Abstract
Despite decades of clinical use, mechanisms of glucocorticoid resistance are poorly understood. We treated primary murine T lineage acute lymphoblastic leukemias (T-ALLs) with the glucocorticoid dexamethasone (DEX) alone and in combination with the pan-PI3 kinase inhibitor GDC-0941 and observed a robust response to DEX that was modestly enhanced by GDC-0941. Continuous in vivo treatment invariably resulted in outgrowth of drug-resistant clones, ~30% of which showed markedly reduced glucocorticoid receptor (GR) protein expression. A similar proportion of relapsed human T-ALLs also exhibited low GR protein levels. De novo or preexisting mutations in the gene encoding GR (Nr3c1) occurred in relapsed clones derived from multiple independent parental leukemias. CRISPR/Cas9 gene editing confirmed that loss of GR expression confers DEX resistance. Exposing drug-sensitive T-ALLs to DEX in vivo altered transcript levels of multiple genes, and this response was attenuated in relapsed T-ALLs. These data implicate reduced GR protein expression as a frequent cause of glucocorticoid resistance in T-ALL.
Similar content being viewed by others
Introduction
Glucocorticoids are a cornerstone of therapeutic protocols for T lineage acute lymphoblastic leukemia (T-ALL) and other lymphoid cancers and a poor initial response to glucocorticoids remains one of the strongest predictors of relapse in ALL [1]. Studies performed in the 1980s revealed low GR protein levels in some diagnostic and relapsed pediatric ALL samples, but uncovered no clear association with treatment response [2,3,4,5]. In T-ALL cell lines exposed to glucocorticoids in vitro, resistance was associated with somatic NR3C1 mutations resulting in impaired GR activity, reduced receptor expression, and failure to upregulate GR in response to glucocorticoid exposure [6,7,8,9,10,11,12]. However, NR3C1 alterations were found only rarely in relapsed human B and T lineage ALLs [13,14,15,16,17] with the exception of relapsed B-cell precursor ALLs, which harbor frequent copy number alterations resulting in NR3C1 deletions [18,19,20,21]. Thus, the molecular mechanisms contributing to glucocorticoid resistance and relapse in ALL remain poorly understood.
Transplanting primary murine acute leukemias generated via retroviral insertional mutagenesis (RIM) in wild-type (WT) and KrasG12D mice and treating them in vivo is an unbiased strategy for elucidating resistance mechanisms [22,23,24,25]. In a previous study, the pan-PI3 kinase (PI3K) inhibitor GDC-0941 exhibited anti-leukemia activity as a single agent and in combination with the MEK inhibitor PD0325901 in RIM-induced T-ALLs [24]. These data and a report that identified the PI3K effector AKT as a negative regulator of GR [26] raised the possibility that PI3K inhibition might be synergistic with glucocorticoid treatment in T-ALL. To address this question, we treated 10 independent T-ALLs with the glucocorticoid dexamethasone (DEX) alone and in combination with GDC-0941. These studies revealed frequent downregulation of GR protein expression in drug-resistant leukemias isolated at relapse, including clones with either preexisting or acquired Nr3c1 mutations that followed different evolutionary trajectories. Directly inactivating NR3C1 induced profound glucocorticoid resistance in drug-sensitive human T-ALL cells, and primary murine leukemias with reduced GR expression showed impaired regulation of glucocorticoid response genes upon in vivo DEX exposure. These data support the existence of diverse molecular mechanisms that converge on reduced GR expression as a frequent cause of glucocorticoid resistance in T-ALL and potentially other lymphoid cancers.
Results
Dexamethasone and GDC-0941 show in vivo efficacy in T-ALL
Treating healthy mice with 15 mg/kg/day of DEX alone and in combination with 125 mg/kg/day of GDC-0941 [24] caused lymphopenia and splenic and thymic atrophy without other toxicities (data not shown). T-ALLs generated by performing RIM with MOL4070 [27] in wild-type mice (KrasWT) and congenic animals harboring a Kras oncogene (KrasG12D) [23, 24] contain frequent insertions and/or somatic mutations in known human T-ALL genes [23, 28]. Ten independent T-ALLs (n = 5 each of KrasWT and KrasG12D) were transplanted into 14 mice each and recipients were assigned to treatment with vehicle (n = 4), DEX (n = 5), or DEX/GDC-0941 (n = 5) (Fig. 1a). Mice were treated for 8 weeks or until disease progression, and bone marrow was obtained at euthanasia.
a Primary T-ALLs were first expanded in a single recipient. After euthanasia, 2 × 106 bone marrow cells were harvested and transplanted into 14 recipient mice, which were then randomly assigned to one of the three indicated treatment groups. b Kaplan–Meier survival analysis of recipient mice transplanted with 10 independent primary T-ALLs (five KrasWT and five KrasG12D) and treated with vehicle (n = 40), DEX (n = 50), or DEX/GDC-0941 (n = 50) indicated that DEX significantly extended survival (p < 0.0001, Log-rank test), which was modestly but not significantly enhanced by GDC-0941 (p = 0.0805, Log-rank test). c Southern blot cropped to show patterns of MOL4070 virus integration in genomic DNA extracted from leukemia cells harvested from individual moribund recipients of T-ALL 20M that were treated with vehicle (V1–V3), DEX (D1-D4), or DEX/GDC-0941 (C1–C5). Novel restriction fragments indicative of retroviral integrations that are not present in the dominant parental clone are visible in relapsed leukemias D3, D4, C1, and C4. d Kaplan–Meier survival analysis of relapsed T-ALLs (dotted lines) treated with vehicle (n = 3) or DEX (n = 4) compared with the corresponding drug-sensitive parental T-ALLs (solid lines) treated with vehicle (n = 4) or DEX (n = 5). Relapsed T-ALLs demonstrated intrinsic drug resistance by the criterion of significantly shorter survival after DEX treatment versus the corresponding parental leukemias; 20M.D1 (p = 0.0039, Log-rank test), 73M.D4 (p = 0.0027, Log-rank test), and JW-81.D3 (p = 0.0025, Log-rank test).
DEX-treated recipient mice showed a significant survival advantage compared with vehicle-treated controls (median 31 versus 20 days; p < 0.0001, Log-rank test) that was modestly enhanced by the addition of GDC-0941 (median 31 versus 40 days; p = 0.0805, Log-rank test) (Fig. 1b). Examining the ten individual leukemias revealed variable sensitivity to DEX and DEX/GDC-0941 (Table S1) that was independent of KrasG12D mutation status (Fig. S1a, b). To characterize genetic differences between parental and relapsed T-ALLs, we isolated leukemia cells from 71 mice that progressed after a significant (p < 0.05, Log-rank test) response to DEX or DEX/GDC-0941 treatment and performed Southern blotting to detect retroviral insertion sites. Twenty-eight relapsed leukemias (39%) exhibited one or more novel integrations compared with the corresponding parental T-ALL (Fig. 1c). Parental and relapsed T-ALLs invariably shared multiple retroviral integrations indicating evolution from a common founder clone, consistent with studies of relapsed human ALLs [13]. Moreover, T-ALLs that relapsed after an initial response to DEX acquired intrinsic drug resistance, confirmed by transplantation into secondary recipients and in vivo re-treatment with DEX (Figs. 1d and S1c). Relapsed T-ALLs with and without clonal evolution as visualized by Southern blotting exhibited DEX resistance, suggesting the existence of both insertional and non-insertional mechanisms.
