than 8 weeks after ASCT in patients with ANC ≥ 1/nl as well as platelets ≥30/nl and was continued until progressive disease (PD) or intolerable toxicity. In the control arm, patients received continuous Rd until PD or intolerable toxicity. If no back-up transplant was available or the treating physician opted for additional apheresis, peripheral blood stem cells (PBSC) were harvested after cyclophosphamide (2 g/m2 on days 1 and 2), G-CSF (filgrastim 10 µg/kg/d or lenograstim 300 µg/m2/day s.c. from day 5 until the end of apheresis) and, if needed, preemptive/rescue plerixafor (240 µg/kg daily until the end of apheresis) following the 3rd Rd cycle in both arms. The details and results of stem cell harvesting within the trial have been published previously [12].
Response and PD were assessed according to IMWG criteria [11] complemented by minimal response (MR) according to EBMT criteria [13] and near-complete remission (nCR) [14]. Assessments were performed at trial sites after the 3rd Rd cycle in both arms, after Rd cycle 5 in the control arm, 2 months after sHDCT/ASCT in the transplant arm, every 3 months thereafter and at PD in both arms.
Bone marrow aspirates were collected at baseline and CD138+ selected plasma cells were submitted to interphase fluorescence in situ hybridization for common cytogenetic aberrations [15]. Translocations t(4;14), t(14;16), deletion 17p13, and gain 1q21 (>3 copies) were considered high-risk cytogenetic aberrations.
Outcomes
The primary endpoint was PFS calculated by time from randomization to PD or death, irrespective of the cause of death. Secondary endpoints included OS (time from randomization to death, irrespective of the cause of death), response rates, time to best response, time to next treatment, and safety and toxicity (adverse events [AE] graded according to CTCAE version 4.0). Grade 1 AE of negligible clinical significance (e.g. fatigue, obstipation, night sweats) did not have to be reported. Hematotoxicity had to be reported only if grade ≥3; leukocytopenia only if grade ≥4. MedDRA system organ class (SOC) terms are reported where suitable; the SOC term “gastrointestinal toxicities” was modified to include “mucosal inflammation”; the SOC term “blood and lymphatic tissue disorders” was modified to include all reports of cytopenias.
Statistical analysis
The number of trial patients (n = 282) required to prove inferiority of PFS in the control vs. the transplant arm with 80% power and at a two-sided type I error of 5% was calculated based on an estimated median PFS of 11 vs. 16.5 months in the control and the transplant arm, respectively, with an HR of 0.67 and 193 expected PFS events and accounting for 15% loss to follow-up/drop-outs.
PFS, OS, and response rates were analyzed in the ITT population as randomized. PFS and OS were compared by two-sided log-rank test and Cox regression. The PFS comparison is confirmative, all other analyses are exploratory. Response rates were compared by Fisher’s exact test. Due to very small randomized strata, results of the unstratified analyses are primarily reported.
The safety population consisted of all patients who received at least one dose of study treatment: 145 patients in the control arm and 135 patients in the transplant arm. Patients were analyzed as treated. Patients randomized to the transplant arm but proceeding in the control arm after induction were analyzed in the control arm. Safety and toxicity variables were analyzed by Fisher’s exact test.
Landmark (LM) analyses for PFS and OS were calculated to assess the effect of sHDCT/ASCT in patients that reached the stage of the randomized intervention. Patients without PD who received sHDCT/ASCT or started the contemporaneous Rd cycle 5 entered the analysis as randomized.
Multivariable proportional hazards regression models were calculated for PFS and OS. Missing values were multiply imputed using the mice algorithm [16].
