Abstract
Hematopoietic stem and progenitor cells (HSPCs) are responsible for lifelong maintenance of hematopoiesis through self-renewal and differentiation into mature blood cell lineages. Traditional models hold that HSPCs guard homeostatic function and adapt to regenerative demand by integrating cell-autonomous, intrinsic programs with extrinsic cues from the niche. Despite the biologic significance, little is known about the active roles HSPCs partake in reciprocally shaping the function of their microenvironment. Here, we review evidence of signals emerging from HSPCs through secreted autocrine or paracrine factors, including extracellular vesicles, and via direct contact within the niche. We also discuss the functional impact of direct cellular interactions between hematopoietic elements on niche occupancy in the context of leukemic infiltration. The aggregate data support a model whereby HSPCs are active participants in the dynamic adaptation of the stem cell niche unit during development and homeostasis, and under inflammatory stress, malignancy, or transplantation.
Similar content being viewed by others
Introduction
A heterogeneous population of hematopoietic stem and progenitor cells (HSPCs) sustain lifelong organ function through the ability to self-renew and differentiate into mature cell lineages. During adult life, operationally-defined, highly specialized core units, termed niches, support HSPC residence and maintain cells in a predominantly quiescent state to prevent exhaustion [1, 2]. In response to systemic demand or injury, a small number of long-lived stem cells emerge to undergo asymmetric cell division, allowing simultaneous self-renewal and hematopoietic differentiation [3]. A central tenet of HSPC biology is that overall function is governed by integrating cell-intrinsic programs with extrinsic cues. These unique capabilities rely upon extensive and tightly controlled cell-to-cell communication between constituent niche components and HSPCs.
A growing body of research, predominantly in murine models, has examined the role of individual elements in the bone marrow (BM) niche in regulating HSPCs. Various non-hematopoietic cells, including BM fibroblasts, endothelial cells, and osteoblasts provide juxtacrine and paracrine signals received by HSPCs, including hematopoietic growth factors, chemokines, cytokines, morphogens, and adhesion molecules [4,5,6]. For example, osteoblast-derived factors such as angiopoietin-1 (ANGPT1) and thrombopoietin (TPO) guard HSPC quiescence by binding the respective cognate TIE2 and MPL receptors expressed on stem cells [7, 8]. Similarly, C-X-C Motif Chemokine Receptor 4 (CXCR4) signaling via contact with perivascular C-X-C motif chemokine 12 (CXCL12)-abundant reticular (CAR) cells is critical to HSPC pool maintenance and expansion [9].
Adding to the apparent complexity of the niche, differentiated progeny such as megakaryocytes and macrophages influence HSPC quiescence, proliferation, or migration. For example, megakaryocyte secretion of CXCL4 directly influences HSPC quiescence through promotion of adhesion to nearby stromal cells and interference with HSPC interleukin (IL)-8 signaling [10]. BM macrophages are also critical for production of HSPC-trophic cytokines and maintenance of HSPCs in the niche [11]. Similar crosstalk is likely to exist between stem cells and multipotent progenitors.
While the prevailing model of HSPCs as passive targets of exocrine signals has been thoroughly vetted, it is conceptually limiting. Evidence continues to accrue in support of a more active role for HSPCs in shaping niche function and maintaining their own multipotency and self-renewal capacities. These observations span both murine and human systems, where immunophenotypic and functional differences exist [12], and across heterogeneous subpopulations of HSPCs with divergent lineage commitment at various stages of maturity. In acknowledging the prevailing uncertainty regarding the immunophenotypic identification of long-term stem cells in human, and the fluid boundaries of lineage commitment in mice, we therefore rely on the “HSPC” designation throughout. Here we review the secreted signals including extracellular vesicles (EVs) employed by HSPCs to regulate their own function directly and indirectly through other cells in a dynamic and functionally adaptive microenvironment (Table 1). This proposed, more inclusive view of reciprocal HSPC-niche crosstalk readily accommodates seminal observations of physiologic cell-cell competition, niche-occupancy, and clonal extinction in addition to providing insight into leukemia cell signaling.
Autocrine secretory signals contribute to HSPC self-renewal and expansion
HSPCs have long been known to play active roles in self-maintenance through autocrine secretory activity. For example, highly enriched human CD34-positive BM-derived cells secrete self-renewal regulatory factors including Kit ligand, TPO, and ANGPT1 that bind to their respective KIT, MPL, and TIE2 receptors on HSPCs (Fig. 1) [13,14,15]. HSPC-derived insulin-like growth factor-1 (IGF-1) may also contribute to auto-protection from apoptotic cell death [14]. Human CD34-positive cells additionally express TNF receptors and CD95 (FAS) while secreting detectable levels of TNF-α and Fas ligand (Fas-L) [13, 14, 16]. Interestingly, soluble Fas-L may stimulate HSPC proliferation, while the relative insensitivity of human HSPCs to FAS-mediated apoptosis may in part be regulated by modulating expression of FLICE inhibitory protein (FLIP) [17]. Emerging evidence also implicates the possibility of autocrine secretion of ATP and its metabolite adenosine in regulating the mobilization of HSPCs through purinergic activation of the Nlrp3 inflammasome [18].