Reduced GR protein expression is frequent in relapsed mouse and human T-ALLs
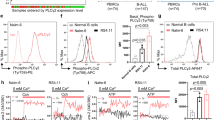
We performed Western blotting to assess Notch1 and Ras-regulated signaling molecules. These studies did not uncover consistent differences in the levels of cleaved Notch1, PTEN, phosphorylated ERK, phosphorylated Akt or phosphorylated S6 between parental and relapsed leukemias (Fig. S2). However, we observed markedly reduced or absent GR protein expression in multiple independent relapsed T-ALLs compared with the corresponding DEX-sensitive parental leukemia, which was independent of Kras mutation status (Fig. 2a). We next performed immunohistochemistry (IHC) to assess GR protein levels in a cohort of 102 diagnostic and relapsed T-ALL patient bone marrow biopsies in order to determine if human leukemias also exhibited less receptor expression at relapse (Fig. 2b). Analysis of Western blotting data in our murine leukemias demonstrated high levels of GR in 80% of parental T-ALLs (n = 10) versus 32% of relapsed leukemias (n = 65), with the remaining samples exhibiting medium (34%) or low (34%) GR protein expression (Fig. 2c; p value = 0.0109, Fisher’s test). Strikingly, blinded IHC analysis of patient samples with comparable disease burdens (Table S2) revealed robust GR protein expression in 90% of diagnostic T-ALL samples (n = 72), whereas 40% of the relapsed human leukemias (n = 30) were either weakly positive (27%) or negative (13%) for GR staining (Fig. 2d; p value = 0.0013, Fisher’s test). Together, these data demonstrate a significant increase in the proportion of T-ALLs with reduced or absent GR protein expression at relapse in large cohorts of mouse and human T-ALLs.
a Western blot cropped to show GR expression in bone marrow lysates from KrasWT and KrasG12D T-ALLs in parental (P), DEX treated (D) and DEX/GDC-0941 combination treated (C) mice at relapse. b Human bone marrow from T-ALL patients was subjected to immunohistochemistry analysis of GR protein expression and scored in a blinded fashion independently by two hematopathologists to classify samples as strongly positive (IV), positive (III), weakly positive (II), or negative (I). Scale bar represents 30 microns. Graphical representation of GR expression levels assessed by Western blot in primary murine T-ALLs (c) and by immunohistochemistry in human T-ALL patient samples (d). GR expression in murine T-ALLs was classified as low (examples in a include 2M.D1, 2M.C2, 20M.D4, 20M.C5), medium (examples include JW-81.C2, 5C.C2, 2M.C4, 20M.C4), or high (examples include 5C.D1, 2M.C1, 2M.C3, 20M.D3). Asterisk in c denotes a significant difference between high and medium/low classifications in parental versus relapsed murine T-ALLs (p value = 0.0109, Fisher’s test). Asterisk in d denotes a significant difference between strongly positive/positive and weakly positive/negative classifications in diagnostic versus relapsed human T-ALLs (p value = 0.0013, Fisher’s test). e Gene expression analysis using a TaqMan assay to measure Nr3c1 transcript levels in four independent parental T-ALLs (P), and corresponding DEX (D) or DEX/GDC-0941 combination (C) treated relapsed T-ALLs. Error bars represent standard error of the mean for technical triplicates. f Western blot cropped to show GR expression in lysates from the CCRF-CEM human T-ALL cell line edited using nucleofection with Cas9 ribonuleoproteins containing two independent sgRNAs targeting the Nr3c1 gene. g Analysis of viability in CCRF-CEM cells CRISPR edited at the GR locus and exposed to increasing doses of DEX. Error bars represent standard deviation of technical triplicates.
In order to assess the relationship between GR protein expression and Nr3c1 transcript levels, we performed quantitative RT-PCR (qPCR) analysis of bone marrow harvested from paired parental and relapsed murine T-ALLs. These studies revealed variably decreased Nr3c1 mRNA levels at relapse (Fig. 2e), suggesting GR protein levels may reflect reduced Nr3c1 transcription or result from post-transcriptional or post-translational mechanisms. We next performed CRISPR-mediated gene editing of Nr3c1 in CCRF-CEM, an established DEX-sensitive human T-ALL cell line [29]. CRISPR editing using ribonucleoproteins (RNPs) containing one of two independent sgRNA molecules targeting the Nr3c1 locus with high efficiency (58.9% for sgGR.1, 64.7% for sgGR.2) resulted in reduced GR protein expression (Fig. 2f) and also rendered CCRF-CEM cells resistant to DEX-induced cell death (Fig. 2g).
Relapsed T-ALLs harbor preexisting or acquired Nr3c1 mutations
To identify genomic alterations that might cause DEX resistance, we performed exome sequencing of 25 relapsed murine T-ALLs, including 22 with reduced or absent GR protein expression. This analysis revealed distinct point mutations or small insertions/deletions (indels) within Nr3c1 coding exons in 18% of relapsed T-ALLs analyzed that emerged after treatment with DEX or DEX/GDC-0941 (n = 22, including four samples from different recipients transplanted with KrasG12D T-ALL 78A that harbored the same indel). Nr3c1 mutations were also detected in mice transplanted with KrasWT T-ALL 5C and KrasG12D T-ALLs 2M and 20M (Fig. 3a and Table S3). Targeted Sanger sequencing of additional drug-treated relapsed clones isolated from recipients of parental T-ALL 78A revealed the same Nr3c1 L282fs indel at a variant allele frequency (VAF) that correlated with GR protein levels (Figs. 3b, S3a), and ultra-deep sequencing at ~30,000× coverage identified the L282fs indel at 1.1% VAF in parental T-ALL 78A (Fig. S3b). By contrast, the Nr3c1 mutations in T-ALLs 5C.C3, 2M.C4, and 20M.D1 were each detected at relapse in only one recipient mouse (n = 8 relapsed clones analyzed for T-ALL 5C; n = 8 for T-ALL 2M; n = 9 for T-ALL 20M). Accordingly, targeted sequencing did not identify preexisting Nr3c1 mutations Q517X and I513fs in parental T-ALLs 5C and 2M, respectively at a minimum sensitivity of <0.1% VAF. As in human T-ALL [30], somatic Notch1 mutations represent late genetic events in this model [23, 28]. T-ALLs 2M and 20M contain somatic Notch1 PEST domain mutations that were retained in resistant clones with or without Nr3c1 mutations, strongly suggesting that the Nr3c1 mutation was acquired later. Together, these data indicate T-ALL cells with Nr3c1 mutations are selected by DEX treatment, either as rare preexisting subclones or after de novo acquisition of this alteration. This observation is consistent with data from human ALL demonstrating both selection of preexisting relapse clones during treatment and development of new mutations that drive drug resistance [13, 16, 31,32,33].
a Dot plots showing mutant allele frequencies for mutations identified in the parental (P, x-axis) and the corresponding DEX-treated (D) or DEX/GDC-0941 combination-treated (C) relapsed T-ALLs (y-axis) highlighting the specific Nr3c1 mutations or indels. b Box and whiskers plot showing allele frequency of the Nr3c1 indel in each relapsed sample as compared with parental T-ALL 78A as determined from Sanger sequencing data (top), and Western blot cropped to show corresponding GR protein expression (bottom). Whiskers represent 10–90 percentile. c Sanger sequencing traces showing the site of the Nr3c1 point mutation (arrow) in parental and relapsed 20M T-ALLs. Note representation of the mutant allele is stable after transplantation in the absence of treatment, but increases markedly in secondary recipients that received DEX. d Western blot cropped to show GR protein expression in the parental (P), relapsed (D) and re-treated 20M T-ALLs shown in c. e Viability of independent CRISPR-edited CCRF-CEM cell clones nucleofected with a scramble guide (S1, S2, S3) or with a guide targeting the homologous region of exon 4 in which the mutation in T-ALL 20M.D was identified (GR1, GR2, GR3) in response to increasing doses of DEX. Error bars represent standard deviation of technical triplicates. f Western blot cropped to show GR expression in lysates from CCRF-CEM cell clones edited with scramble or Nr3c1-targeting sgRNAs.