Results
Between December 02, 2010 and March 18, 2016, 282 patients were randomized (Fig. 1). The ITT population consisted of 139 patients in the transplant arm and 138 patients in the control arm; five randomized patients (transplant arm: three; control arm: two) were excluded from the ITT population due to violations of major inclusion/exclusion criteria. In the transplant arm, 127 patients (91%) and in the control arm, 132 patients (96%) completed reinduction therapy with 3 Rd cycles per protocol. PBSC mobilization was initiated in 40 patients of whom 31 (78%) collected ≥2 × 106 CD34+ cells/kg. Preemptive or rescue plerixafor was administered in 20 of 40 patients (50%). Four patients who did not harvest sufficient stem cells and one patient due to sepsis in the transplant arm continued treatment according to the control arm. In the transplant arm, 98/139 patients (71%) underwent the assigned sHDCT/ASCT. Reasons for drop-outs before sHDCT/ASCT are given in Supplementary Table 1. Maintenance lenalidomide was started in 97/98 patients (99%) who underwent sHDCT/ASCT. At data cutoff, 32/139 patients (23%) in the transplant arm and 36/138 patients (26%) in the control arm continued trial treatment. The median time on trial was 14.4 months (IQR 19.9) in the transplant arm and 16.9 months (IQR 16.4) in the control arm (p = 0.19).
Baseline characteristics (Table 1) were balanced between trial arms. At randomization the median age of all patients was 61.6 years (IQR 12.4) and the median interval from diagnosis of MM was 4.0 years (IQR 3.2). Almost all patients had received only one prior line of therapy (260/277 [94%]) and had received frontline HDCT/ASCT (259/277 [94%]). The majority had previously been exposed to bortezomib (213/277 [77%]) and only a minority to lenalidomide (30/277 [11%]). The most frequent induction regimens in the course of frontline HDCT/ASCT were bortezomib/doxorubicin/dexamethasone (PAD) or bortezomib/cyclophosphamide/dexamethasone (VCD) in 184 patients and vincristine/doxorubicin/dexamethasone (VAD) in 46 patients. High-risk cytogenetic aberrations were present in 39/91 (43%) and 31/98 (32%) of patients in the transplant and control arm, respectively (p = 0.13)
Response after Rd reinduction was comparable between trial arms (Table 2). A higher ORR of 82% (84/102) was observed in the transplant arm after sHDCT/ASCT as compared to 71% (80/113) in the control arm after Rd cycle 5 (p = 0.05); conversely, fewer patients developed PD in the transplant arm at this stage of the trial (2/102 [2%] vs. 10/113 [9%]). Responses continued to deepen during lenalidomide maintenance and Rd cycles ≥ 6 (Supplementary Fig. 1). Best response during the complete trial did not differ between the transplant and control arm (ORR: 78% [106/136] vs. 75% [103/138], p = 0.57; ≥VGPR: 49% [67/136] vs. 47% [65/138], p = 0.81).
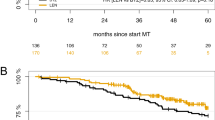
Median follow-up at data cutoff (June 31, 2017) was 36.8 months and 183 patients had a PFS event (transplant arm: 88/139 [63%], control arm: 95/138 [69%]). The primary endpoint, median PFS, was 20.7 months in the transplant arm and 18.8 months in the control arm (HR 0.87; 95% CI 0.65–1.16; p = 0.34; Fig. 2a). PFS stratified according to frontline HDCT/ASCT (HR 0.86; 95% CI 0.64–1.16; p = 0.32) and time to next anti-MM treatment (Supplementary Fig. 2) were also not significantly different between trial arms.
Overall 76 patients died, 35/139 (25%) in the transplant arm and 41/138 (30%) in the control arm; the main cause of death was MM (21/35 [60%] and 24/41 [59%]). Median OS did not differ significantly between trial arms (not reached [NR] in the transplant arm vs. 62.7 months in the control arm; HR 0.81; 95% CI 0.52–1.28; p = 0.37; Fig. 2b). OS at 3 years was 71.8% (95% CI 63.3–81.4%) in the transplant and 71.9% (95% CI 63.4–81.5%) in the control arm.