Numerous ligand and receptor interactions participate in driving HSPC behavior. (1) Self-signaling events occur in part via VPS33B-dependent extracellular vesicle (EV) secretion of factors including thrombopoietin (TPO), angiopoietin-like protein (ANGPTL)-2 and ANGPTL3. (2) Positive feedback loops enhance cooperative signals, such as TPO and hypoxia-inducible factor 1 alpha (HIF-1α)-driven transcription of vascular endothelial growth factor (VEGF; see main text). (3) Autocrine ligand-receptor signals converge on key common transduction pathways to regulate a balance between HSPC survival, quiescence, proliferation, and differentiation. (4) Key transcription factors additionally regulate cell autonomous responses. For example, binding of Kit ligand (KL) to its receptor, KIT, results in activation of the transcription factor SCL, which in turns upregulates KIT and enhances HSPC sensitivity to secreted KL. MVB multivesicular body, TNFα tumor necrosis factor alpha, TNFR TNF receptor, ANGPT1 angiopoietin-1, IGF-1 insulin-like growth factor-1, IGF-1R IGF-1 receptor, FLT3(-L) fms-related receptor tyrosine kinase 3 (ligand), VEGFR VEGF receptor, MAPK mitogen-activated protein kinase, PI3K phosphatidylinositol-3-kinase, mTOR mammalian target of rapamycin, JAK/STAT Janus kinase/signal transducers and activators of transcription, NF-κB Nuclear factor-κB.
In a mechanism distinct from its well described role in angiogenesis, vascular endothelial growth factor (VEGF) also promotes HSPC survival and repopulation via an internal autocrine loop (Fig. 1) [19]. Mechanistically unique, VEGF-induced HSPC renewal is not inhibited by extracellularly-acting inhibitors, but rather necessitates the interaction of an internally acting ligand with its receptors VEGFR1 and VEGFR2, possibly at an endosomal membrane. These signaling events may be further amplified by HSPC production of secreted TPO, which stimulates glycolysis through interaction with its receptor MPL on the cell surface, generating increased reactive oxygen species with subsequent activation of the VEGF transcription factor, hypoxia-inducible factor 1alpha (HIF-1α) (Fig. 1) [20]. In a similar pre-secretory mechanism, IL-3 may drive autocrine growth by interaction with its receptor in HSPCs prior to surface display [21].
Additional autocrine factors likely involved in various HSPC functions include hepatocyte growth factor (HGF), RANTES (CCL5), IL-12, granulocyte-macrophage colony-stimulating factor (GM-CSF), angiopoietin-2 (ANGPT2), IL-8, BMP6, SPP1, and TNFSF10 [22, 23]. Several non-overlapping discovery approaches including gene expression profiling and computational modeling suggest that more putative autocrine regulatory loops may exist. For example, HSPCs express dual receptor and ligand gene products, including FGF/FGFR, PDGF/PDGFR, IL-4/IL-4R, Wnt/Frz, Dll1/Notch1, and Jag2/Notch1 [24].
Autocrine TGF-β signals
As critical developmental morphogens and HSPC quiescence factors, secreted transforming growth factor (TGF)-β1 and TGF-β2 play pleiotropic roles in regulating HSPC proliferation. Several early studies suggest a role for TGF-β1 in negative regulation of cell cycling in HSPCs in vitro [25]. Experimental blockade with anti-TGF-β1 antibody significantly increased in vitro HSPC cycling and accelerated subsequent HSPC engraftment. In contrast, a biphasic dose response of TGF-β2 on HSPC proliferation may exist, such that low concentrations of TGF-β2 yield a stimulatory effect on cell expansion (perhaps mirroring in vivo phenomena) while higher concentrations are inhibitory [26]. Although not well understood, autocrine and paracrine signaling mechanisms possibly differ with respect to TGF-β functions [27]. For instance, in vivo HSPCs from conditional Tgf-β type I receptor gene knockout mice were found to exhibit normal cell cycling in the context of an otherwise intact BM niche, and retained a similar ability to repopulate recipient mice following BM transplantation [28]. Moreover, autocrine activity by distinct HSPC subtypes may respond differently to TGF-β, where myeloid-biased HSPCs undergo growth stimulation, yet lymphoid-biased HSPCs are growth inhibited [29]. Conceptually, dynamic receptor expression, alternative splice variants, surface shedding, degradation, and endosomal receptor reuptake provide a system for continuous tuning that can account for highly context dependent effects of secreted TGF-β on HSPCs, as evident in other tissues [30, 31].