Loss of Nr3c1 enhances clonal fitness and confers dexamethasone resistance
To determine if Nr3c1 mutations identified in relapsed T-ALLs are sufficient to induce DEX resistance, we performed functional studies in both relapsed T-ALL 20M.D1 and CCRF-CEM cells. Relapsed T-ALL 20M.D1 harbors a truncating Nr3c1 K514X stop-gain mutation at ~70% VAF, and exhibited phenotypic resistance upon DEX re-treatment in secondary recipients (Fig. 1d). In addition, in vivo re-treatment of relapsed clone 20M.D1 with DEX, but not the control vehicle, strongly selected for outgrowth of Nr3c1-mutant T-ALL cells (Fig. 3c) that exhibited profoundly reduced GR protein expression (Fig. 3d). We also performed nucleofection in CCRF-CEM cells with RNPs containing sgRNAs targeting the homologous region of Nr3c1 harboring the K514X mutation identified in T-ALL 20M.D1, then used limiting dilution to isolate multiple independent single-cell clones that exhibited bi-allelic editing. Whereas three CRISPR-edited clones exhibited loss of GR protein expression and resistance to DEX, three clones isolated after nucleofection with a control RNP containing a scrambled sgRNA showed no change in GR levels and remained drug-sensitive (Fig. 3e, f). Together, these studies indicate the Nr3c1 K514X stop-gain mutation identified in T-ALL 20M.D1 causes loss of GR protein expression and enhances the fitness of primary leukemia cells exposed to DEX in vivo, and also show that targeted disruption of Nr3c1 is sufficient to induce glucocorticoid resistance in vitro.
Relapsed T-ALLs with preexisting or acquired Nr3c1 mutations follow distinct evolutionary trajectories
The experimental strategy of transplanting primary T-ALLs into multiple independent recipient mice and treating them in vivo (Fig. 1a) provided a unique opportunity to explore the genetic relatedness of individual relapsed clones with either a preexisting or acquired Nr3c1 mutation. We analyzed retroviral integration and exome sequencing data from relapsed clones isolated from different recipients transplanted with parental T-ALLs 78A (n = 4) or 20M (n = 6). Consistent with rare preexisting T-ALL 78A cells bearing an Nr3c1 L282fs indel that conferred DEX resistance, Southern blotting revealed an identical pattern of MOL4070 integrations in the parental leukemia and all of the relapsed subclones (data not shown). The majority of mutations identified in T-ALL 78A, including the engineered KrasG12D mutation and a Notch1 indel, were also present at similar VAFs in all four relapsed clones analyzed by exome sequencing (78A.D1, 78A.D4, 78A.C2, 78A.C3) (Fig. 4a). A subclone harboring the Nr3c1 L282fs indel was positively selected during treatment and emerged in all four recipients analyzed with relatively few additional relapse-specific mutations identified (Fig. 4b and Table S3). The limited genetic diversity of T-ALL 78A relapsed clones is fully consistent with the existence of a rare, preexisting drug resistance mutation in the parental leukemia.
a Graphical representation of the allele frequencies of missense mutations and indels shared between the parental T-ALL 78A (x-axis) and four independent relapsed leukemias (y-axis; gray), present only in the parental leukemia (green), shared only with other relapsed leukemias (blue), significantly enriched in the relapsed leukemia (purple), or specific to one of the relapsed leukemias (red). b Analysis of the evolutionary relationships among genetic alterations identified by exome sequencing in T-ALL 78A indicated a linear pattern of clonal evolution. c Graphical representation of the mutant allele frequencies shared between the parental T-ALL 20M (x-axis) and six independent relapsed leukemias (y-axis; gray), present in the parental and a subset of relapsed leukemias (yellow), shared only with other relapsed leukemias (blue), or specific to one of the relapsed leukemias (red). d Evolutionary analysis of the genetic alterations identified by exome sequencing in T-ALL 20M indicates a branching pattern of clonal dynamics involving multiple ancestral populations. Legends indicate the type of mutation represented by each dot shown in a and b (left), and each dot and branch shown in c and d (right). The same color scheme is used in a–d.
In contrast to T-ALL 78A, the Nr3c1 K514X mutation was only detected in relapsed T-ALL 20M.D1. Interestingly, leukemia cells isolated from four other recipient mice that relapsed during treatment with DEX or DEX/GDC-0941 exhibited clonal evolution by Southern blot. T-ALLs 20M.D3 and 20M.D4 harbored two novel insertions also present in 20M.C1 (Fig. 1c), indicating evolution from a common preexisting founder clone. Exome sequencing of relapsed T-ALLs with (20M.D4, 20M.C1) or without (20M.C3, 20M.C5, 20M.D1, 20M.D2) novel retroviral integrations (Fig. 1c) revealed multiple mutations present at similar VAFs as in the parental leukemia (Fig. 4c, gray circles). In addition, relapsed clones 20M.D4, 20M.C1, 20M.C3, and 20M.C5 shared mutations that were absent in the parental leukemia (Fig. 4c; blue circles and Table S3), indicating derivation from a common ancestral clone. Within this group, relapsed clones 20M.C1 and 20M.C3 exhibited complete or partial loss of numerous mutations identified in the parental T-ALL (Fig. 4c, yellow circles), and thus arose prior to their acquisition. In addition, 20M.D4, 20M.C3, and 20M.C5 represent multi-lineage relapses harboring at least two subclones based on relapse-specific and partially shared VAFs lower than shared VAFs. The Nr3c1 K514X mutation is among the alterations found in a single relapsed clone (20M.D1), with additional unique mutations identified in the other relapsed leukemias analyzed (Fig. 4d and Table S3). Together, these data indicate that DEX resistance in T-ALL 20M is characterized by complex clonal dynamics with outgrowth from distinct but related preexisting subclones that undergo further genetic evolution upon in vivo drug exposure. These data are most consistent with the existence of an ancestral drug-tolerant clone that persists during treatment, but must acquire additional somatic mutations to cause relapse.