The overall rates of AE, grade ≥ 3 AE (Table 3) and serious AE (SAE; 56% vs. 50%; p = 0.4) were comparable between trial arms. More grade ≥ 3 blood and lymphatic system disorders were reported in the transplant arm (70% [94/135] vs. 35% [50/145]; p < 0.0001) primarily based on a higher rate of grade ≥ 3 leukopenia/neutropenia (62% [83/135] vs. 25% [36/145]; p < 0.001). This difference was not solely attributable to immediate aplasia following sHDCT/ASCT, since higher rates of grade ≥ 3 leukopenia/neutropenia were also observed during lenalidomide maintenance as compared to the contemporaneous Rd cycles ≥ 6 (27% [26/98] vs. 10% [12/116]; p = 0.0023). The increase in grade ≥ 3 leukopenia/neutropenia did not translate into a significant increase in grade ≥ 3 infections in the transplant arm overall (33% [45/135] vs. 28% [40/145]; p = 0.3) or during lenalidomide maintenance (17% [17/98] vs. 20% [23/116]; p = 0.73). More grade ≥ 3 gastrointestinal disorders were reported in the transplant arm (19% [25/135] vs. 6% [8/145]; p < 0.001) due to events during sHDCT/ASCT. More grade ≥ 3 eye disorders (6% [9/145] vs. 0% [0/135]; p = 0.0036) and respiratory/thoracic/mediastinal disorders (6% [9/145] vs. 1% [1/135]; p = 0.02) were reported in the control arm, mainly during Rd cycles ≥ 6. No significant differences were observed regarding grade ≥ 3 thromboembolic events (3% [5/145] vs. 1% [1/135]; p = 0.22) and second primary malignancies (SPM; transplant arm: n = 10; control arm n = 11; Supplementary Table 2).
The melphalan dose was adjusted in 5/98 patients (5%) due to patient’s condition (n = 2) and renal insufficiency (n = 3). Dose modifications (delay, interruption, reduction, or discontinuation) of lenalidomide due to any toxicity were required in 56% (74/133) and 49% (70/143) of patients in the transplant and control arm, respectively (p = 0.28). In 21% (28/133) and 9% (13/143) of patients, the dose was modified due to neutropenia (p = 0.0064); this difference was driven by a 27% (26/97 patients) rate of lenalidomide dose modifications due to neutropenia during lenalidomide maintenance in the transplant arm. Lenalidomide maintenance was permanently discontinued due to AE in 24% (23/97) of patients, most frequently due to fatigue (n = 5), cytopenia (n = 4), rash (n = 4), and diarrhea (n = 3). Overall, more patients in the transplant arm went off protocol due to AE (33/139 [24%] vs. 8/138 [6%]) and less due to PD (58/139 [42%] vs. 74/138 [54%]). Overall, 11 patients died on study or within 30 days after the end of study, 4/135 (3%) in the transplant arm and 7/145 (5%) in the control arm. No deaths occurred during the sHDCT/ASCT phase.
Forty-one of one hundred and thirty-nine patients (29%) in the transplant arm did not receive the planned sHDCT/ASCT. Post hoc LM analyses of survival from sHDCT (median interval from randomization of 3.8 months [IQR 1.2]) and the contemporaneous Rd cycle 5 (median interval from randomization 4.0 months [IQR 0.7]) were calculated to assess the treatment effect in patients that reached the sHDCT/ASCT phase of the trial. In the transplant arm, 103 patients entered the LM analyses (98 [95%] undergoing sHDCT/ASCT plus 5 patients in the transplant arm treated according to the control arm due to lack of PBSC transplants [n = 4] and sepsis [n = 1]). In the control arm, 114 patients entered the LM analyses. The main reasons for drop-outs before the LM were PD (n = 17 vs. 15), AE (n = 8 vs. n = 3), and withdrawal of consent (n = 7 vs. n = 4; Supplementary Table 1). Baseline characteristics of patients in the LM analyses were balanced as in the overall ITT population (Supplementary Table 3). LM analyses showed a trend towards superior PFS (median 23.3 vs. 20.1 months; HR 0.74; 95% CI 0.52–1.04; p = 0.09; Fig. 3a) and significantly superior OS (median NR vs. 57 months; HR 0.56; 95% CI 0.32–0.99; p = 0.046; Fig. 3b) in the transplant arm.
Multivariate Cox regression models for PFS and OS taking into account prognostically relevant baseline factors were calculated to further assess the treatment effect. In the LM population (Table 4) the transplant arm was significantly associated with superior PFS (HR 0.6; 0.41–0.88; p = 0.0087) and OS (HR 0.39; 0.2–0.76; p = 0.0057); similar to univariate analyses this association did not reach statistical significance in the complete ITT population (PFS: HR 0.74; 0.54–1.01; p = 0.062; OS: HR 0.67; 0.41–1.11; p = 0.12; Supplementary Table 4). The most prominent negative prognostic factor for both PFS and OS were high-risk cytogenetic aberrations in the LM population (PFS: HR 2.71; 1.72–4.27; p < 0.001; OS: HR 4.22; 1.98–8.99; <0.001) as well as in the complete ITT population (PFS: HR 2.28; 1.54–3.36; p < 0.001; OS: HR 2.88; 1.56–5.3; <0.001).
Discussion
The ReLApsE trial is the first randomized trial that compared sHDCT/ASCT to continuous novel agent treatment until disease progression. No significant PFS or OS benefit was observed in the primary analysis.
The observed treatment effect was lower than expected (HR 0.87 vs. 0.67). In part this was related to better outcome of Rd in the control arm with median PFS of 18.8 months compared to the expected 11 months based on published data at the time of trial conception (CC-5013-MM-009 [17] and CC-5013-MM-010 [18] trials). Patients in the CC-5013-MM-009/010 trials were more heavily pretreated with ≥2 lines of prior therapy in the majority of patients as compared to only 1 prior line of therapy in our trial. Moreover, dexamethasone (40 mg) was administered on days 1–4, 9–12, and 17–20 of the first four cycles in the CC-5013-MM-009/010 trials compared to once weekly (days 1, 8, 15, and 22) in our trial. Better outcome with Rd in patients with less extensive pretreatment [19] and using the lower dexamethasone dose has been reported [20]. In addition, increased experience with management of patients on Rd may have contributed to better outcome in the control arm of our trial.
The significant fraction of 29% of patients in the transplant arm that did not receive the assigned sHDCT/ASCT is a limitation of our trial. This was related to our trial design which randomized patients at baseline. A similar fraction of patients not undergoing an assigned HDCT/ASCT (30%) was reported in a phase III trial in newly diagnosed, elderly patients [21] and may represent the rate of drop-outs that has to be expected with HDCT/ASCT in a population at increased risk for adverse outcomes due to age or relapsed disease. Our intention behind upfront randomization was to compare the two treatment strategies without limiting the generalizability of the results to the more narrow population of patients that undergo a postponed randomization after completion of reinduction. The latter population might have been skewed due to e.g., patients that have already attained deep remissions with reinduction and choose to avoid embarking on a trial with a 50% chance of receiving treatment intensification with sHDCT/ASCT at this point. In the NCRI Myeloma X Relapse trial [8], 41% of patients left the trial after reinduction and before randomization to sHDCT/ASCT. The early drop-outs before sHDCT/ASCT that we observed with our strategy were due to PD in roughly half of the cases, suggesting a more active induction regimen may increase the fraction of patients that are able to receive an sHDCT/ASCT. Withdrawal of consent before sHDCT/ASCT played only a minor role (19% of drop-outs before HDCT/ASCT).
Post hoc LM analyses from the time of sHDCT/ASCT and the corresponding point in time in the control arm were performed in an attempt to assess the treatment effect in patients that reached the stage of the randomized intervention. Patients in the transplant arm showed a trend towards superior PFS and had significantly superior OS from the LM. The PFS benefit achieved statistical significance in multivariate analysis accounting for relevant prognostic baseline factors including high-risk cytogenetic aberrations, which were slightly more prevalent in the transplant arm. The treatment effect is also apparent in the higher ORR in the transplant arm after the sHDCT/ASCT. Potential selection bias introduced by nonrandom drop-outs before the LM is a limitation of the LM analyses. More drop-outs occurred in the transplant arm, however, reasons for drop-outs were diverse with disease progression as the major reason in both trial arms and baseline characteristics of the LM cohort remained balanced.