Relapsed T-ALLs show attenuated transcriptional responses to in vivo dexamethasone exposure
We sought to define common transcriptional changes within genetically diverse drug-sensitive T-ALLs following glucocorticoid treatment, which has pleiotropic and cell context-dependent effects [34]. Each parental leukemia was first transplanted into a cohort of recipient mice, which received a single dose of DEX (15 mg/kg) or control vehicle when they became moribund. The mice were euthanized 4 h later and bone marrow was harvested for RNA sequencing (RNA-seq). We observed modulation of known GR transcriptional targets after short-term DEX exposure in drug-sensitive leukemias. Notably, Myc and several of its target genes were consistently downregulated upon DEX exposure, while primary GR target genes (Fkbp5, Tsc22d3, Nr3c1) and pro-apoptotic genes (Bcl2l11, Txnip, Bmf) were consistently upregulated (Fig. 5a). We used qPCR to validate a subset of the transcriptional changes identified by RNA-seq, and then assessed whether short-term in vivo DEX treatment also modulated expression of GR-responsive genes in relapsed T-ALLs with low GR protein levels (Fig. 5b and Fig. S4a, b). Analysis of T-ALL 20M revealed a blunted transcriptional response to DEX in the relapsed sample harboring the Nr3c1 K514X mutation (20M.D1) as compared with the corresponding parental leukemia. Interestingly, four additional relapsed samples from T-ALL 20M that express low GR protein levels but lack Nr3c1 mutations (20M.D2, 20M.D4, 20M.C1, 20M.C5) also showed a blunted transcriptional response to DEX (Fig. 5b). Similarly, DEX treatment of relapsed subclones from T-ALLs 2M and 73A induced a pattern of target gene expression distinct from that observed in the corresponding parental T-ALL (Fig. S4a, b). These data demonstrate a lack of the expected transcriptional response to in vivo DEX treatment in relapsed T-ALLs with reduced GR protein levels that either harbor or lack a Nr3c1 mutation.
a Heatmap showing fold increased (p value < 0.001, FDR = 0.002) or decreased (p value < 0.001, FDR = 0.050) expression of selected GR-responsive genes after short-term in vivo DEX treatment measured by RNA-seq. b Gene expression analysis using TaqMan assays for each indicated gene in parental (P, gray bars) and relapsed (D) 20M T-ALLs with low GR protein expression with (blue bars) or without (red bars) a Nr3c1 mutation showing transcript levels of GC response genes as compared with vehicle-treated controls. Error bars represent standard error of the mean for technical triplicates.
Discussion
Delineating how individual drugs contribute to resistance and relapse is challenging in the context of the multi-agent chemotherapy protocols used in human ALL. To overcome this limitation, we transplanted and treated genetically heterogeneous primary T-ALLs with DEX as a single agent or in combination with a PI3K inhibitor and performed molecular and functional analyses of relapsed leukemias. Of 65 T-ALLs that relapsed after initially responding to glucocorticoid treatment in vivo, 68% showed reduced or absent GR protein expression. Importantly, GR protein levels were also reduced in 40% of relapsed human T-ALL samples, implicating loss of GR expression as a major cause of glucocorticoid resistance. This observation is consistent with clinical data correlating poor response to glucocorticoid treatment during induction with an elevated risk of relapse [1]. Although previous studies examining ALL patient samples have generated conflicting data regarding the correlation between GR expression level and glucocorticoid resistance [35,36,37], functional studies in human T-ALL cell lines have demonstrated that modulating GR expression level affects glucocorticoid response and resistance in vitro [38] and in xenograft models [39]. Consistent with these data, our unbiased forward genetic screen identified loss of GR expression as a mechanism of glucocorticoid resistance in vivo, which we functionally validated using CRISPR/Cas9 gene editing in vitro.
We identified Nr3c1 mutations in 18% of relapsed murine T-ALLs analyzed, and showed those with reduced or absent GR protein expression with or without a Nr3c1 mutation were resistant to DEX upon re-transplant and re-treatment. Whereas previous studies including relatively small numbers of relapsed ALLs uncovered NR3C1 mutations or deletions either rarely [16] or not at all [13, 15], a very recent study that performed comprehensive serial sequencing of diagnostic and relapsed samples from 103 Chinese pediatric ALL patients identified NR3C1 mutations as the most frequent relapse-specific alteration, occurring in 14% of cases [40]. Copy number alterations leading to deletion of NR3C1 have also been identified in 9% of relapsed B-cell precursor ALLs [21], with one study reporting NR3C1 deletions in 28% of the ETV6/RUNX1-positive subgroup [18]. Importantly, NR3C1 deletions were consistently associated with inferior treatment response and adverse outcome in B-cell precursor ALL [18,19,20,21].
Non-mutational mechanisms have also been implicated in causing reduced GR expression and glucocorticoid resistance in ALL. Paugh and colleagues identified caspase-induced cleavage of GR due to epigenetic upregulation of the NALP inflammasome as a glucocorticoid resistance mechanism, and showed this reduction in GR protein levels induced substantial DEX resistance in cell line models [41]. More recently, a preliminary report showed T-ALL cells with a mutation in the histone methyl transferase NSD2 exhibit both reduced GR expression and impaired upregulation after glucocorticoid treatment, resulting in a lack of GR binding to DNA and a profound reduction in the subsequent transcriptional response [42]. Together with our studies of human and mouse leukemias, these emerging data indicate reduced GR expression is common in relapsed T-ALL and may occur in the absence of Nr3c1/NR3C1 mutations.
Recent serial targeted ultra-deep sequencing of a large human ALL cohort showed that most NR3C1 mutations detected at relapse are likely acquired during treatment and arise in patients that relapse later than those harboring a preexisting drug resistance mutation [40]. Similarly, three of the four Nr3c1 mutations we identified in relapsed mouse T-ALLs were not detected in the corresponding parental leukemia, and were only present in a single recipient mouse that relapsed later than recipients of the T-ALL harboring a preexisting Nr3c1 mutation. Gain-of-function PRPS1 and NT5C2 mutations that drive thiopurine resistance in ALL were also not detected at diagnosis by high-depth sequencing [31, 43]. A recent study showing NT5C2 mutations reduce fitness of ALL cells in the absence of drug treatment provided an elegant mechanistic explanation for this observation [33]. The ability of NR3C1 mutations to alter the competitive fitness of leukemia-initiating cells may be context-dependent, particularly given the diverse physiologic roles of glucocorticoid signaling at different stages of lymphoid development [44].
Several sequencing studies comparing diagnostic and relapsed ALL patient samples have identified preexisting drug-resistant cells that are selected during treatment [13, 16, 32]. An alternative mechanism in which drug-tolerant “persister” cells survive treatment and then acquire additional alterations rendering them fully resistant was first described in solid tumor cell lines [45,46,47,48,49], and has been observed in γ-secretase inhibitor resistant T-ALL cell lines in which drug-tolerant cells with an altered chromatin state exhibit sensitivity to inhibition of the epigenetic reader protein BRD4 [50]. However, the role of drug-tolerant persister cells in primary cancers and in the context of treatment with conventional chemotherapy agents remains unclear. Our analysis of evolutionary trajectories in multiple relapsed murine T-ALLs derived from the same parental leukemia including one with a somatic Nr3c1 mutation uncovered remarkable genetic diversity in comparison to the T-ALL with a preexisting Nr3c1 mutation. These data support a two-step model of glucocorticoid resistance whereby preexisting drug-tolerant clones survive induction, but must acquire additional alterations that confer full resistance to DEX and drive relapse. Furthermore, our data indicating the majority of Nr3c1 mutations were acquired during treatment suggest this resistance mechanism may be more common than selection of a preexisting resistant clone in ALL. Similarly, deep genomic analysis of relapsed pediatric ALLs supports acquired mutations in genes involved in drug action within a drug-tolerant cell population as a frequent mechanism of resistance, and demonstrated a comparable evolutionary trajectory as we observed in relapsed mouse leukemias [40].
Our data provide a strong rationale for implementing treatment strategies to eradicate drug-tolerant clones that survive induction and ultimately give rise to resistance in patients with sub-optimal responses to front-line therapies. Although the addition of a PI3K inhibitor enhanced survival in some T-ALLs as compared with treatment with DEX alone, this combination therapy was not sufficient to overcome DEX resistance across our cohort of primary murine leukemias. Newer compounds targeting specific PI3K isoforms [51] or inhibitors of downstream effectors [52] may provide more effective suppression of the pathway and enhance survival in combination with glucocorticoids. In addition, recent studies suggest that targeting interleukin 7 receptor signaling [53, 54] and developing methods for antagonizing aberrant JDP2 expression through MCL1 inhibition [55] are potential strategies for overcoming intrinsic glucocorticoid resistance in select patients.
As relapsed/refractory T-ALLs with NR3C1 mutations or loss of GR protein expression are unlikely to respond to glucocorticoids, this knowledge may identify patients who will not benefit from additional glucocorticoid therapy and could thereby avoid potential toxicities. Similarly, developing clinical tests to identify NR3C1 mutations and/or low GR protein expression in the leukemic blasts of patients with detectable minimal residual disease during treatment may identify those at high risk for relapse who could benefit from alternative or novel therapies. Intriguingly, restoring glucocorticoid sensitivity may prove feasible with combination therapy in some leukemias that exhibit reduced GR protein expression due to non-mutational mechanisms, potentially through the use of drugs targeting epigenetic modification [56, 57].
Materials and methods
Preclinical trials
Retroviral insertional mutagenesis was performed using MOL4070LTR in wild-type (KrasWT) or Mx1-Cre, Lox-STOP-Lox (LSL)-KrasG12D (KrasG12D) mice on a C57BL/6 x 129Sv/Jae (F1) strain background as described previously [23]. Cryopreserved primary T-ALL cells were transplanted into sublethally irradiated (450 rads) recipient mice for in vivo expansion, then (2 × 106) bone marrow cells from these mice were intravenously or retro-orbitally injected into sublethally irradiated 8–12 week old male F1 recipient mice for the preclinical trial. Starting four days post transplant, GDC-0941 (125 mg/kg/day) or control vehicle (0.5% hydroxypropyl methylcellulose, 0.2% Tween 80) was administered by oral gavage, and DEX (15 mg/kg/day) or control vehicle (phosphate buffered saline) was administered by intraperitoneal injection without blinding at any stage of the study. For the trial cohort, 14 mice were randomly assigned to receive vehicle (n = 4), DEX (n = 5), or DEX/GDC-0941 (n = 5). To confirm drug resistance in relapsed T-ALLs, seven secondary recipients were randomly assigned to receive vehicle (n = 3) or DEX (n = 4). Mice were euthanized when they appeared moribund and survival was calculated from day of transplant. The time to death for vehicle-treated mice transplanted with individual T-ALLs was highly consistent, allowing determination of significant differences in the survival of drug-treated mice. GraphPad Prism Software was used generate Kaplan–Meier survival curves. Statistical significance was calculated by comparing individual trial arms using a two-tailed Log-rank test. Data analysis from previous studies has verified the statistical power of these cohort sizes to reliably detect significant differences [22,23,24,25, 58]. All animals assigned to treatment groups were included in the survival analysis. Very rarely (n = 3 mice in the original preclinical trial cohort), animals were censored due to a mortality that was not caused by leukemia but rather an unrelated event including complications during drug administration or lack of leukemia engraftment leading to bone marrow failure. Dexamethasone sodium phosphate for injection (NDC 63323-516-10) was purchased through the University of California, San Francisco (UCSF) pharmacy. GDC-0941 was obtained through a Materials Transfer Agreement with Genentech, Inc. All animal studies were approved by the UCSF Institutional Animal Care and Use Committee (AN179357).
Southern blot analysis and MOL4070LTR integration cloning
Restriction enzyme digestion with HindIII or BamHI of genomic DNA from primary mouse T-ALLs was followed by gel electrophoresis and hybridization with a 32P-radiolabeled MOLO4070LTR-specific probe as described previously [22]. Retroviral junction fragments were cloned and sequenced using a linker-based approach [59].
Western blot analysis and immunohistochemistry
Bone marrow from moribund T-ALL recipients was subjected to red blood cell lysis, then resuspended in RIPA buffer to generate lysates. Protein concentration was determined using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific), then equivalent amounts of protein were mixed with Laemmli Sample Buffer (Bio-Rad Laboratories) followed by separation on an 8–20% Criterion TGX Precast Gel (Bio-Rad Laboratories). Immunoblots were assayed using primary antibodies listed in Table S4. IRDye secondary antibodies were used for detection and Western blots were imaged using an Odyssey Imaging System (LI-COR Biosciences). GR expression level for each leukemia was assigned an unblinded score of high (comparable to the corresponding parental T-ALL), medium (reduced as compared with the corresponding parental T-ALL), or low (nearly or completely absent). The level of GR protein expression for each T-ALL was assessed in bone marrow from at least two independent recipient mice with comparable results. Immunohistochemistry was performed on de-identified human bone marrow samples from children and adults of both sexes diagnosed with T-cell acute lymphoblastic leukemia using GR antibody at 1:400. Diagnostic and relapsed samples were unpaired, with the exception of two patients. T-ALL samples subjected to IHC analysis were scored blindly by two hematopathologists. A detailed protocol for immunohistochemistry and image acquisition in human T-ALLs is provided in the Supplementary Materials and methods. GraphPad Prism Software was used for graphical representation of the data. The two-tailed Fisher’s exact test was used to determine the significance of association with respect to GR expression levels in parental/diagnostic versus relapsed mouse and human T-ALLs. De-identified human tissue samples were obtained with Institutional Review Board approval from UCSF (10-01080), Brigham and Women’s Hospital (2010P002736) and Boston Children’s Hospital (P00025328).
CRISPR/Cas9 modification of CCRF-CEM cells
CCRF-CEM cells were purchased through the UCSF Cell Culture Facility (ATCC, CCL-119) and cultured in RPMI media supplemented with 10% fetal bovine serum and glutamine. All cell lines described in this study were authenticated through the UC Berkeley DNA Sequencing Facility and tested negative for mycoplasma. Cas9 protein was purchased from the University of California, Berkeley. tracrRNA, sgRNAs, and the HDR template were purchased from Dharmacon, and sequences are listed in Table S5. Transfection was performed using Amaxa Cell Line Nucleofector Kit C (Lonza, VACA-1004) in an Amaxa Nucleofector II Device. Editing efficiencies were determined using TIDE analysis software [60]. CRISPR-edited clones were generated using limiting dilution in conjunction with TIDE analysis to confirm editing of all alleles and Sanger sequencing to confirm HDR template incorporation.