Regarding OS, follow-up is still short and results will be updated in a future long-term follow-up analysis. An important benefit of sHDCT/ASCT that affects OS but not PFS may be the additional line of therapy that it provides. Patients in the transplant arm received a substantially lower cumulative lenalidomide dose and left the trial less frequently due to PD on a lower dose of lenalidomide. This likely avoids full refractoriness to lenalidomide and may increases their sensitivity also to other IMiD-based regimens. Another key factor to influence OS may be the rate of sHDCT/ASCT as a further line of therapy in the control arm which will also be assessed with more follow-up.
The only randomized trial on sHDCT/ASCT performed in the past (NCRI Myeloma X Relapse [8]) demonstrated a median PFS benefit from randomization of 19 vs. 11 months. In contrast to our trial, Cook et al. randomized patients after completion of PAD reinduction treatment and stem cell collection. Consequently, 93% of patients (83/89) in their trial received the assigned sHDCT/ASCT. While the PFS effect of the sHDCT/ASCT in the Myeloma X Relapse trial (HR 0.45) is substantially larger than in our trial, weekly cyclophosphamide is no longer considered a standard comparator. Rd is a more effective treatment and has been the most important comparator in recent randomized phase III trials leading to the approval of novel triplet regimens for RRMM [22,23,24,25]. These triplet regimens combining Rd with another novel agent (daratumumab, elotuzumab, carfilzomib, or ixazomib) have now become a new standard of care for RRMM. Based on our LM analyses the PFS benefit in patients who actually received the sHDCT/ASCT lies at the upper limit of what has been reported for the triplet regimens (HR 0.37–0.74), potentially further challenging the impact of sHDCT/ASCT. Future randomized trials integrating sHDCT/ASCT into the novel triplet regimens will be needed to further define its role in today’s treatment landscape for RRMM. Importantly, such trials should perform randomization in close proximity to the randomized intervention (i.e. directly before the sHDCT/ASCT step) in order to avoid significant early drop-out rates that may obscure a treatment effect.
The absence of transplant related mortality in our trial despite inclusion of patients up to an age of 75 years is reassuring of the safety of sHDCT/ASCT. All except one patient that underwent sHDCT/ASCT were able to start lenalidomide maintenance treatment. However, a ~25% rate of higher grade neutropenia occurred during lenalidomide maintenance and ~25% of patients discontinued maintenance due to a diverse set of AE. This is in line with findings from trials on lenalidomide maintenance after HDCT/ASCT in the frontline setting [26,27,28]. The increase in higher grade neutropenia as compared to standard Rd during the corresponding phase of the trial may indicate elevated bone marrow vulnerability of patients in the early phase after sHDCT/ASCT. Importantly, no corresponding increase in higher grade infections during maintenance treatment was observed and discontinuation of maintenance due to cytopenia was necessary only in 4% of patients.
In conclusion, the ReLApsE trial is the first randomized controlled trial of sHDCT/ASCT versus continuous novel agent treatment, specifically Rd. No survival benefit was observed in the primary analysis under the limitation of a ~30% drop-out rate before sHDCT/ASCT. Improved PFS and OS in patients that received the assigned sHDCT/ASCT suggest that some benefit may still persist in the era of continuous novel agent treatment.
References
Gay F, Engelhardt M, Terpos E, Wäsch R, Giaccone L, Auner HW, et al. From transplant to novel cellular therapies in multiple myeloma: EMN guidelines and future perspectives. Haematologica. 2017;103:197-211.
Kumar SK, Callander NS, Alsina M, Atanackovic D, Biermann JS, Chandler JC, et al. Multiple myeloma, version 3.2017, NCCN Clinical Practice Guidelines in oncology. J Natl Compr Canc Netw. 2017;15:230–69.
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335:91–7.
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20.
Cavo M, Palumbo Antonio, Zweegman Sonja, Dimopoulos Meletios A, Hajek Roman, Pantani Lucia, et al. Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): a randomized phase 3 study of the European Myeloma Network (EMN02/HO95 MM trial). J Clin Oncol. 2016;34:8000-8000.