In vitro dexamethasone dose response assays
Unperturbed or CRISPR-edited CCRF-CEM cells were seeded into 96-well round-bottom tissue culture plates in triplicate (50,000 cells/well) and exposed to increasing concentrations of DEX (Sigma; D4902) or vehicle (DMSO). Cells were resuspended in Hoechst viability dye after 72 h and analyzed on a BD FACSVerse 8 color Flow Cytometer. FlowJo software was used to determine the percentage of live cells at each DEX concentration. GraphPad Prism Software was used for graphical representation of the data. Each dose response assay was repeated three times with comparable results.
Exome and targeted sequencing
Genomic DNA was isolated from the bone marrow of moribund T-ALL recipient mice after prolonged in vivo drug exposure using a Puregene DNA Isolation Kit (Qiagen), quantified with PicoGreen and quality controlled by Agilent BioAnalyzer, and 264–500 ng of DNA were used to prepare libraries using the KAPA Hyper Prep Kit (Kapa Biosystems KK8504) with 8 PCR cycles. After sample barcoding, 500–2100 ng of library were captured by hybridization using SureSelectXT Mouse All Exon (Agilent #5190-4641) according to the manufacturer’s protocol. In total, 8–10 cycles of PCR amplification of the post-capture libraries was undertaken after which samples were sequenced on either HiSeq 4000 or HiSeq 2500 instrumentation in 100 bp paired end format using the HiSeq 3000/4000 SBS Kit or HiSeq Rapid SBS Kit v2 (Illumina). Normal and tumor samples were covered to an average of 86- and 173-fold, respectively. Detailed descriptions of the targeted sequencing approach, exome sequencing analysis, and Sanger sequencing validation methods are described in the Supplementary Materials and methods. Primers used to validate Nr3c1 mutations identified by exome sequencing are listed in Table S6. The exome sequencing data presented in Figs. 3 and 4 of this manuscript were deposited into the DNA Data Bank for Japan (https://www.ddbj.nig.ac.jp/dra/index-e.html) under accession code DRA009410.
Short-term dexamethasone treatment and RNA-seq
Moribund T-ALL recipient mice received a single dose of vehicle (phosphate buffered saline) or DEX (15 mg/kg), and were euthanized 4 h later. Bone marrow cells from these animals were subjected to red blood cell lysis, then frozen in Trizol. Total RNA was extracted and assessed for quality and quantity using the Eukaryote Total RNA Nano Bioanalyzer chip (Agilent Technologies, Inc) and Qubit fluorometer (Thermo Fisher), respectively. RNA-Seq libraries were prepared on the Sciclone liquid handling workstation (PerkinElmer) from 1 µg of total RNA per sample using the TruSeq Stranded Total RNA kit (Illumina, Inc.) following the manufacturer’s recommendations. RNA-Seq libraries were clustered on the cBot and sequenced on HiSeq 2000 and HiSeq 2500 sequencers at 2 × 100 bp using HiSeq v4 sequencing reagents (Illumina, Inc.). RNA was isolated from bone marrow of one vehicle- or DEX-treated mouse per T-ALL and used to perform RNA-seq. Detailed analysis methods are described in the Supplementary Materials and methods. False discovery rate and p values were calculated from RNA-seq data to assess the significance of gene expression changes. RNA-sequencing data presented in Fig. 5 of this manuscript was deposited into the National Center for Biotechnology Information Gene Expression Omnibus (NCBI Geo; https://www.ncbi.nlm.nih.gov/geo/) under accession code GSE141967.
Quantitative RT-PCR validation of transcriptome analysis
Bone marrow cells collected after short-term DEX treatment as described above were subjected to red blood cell lysis, then total RNA was isolated using the RNeasy Mini Kit (Qiagen) and used to generate cDNA using Superscript III (Thermo Fisher Scientific). TaqMan Gene Expression Assays (Applied Biosystems) are listed in Table S7, and qPCR was performed on a QuantStudio 5 Real-Time PCR Instrument (Applied Biosystems). qPCR was performed on bone marrow from one vehicle- or DEX-treated mouse per T-ALL using technical triplicates. GraphPad Prism Software was used for graphical representation of the data.
Statistical analysis
The two-tailed Log-rank test was used to perform pairwise comparisons of Kaplan–Meier survival analyses in order to determine significance. The two-tailed Fisher’s exact test was used to determine the significance of association with respect to GR expression levels in parental/diagnostic versus relapsed mouse and human T-ALLs. False discovery rate and p-values were calculated to assess the significance of gene expression changes as determined by RNA-seq analysis of murine T-ALLs. Standard deviation was calculated using technical triplicates for in vitro assessment of DEX sensitivity in human T-ALL cell lines. Standard error of the mean was calculated using technical triplicates for qPCR in primary murine T-ALLs.
References
Gao J, Liu WJ. Prognostic value of the response to prednisone for children with acute lymphoblastic leukemia: a meta-analysis. Eur Rev Med Pharmacol Sci. 2018;22:7858–66.
Pui CH, Dahl GV, Rivera G, Murphy SB, Costlow ME. The relationship of blast cell glucocorticoid receptor levels to response to single-agent steroid trial and remission response in children with acute lymphoblastic leukemia. Leuk Res. 1984;8:579–85.
Pui CH, Ochs J, Kalwinsky DK, Costlow ME. Impact of treatment efficacy on the prognostic value of glucocorticoid receptor levels in childhood acute lymphoblastic leukemia. Leuk Res. 1984;8:345–50.
Pui CH, Costlow ME. Sequential studies of lymphoblast glucocorticoid receptor levels at diagnosis and relapse in childhood leukemia: an update. Leuk Res. 1986;10:227–9.
Lauten M, Cario G, Asgedom G, Welte K, Schrappe M. Protein expression of the glucocorticoid receptor in childhood acute lymphoblastic leukemia. Haematologica. 2003;88:1253–8.
Ashraf J, Thompson EB. Identification of the activation-labile gene: a single point mutation in the human glucocorticoid receptor presents as two distinct receptor phenotypes. Mol Endocrinol. 1993;7:631–42.
Powers JH, Hillmann AG, Tang DC, Harmon JM. Cloning and expression of mutant glucocorticoid receptors from glucocorticoid-sensitive and -resistant human leukemic cells. Cancer Res. 1993;53:4059–65.
Strasser-Wozak EM, Hattmannstorfer R, Hala M, Hartmann BL, Fiegl M, Geley S, et al. Splice site mutation in the glucocorticoid receptor gene causes resistance to glucocorticoid-induced apoptosis in a human acute leukemic cell line. Cancer Res. 1995;55:348–53.
Geley S, Hartmann BL, Hala M, Strasser-Wozak EM, Kapelari K, Kofler R. Resistance to glucocorticoid-induced apoptosis in human T-cell acute lymphoblastic leukemia CEM-C1 cells is due to insufficient glucocorticoid receptor expression. Cancer Res. 1996;56:5033–8.
Hala M, Hartmann BL, Bock G, Geley S, Kofler R. Glucocorticoid-receptor-gene defects and resistance to glucocorticoid-induced apoptosis in human leukemic cell lines. Int J Cancer. 1996;68:663–8.
Riml S, Schmidt S, Ausserlechner MJ, Geley S, Kofler R. Glucocorticoid receptor heterozygosity combined with lack of receptor auto-induction causes glucocorticoid resistance in Jurkat acute lymphoblastic leukemia cells. Cell Death Differ. 2004;11(Suppl 1):S65–72.