Atanackovic D, Schilling G. Second autologous transplant as salvage therapy in multiple myeloma. Br J Haematol. 2013;163:565–72.
Cook G, Williams C, Brown JM, Cairns DA, Cavenagh J, Snowden JA, et al. High-dose chemotherapy plus autologous stem-cell transplantation as consolidation therapy in patients with relapsed multiple myeloma after previous autologous stem-cell transplantation (NCRI Myeloma X Relapse [Intensive trial]): a randomised, open-label. Lancet Oncol. 2014;15:874–85.
Cook G, Ashcroft AJ, Cairns DA, Williams CD, Brown JM, Cavenagh JD, et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): a randomised, open-label, phase 3 trial. Lancet Haematol. 2016;3:e340–51.
Baertsch M-A, Schlenzka J, Mai EK, Merz M, Hillengaß J, Raab MS, et al. Rationale and design of the German-Speaking Myeloma Multicenter Group (GMMG) trial ReLApsE: a randomized, open, multicenter phase III trial of lenalidomide/dexamethasone versus lenalidomide/dexamethasone plus subsequent autologous stem cell transplantatio. BMC Cancer. 2016;16:290.
Durie BGM, Harousseau J-L, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
Baertsch M-A, Schlenzka J, Lisenko K, Krzykalla J, Becker N, Weisel K, et al. Cyclophosphamide-based stem cell mobilization in relapsed multiple myeloma patients: a subgroup analysis from the phase III trial ReLApsE. Eur J Haematol. 2017;99. https://doi.org/10.1111/ejh.12888.
Bladé J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–23.
Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N. Engl J Med. 2003;348:2609–17.
Neben K, Jauch A, Bertsch U, Heiss C, Hielscher T, Seckinger A, et al. Combining information regarding chromosomal aberrations t(4;14) and del(17p13) with the International Staging System classification allows stratification of myeloma patients undergoing autologous stem cell transplantation. Haematologica. 2010;95:1150–7.
van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat softw. 2011;45:1–67.
Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–42.
Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau J-L, Dmoszynska A, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–32.
Stadtmauer EA, Weber DM, Niesvizky R, Belch A, Prince MH, San Miguel JF, et al. Lenalidomide in combination with dexamethasone at first relapse in comparison with its use as later salvage therapy in relapsed or refractory multiple myeloma. Eur J Haematol. 2009;82:426–32.
Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37.
Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): a randomised trial. Lancet. 2007;370:1209–18.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–31.
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2014;372:141206080130007.
Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621–31.
Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral Ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621–34.
Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1782–91.
McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1770–81.
McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279–89.
Acknowledgements
The trial was designed and conducted by the German Myeloma Multicenter Group (GMMG). We thank all investigators, study nurses, research staff, the coordination centers for clinical trials (KKS) in Heidelberg and Leipzig, and—most importantly—the participating patients and their families. Furthermore we thank the Dietmar Hopp-Stiftung, Celgene, Chugai, and Amgen for their support of the trial.
Author information
Authors and Affiliations
Consortia
Contributions
HG designed the trial and HG, MAB, JS, NB, CH and TH analyzed the data. HG, MAB, JS, MSR, JH, AJ, PB, MG, SK, MS-H, PR, UG, RF, MH, HM, HWL, CS, AN, HS, RN, and KW collected data. MAB wrote and all co-authors revised and approved the article.