Schmidt S, Irving JA, Minto L, Matheson E, Nicholson L, Ploner A, et al. Glucocorticoid resistance in two key models of acute lymphoblastic leukemia occurs at the level of the glucocorticoid receptor. FASEB J. 2006;20:2600–2.
Mullighan CG, Phillips LA, Su X, Ma J, Miller CB, Shurtleff SA, et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008;322:1377–80.
Hogan LE, Meyer JA, Yang J, Wang J, Wong N, Yang W, et al. Integrated genomic analysis of relapsed childhood acute lymphoblastic leukemia reveals therapeutic strategies. Blood. 2011;118:5218–26.
Kunz JB, Rausch T, Bandapalli OR, Eilers J, Pechanska P, Schuessele S, et al. Pediatric T-cell lymphoblastic leukemia evolves into relapse by clonal selection, acquisition of mutations and promoter hypomethylation. Haematologica. 2015;100:1442–50.
Oshima K, Khiabanian H, da Silva-Almeida AC, Tzoneva G, Abate F, Ambesi-Impiombato A, et al. Mutational landscape, clonal evolution patterns, and role of RAS mutations in relapsed acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2016;113:11306–11.
Spinella JF, Richer C, Cassart P, Ouimet M, Healy J, Sinnett D. Mutational dynamics of early and late relapsed childhood ALL: rapid clonal expansion and long-term dormancy. Blood Adv. 2018;2:177–88.
Kuster L, Grausenburger R, Fuka G, Kaindl U, Krapf G, Inthal A, et al. ETV6/RUNX1-positive relapses evolve from an ancestral clone and frequently acquire deletions of genes implicated in glucocorticoid signaling. Blood. 2011;117:2658–67.
Bokemeyer A, Eckert C, Meyr F, Koerner G, von Stackelberg A, Ullmann R, et al. Copy number genome alterations are associated with treatment response and outcome in relapsed childhood ETV6/RUNX1-positive acute lymphoblastic leukemia. Haematologica. 2014;99:706–14.
Grausenburger R, Bastelberger S, Eckert C, Kauer M, Stanulla M, Frech C, et al. Genetic alterations in glucocorticoid signaling pathway components are associated with adverse prognosis in children with relapsed ETV6/RUNX1-positive acute lymphoblastic leukemia. Leuk Lymphoma. 2016;57:1163–73.
Irving JA, Enshaei A, Parker CA, Sutton R, Kuiper RP, Erhorn A, et al. Integration of genetic and clinical risk factors improves prognostication in relapsed childhood B-cell precursor acute lymphoblastic leukemia. Blood. 2016;128:911–22.
Lauchle JO, Kim D, Le DT, Akagi K, Crone M, Krisman K, et al. Response and resistance to MEK inhibition in leukaemias initiated by hyperactive Ras. Nature. 2009;461:411–4.
Dail M, Li Q, McDaniel A, Wong J, Akagi K, Huang B, et al. Mutant Ikzf1, KrasG12D, and Notch1 cooperate in T lineage leukemogenesis and modulate responses to targeted agents. Proc Natl Acad Sci USA. 2010;107:5106–11.
Dail M, Wong J, Lawrence J, O'Connor D, Nakitandwe J, Chen SC, et al. Loss of oncogenic Notch1 with resistance to a PI3K inhibitor in T-cell leukaemia. Nature. 2014;513:512–6.
Burgess MR, Hwang E, Mroue R, Bielski CM, Wandler AM, Huang BJ, et al. KRAS allelic imbalance enhances fitness and modulates MAP kinase dependence in cancer. Cell. 2017;168:817–29. e15.
Piovan E, Yu J, Tosello V, Herranz D, Ambesi-Impiombato A, Da Silva AC, et al. Direct reversal of glucocorticoid resistance by AKT inhibition in acute lymphoblastic leukemia. Cancer Cell. 2013;24:766–76.
Wolff L, Koller R, Hu X, Anver MR. A Moloney murine leukemia virus-based retrovirus with 4070A long terminal repeat sequences induces a high incidence of myeloid as well as lymphoid neoplasms. J Virol. 2003;77:4965–71.
Huang BJ, Wandler AM, Meyer LK, Dail M, Daemen A, Sampath D, et al. Convergent genetic aberrations in murine and human T lineage acute lymphoblastic leukemias. PLoS Genet. 2019;15:e1008168.
Norman MR, Thompson EB. Characterization of a glucocorticoid-sensitive human lymphoid cell line. Cancer Res. 1977;37:3785–91.
Mansour MR, Duke V, Foroni L, Patel B, Allen CG, Ancliff PJ, et al. Notch-1 mutations are secondary events in some patients with T-cell acute lymphoblastic leukemia. Clin Cancer Res. 2007;13:6964–9.
Tzoneva G, Perez-Garcia A, Carpenter Z, Khiabanian H, Tosello V, Allegretta M, et al. Activating mutations in the NT5C2 nucleotidase gene drive chemotherapy resistance in relapsed ALL. Nat Med. 2013;19:368–71.
Ma X, Edmonson M, Yergeau D, Muzny DM, Hampton OA, Rusch M, et al. Rise and fall of subclones from diagnosis to relapse in pediatric B-acute lymphoblastic leukaemia. Nat Commun. 2015;6:6604.
Tzoneva G, Dieck CL, Oshima K, Ambesi-Impiombato A, Sanchez-Martin M, Madubata CJ, et al. Clonal evolution mechanisms in NT5C2 mutant-relapsed acute lymphoblastic leukaemia. Nature. 2018;553:511–4.
Reddy TE, Pauli F, Sprouse RO, Neff NF, Newberry KM, Garabedian MJ, et al. Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Res. 2009;19:2163–71.
Schwartz CL, Thompson EB, Gelber RD, Young ML, Chilton D, Cohen HJ, et al. Improved response with higher corticosteroid dose in children with acute lymphoblastic leukemia. J Clin Oncol. 2001;19:1040–6.
Haarman EG, Kaspers GJ, Pieters R, Rottier MM, Veerman AJ. Glucocorticoid receptor alpha, beta and gamma expression vs in vitro glucocorticod resistance in childhood leukemia. Leukemia. 2004;18:530–7.
Tissing WJ, Meijerink JP, Brinkhof B, Broekhuis MJ, Menezes RX, den Boer ML, et al. Glucocorticoid-induced glucocorticoid-receptor expression and promoter usage is not linked to glucocorticoid resistance in childhood ALL. Blood. 2006;108:1045–9.
Gruber G, Carlet M, Turtscher E, Meister B, Irving JA, Ploner C, et al. Levels of glucocorticoid receptor and its ligand determine sensitivity and kinetics of glucocorticoid-induced leukemia apoptosis. Leukemia. 2009;23:820–3.
Xiao H, Ding Y, Gao Y, Wang LM, Wang H, Ding L, et al. Haploinsufficiency of NR3C1 drives glucocorticoid resistance in adult acute lymphoblastic leukemia cells by down-regulating the mitochondrial apoptosis axis, and is sensitive to Bcl-2 blockage. Cancer Cell Int. 2019;19:218.
Li B, Brady SW, Ma X, Shen S, Zhang Y, Li Y, et al. Therapy-induced mutations drive the genomic landscape of relapsed acute lymphoblastic leukemia. Blood. 2020;135:41–55.