Corresponding author
Ethics declarations
Conflict of interest
HG—Amgen: consultancy, research funding; Novartis: honoraria, research funding; ArtTempi: honoraria; Janssen: consultancy, honoraria, research funding; Sanofi: consultancy, research funding; Mundipharma: research funding; Takeda: consultancy, research funding; Celgene: consultancy, honoraria, research funding; Bristol-Myers Squibb: consultancy, honoraria, research funding; Adaptive Biotechnology: consultancy; Chugai: honoraria, research funding. MAB—Takeda: consultancy, honoraria; Novartis: consultancy, research funding. Travel support: Celgene, Amgen, and Janssen. M-SB: Celgene: consultancy, honoraria; Novartis: consultancy, honoraria, research funding; BMS: consultancy, honoraria, research funding; Amgen: consultancy, honoraria, research funding. JH—Janssen: honoraria, advisory board; Amgen: advisory board; BMS: honoraria, advisory board, research funding; Oncotracker: advisory board; Adaptive Biotech: advisory board; GSK: advisory board. Celgene: consultancy, honoraria, Other: advisory board, research funding. CM-T—research funding: Pfizer, Daiichi Sankyo, BiolineRx, Bayer; advisory boards: Pfizer, Janssen. MS-H—consultancy: Celgene; financial support of educational meetings: Janssen, Takeda, Novartis, Pfizer, Roche, Vifor, Celgene. PR—Honoraria: Takeda, BMS, Roche, Celgene, Sanofi-Aventis; travel support: Celgene, Takeda, Abbvie. UG—honoraria: Sirtex, Daiichi Sankyo, Boehringer Ingelheim, Amgen, Servier, AstraZeneca; consultancy, advisory boards: Merck, BMS, Hexal, Amgen, Celgene, Johnson & Johnson, MSD; travel support: Merck, Amgen, Boehringer Ingelheim. RF—Takeda: honoraria; Bristol-Meyers Squibb: honoraria, Other: travel grant; Celgene: honoraria, Other: travel grant, research funding; Janssen: honoraria; Amgen: honoraria. MH—Novartis: honoraria; Roche: honoraria; Amgen: honoraria; Takeda: honoraria. CS—Novartis: honoraria, research funding; Takeda: honoraria, research funding; Janssen: honoraria, research funding; Celgene: honoraria; BMS: honoraria; Amgen: honoraria; GSK: honoraria. AN—honoraria: Celgene, Takeda, Amgen, Alexion, Sanofi, Janssen, BMS. Research funding: Celgene, Janssen, Takeda. Travel support: Celgene, Takeda, Alexion. HS—Celgene: honoraria, Other: travel suppport, research funding; Janssen: honoraria, Other: travel support, research funding; Novartis: honoraria, Other: travel suppport, research funding; Takeda: honoraria; Amgen: honoraria, Other: travel suppport, research funding; Bristol-Myers Squibb: honoraria, Other: travel suppport, research funding. BB—Janssen: honoraria. KW—Amgen, Celgene, Janssen, and Sanofi: research funding; Amgen, BMS, Celgene, Janssen, Takeda, Adaptive Biotech: honoraria; Amgen, Adaptive Biotech, BMS, Celgene, Janssen, Juno, Sanofi, GSK, Karyopharm and Takeda: consultancy, membership on an entity’s Board of Directors or advisory committees.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Goldschmidt, H., Baertsch, MA., Schlenzka, J. et al. Salvage autologous transplant and lenalidomide maintenance vs. lenalidomide/dexamethasone for relapsed multiple myeloma: the randomized GMMG phase III trial ReLApsE. Leukemia 35, 1134–1144 (2021). https://doi.org/10.1038/s41375-020-0948-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0948-0
- Springer Nature Limited
This article is cited by
-
Health-related quality of life and quality-adjusted progression free survival for carfilzomib and dexamethasone maintenance following salvage autologous stem-cell transplantation in patients with multiple myeloma: a randomized phase 2 trial by the Nordic Myeloma Study Group
Journal of Patient-Reported Outcomes (2024)
-
Treatment pattern and outcomes of re-induction therapy prior to stem cell transplantation in patients with relapsed/refractory multiple myeloma in Germany
Bone Marrow Transplantation (2024)
-
Bridging advanced myeloma patients to subsequent treatments and clinical trials with classical chemotherapy and stem cell support
Bone Marrow Transplantation (2023)
-
Non-interventional Study Evaluating the Mobilization of Stem Cells by Plerixafor Before Salvage Autologous Stem Cell Transplant in Relapsed Multiple Myeloma (IFM-2015-03)
Clinical Hematology International (2023)
-
Carfilzomib, lenalidomide and dexamethasone followed by a second ASCT is an effective strategy in first relapse multiple myeloma: a study on behalf of the Chronic malignancies working party of the EBMT
Bone Marrow Transplantation (2023)