Paugh SW, Bonten EJ, Savic D, Ramsey LB, Thierfelder WE, Gurung P, et al. NALP3 inflammasome upregulation and CASP1 cleavage of the glucocorticoid receptor cause glucocorticoid resistance in leukemia cells. Nat Genet. 2015;47:607–14.
Li J, Troche C, Swaroop A, Kulis M, Oyer J, Will C, et al. A gain of function mutation in the NSD2 histone methyltransferase drives glucocorticoid resistance of acute lymphoblastic leukemia. Blood. 2018;132(Suppl 1):653.
Li B, Li H, Bai Y, Kirschner-Schwabe R, Yang JJ, Chen Y, et al. Negative feedback-defective PRPS1 mutants drive thiopurine resistance in relapsed childhood ALL. Nat Med. 2015;21:563–71.
Jamieson CA, Yamamoto KR. Crosstalk pathway for inhibition of glucocorticoid-induced apoptosis by T cell receptor signaling. Proc Natl Acad Sci USA. 2000;97:7319–24.
Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80.
Ramirez M, Rajaram S, Steininger RJ, Osipchuk D, Roth MA, Morinishi LS, et al. Diverse drug-resistance mechanisms can emerge from drug-tolerant cancer persister cells. Nat Commun. 2016;7:10690.
Raha D, Wilson TR, Peng J, Peterson D, Yue P, Evangelista M, et al. The cancer stem cell marker aldehyde dehydrogenase is required to maintain a drug-tolerant tumor cell subpopulation. Cancer Res. 2014;74:3579–90.
Hata AN, Niederst MJ, Archibald HL, Gomez-Caraballo M, Siddiqui FM, Mulvey HE, et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med. 2016;22:262–9.
Hangauer MJ, Viswanathan VS, Ryan MJ, Bole D, Eaton JK, Matov A, et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551:247–50.
Knoechel B, Roderick JE, Williamson KE, Zhu J, Lohr JG, Cotton MJ, et al. An epigenetic mechanism of resistance to targeted therapy in T cell acute lymphoblastic leukemia. Nat Genet. 2014;46:364–70.
Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer. 2015;15:7–24.
Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44.
Delgado-Martin C, Meyer LK, Huang BJ, Shimano KA, Zinter MS, Nguyen JV, et al. JAK/STAT pathway inhibition overcomes IL7-induced glucocorticoid resistance in a subset of human T-cell acute lymphoblastic leukemias. Leukemia. 2017;31:2568–76.
Meyer LK, Huang BJ, Delgado-Martin C, Roy RP, Hechmer A, Wandler AM, et al. Glucocorticoids paradoxically facilitate steroid resistance in T-cell acute lymphoblastic leukemias and thymocytes. J Clin Investig. 2020;130:863–76.
Mansour MR, He S, Li Z, Lobbardi R, Abraham BJ, Hug C, et al. JDP2: An oncogenic bZIP transcription factor in T cell acute lymphoblastic leukemia. J Exp Med. 2018;215:1929–45.
Mansour MR, Abraham BJ, Anders L, Berezovskaya A, Gutierrez A, Durbin AD, et al. Oncogene regulation. An oncogenic super-enhancer formed through somatic mutation of a noncoding intergenic element. Science. 2014;346:1373–7.
Jing D, Huang Y, Liu X, Sia KCS, Zhang JC, Tai X, et al. Lymphocyte-specific chromatin accessibility pre-determines glucocorticoid resistance in acute lymphoblastic leukemia. Cancer Cell. 2018;34:906–21. e8.
Burgess MR, Hwang E, Firestone AJ, Huang T, Xu J, Zuber J, et al. Preclinical efficacy of MEK inhibition in Nras-mutant AML. Blood. 2014;124:3947–55.
Uren AG, Mikkers H, Kool J, van der Weyden L, Lund AH, Wilson CH, et al. A high-throughput splinkerette-PCR method for the isolation and sequencing of retroviral insertion sites. Nat Protoc. 2009;4:789–98.
Brinkman EK, Chen T, Amendola M, van Steensel B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014;42:e168.
Acknowledgements
We thank members of the Integrated Genomics Operation in the Marie-Josee and Henry R. Kravis Center for Molecular Oncology at the Memorial Sloan-Kettering Cancer Center, and the Genome Sequencing Lab at St. Jude Children’s Research Hospital for assistance with sequencing; the UCSF Biorepository and Tissue Biomarker Technology Core and the DF/HCC Research Pathology Cores at Harvard Medical School for performing immunohistochemistry analysis. This study was supported in part by the UCSF Helen Diller Family Comprehensive Cancer Center Laboratory for Cell Analysis Shared Resource Facility through a grant from the NIH (P30CA082103). We obtained KrasG12D mice from David Tuveson and Tyler Jacks, and Linda Wolff provided the MOL4070LTR retrovirus. This work was supported in part by National Institutes of Health awards R37 CA72614 (KS), R01 CA180037 (KS); P30 CA008748, U54 OD020355 (BST), R01 CA204749 (BST), and awards from the St. Baldrick’s Foundation (KS), the American Cancer Society (RSG-15-067-01-TBG) (KS), the Anna Fuller Fund, and the Robertson Foundation (BST). AMW was supported by a Postdoctoral Fellowship (PF-14-070-01-TBG) from the American Cancer Society including a supplement from the Hillcrest Committee, and an Alex’s Lemonade Stand Foundation Young Investigator Grant including support from Northwestern Mutual. KS is an American Cancer Society Research Professor (RP 10-078-01-TBE).
Author information
Authors and Affiliations
Contributions
AMW, BJH, JWC, SCK, JCA, BST and KS designed experiments and analyzed data. AMW, JWC, KH, HY, LKM, AS, GM and JN performed experiments. BJH, PJ and BST performed bioinformatics analysis. MD, QL, JCW, OW, RPH, SCK, DS and JCA provided reagents. KY, JRD, JZ and MD provided conceptual advice. AMW and KS wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Deepak Sampath and Monique Dail were employees and shareholders of Genentech, Inc. when this work was performed. The remaining authors of this paper declare no financial interest related to this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wandler, A.M., Huang, B.J., Craig, J.W. et al. Loss of glucocorticoid receptor expression mediates in vivo dexamethasone resistance in T-cell acute lymphoblastic leukemia. Leukemia 34, 2025–2037 (2020). https://doi.org/10.1038/s41375-020-0748-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0748-6
- Springer Nature Limited
This article is cited by
-
Glucocorticoid Therapy in Acute Lymphoblastic Leukemia: Navigating Short-Term and Long-Term Effects and Optimal Regimen Selection
Current Hematologic Malignancy Reports (2024)
-
Exploring the nexus of nuclear receptors in hematological malignancies
Cellular and Molecular Life Sciences (2024)
-
Epigenomic profiling of glucocorticoid responses identifies cis-regulatory disruptions impacting steroid resistance in childhood acute lymphoblastic leukemia
Leukemia (2022)
-
Characterization of a novel glucocorticoid-resistant human B-cell acute lymphoblastic leukemia cell line, with AMPK, mTOR and fatty acid synthesis pathway inhibition
Cancer Cell International (2021)
-
The Aurora kinase/β-catenin axis contributes to dexamethasone resistance in leukemia
npj Precision Oncology (2021)