Shared cellular signaling nodes
Downstream of canonical cell surface ligand-receptor binding, many autocrine events converge on shared core pathways including phosphatidylinositol-3-kinase (PI3K)/AKT, mammalian target of rapamycin (mTOR), mitogen-activated protein kinase (MAPK)/ERK, and Janus kinase/signal transducers and activators of transcription (JAK/STAT) to balance cell survival, renewal, and quiescence (Fig. 1). Several key transcription factors modulated by these transduction pathways regulate HSPC quiescence. For example, the proto-oncogene PBX1 positively regulates HSPC quiescence by sensitizing cells to TGF-β signaling [32]. Loss of transcription factors including GATA-2 and STAT5 downstream of ERK and JAK/STAT signaling respectively has been demonstrated to lead to decreased HSPC quiescence [33, 34]. SCL/TAL1, a pivotal hematopoietic transcriptional regulator, establishes a positive feedback loop with KIT to control quiescence in adult HSPCs (Fig. 1) [35]. In embryonic HSPCs, SCL is believed to alternatively contribute to cellular proliferation through induction of mTOR-driven protein synthesis [36]. Finally, activation of MYC downstream of ERK signaling coincides with downregulation of adhesion factors on HSPCs, enabling release of cells from their differentiation-suppressive niche [37].
Altogether, secretory activity is a fundamental HSPC property that enables critical autocrine signals to balance core functions, such as quiescence, proliferation, and differentiation in support of long-term pool integrity. HSPCs equipped for self-communication also allow for the possibility of neighbor crosstalk. In the context of bystander cells in the niche sharing similar ligand-receptor interactions as HSPCs, relative receptor affinity and surface density may become key mechanisms to segregate autocrine from density-dependent paracrine (quorum sensing) activities [38]. It is also widely recognized that epigenetic mechanisms regulate fundamental HSPC properties, but how self-signals are integrated with both epigenetic programs and extrinsic signals to sustain regenerative potency and self-renewal of individual clones remains unknown [39, 40].
HSPC signals directly shape the function of the hematopoietic microenvironment
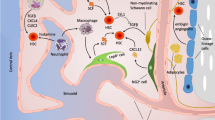
Given the close spatial proximity, it is not surprising that HSPC signals regulate non-hematopoietic, heterotypic cells in the niche via paracrine mechanisms (Fig. 2). Evidence for a close interdependence may be strongest between HSPCs and osteoblasts. Several expressed or secreted factors from osteoblasts impact HSPC function including the Notch ligand Jagged 1 and IL-6 [41]. Interestingly, co-culture with CD34-positive BM cells in turn increases IL-6 and leukemia inhibitory factor synthesis in osteoblasts, but without a requirement for cell-cell contact [42]. Recently, an additional contact-dependent transfer of small membrane microdomains from HSPCs to osteoblasts was also described [43]. Enriched in CD63 and CD133, these membrane microdomains are internalized into osteoblast signaling endosomes, driving downregulation of Smad signaling, and thereby augmenting osteoblast production of CXCL12, a chemokine essential in HSPC migration and quiescence. Further promoting interdependence, HSPCs direct mesenchymal stem cell differentiation toward the osteoblastic lineage by bone morphogenic proteins BMP-2 and BMP-6 secretion, a phenomenon that is enhanced under BM stress [44]. Hematopoietic-derived osteoclasts may additionally influence HSPC mobilization and niche maintenance, though the exact mechanistic impact is uncertain [45, 46]. In part, secretion of macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa-Β ligand (RANKL) by osteoblasts and other BM stromal cells promotes differentiation of osteoclasts from hematopoietic precursors, thereby contributing to osteoclast-driven niche remodeling. Altogether, reciprocal communication between these cells reinforces the close relationship between hematopoiesis and bone turnover.
While numerous studies have focused on the impact of bone marrow (BM) niche cells on HSPC function, little is known about the reciprocol activities driven by HSPCs in modulating a permissive microenvironment. It is clear that HSPCs trigger endothelial remodeling and proliferation through secreted factors such as vascular endothelial growth factor (VEGF) and angiopoietin-1 (ANGPT1). Under stress, ANGPT1 is required to regenerate intact BM vasculature. Bone turnover and hematopoiesis are also closely linked. Through unknown secreted factors, HSPCs drive osteoblast secretion of IL-6, which among many functions, promotes osteoclastogenesis from precursor hematopoietic cells and subsequent bone resorption. Physiologic bone turnover is critical for HSPC engraftment, proliferation, differentiation, and mobilization. To a lesser extent, HSPCs also stimulate osteoblast secretion of leukemia inhibitory factor (LIF), thereby participating in the regulation of HSPC differentiation. HSPC-derived bone morphogenic proteins (BMP-2 and BMP-6) promote mesenchymal stem cell (MSC) differentiation into mature osteoblasts, forming a positive paracrine loop between HSPC and osteoblast development and activation. Finally, HSPCs are in direct contact with stromal cells, including CXCL12-abundant reticular cells (CARs), which may be important for cell orientation during HSPC division. Transfer of reactive oxygen species (ROS) to stromal cells through connexin-43 (Cx43)-dependent gap junctions (dotted lines) promotes HSPC survival and self-renewal.
HSPCs and mesenchymal cells also communicate directly via gap junctions [47]. Under stress, HSPC-derived connexin-43, a major constituent of gap junctions, supports the proliferative rebound following injury of HSPCs by facilitating extrusion and transfer of potentially toxic reactive oxygen species to neighboring stromal cells, thereby minimizing apoptosis or induced senescence [48].
HSPC secreted factors are also important for angiogenesis and vascular remodeling of the metabolically unique BM microenvironment. In addition to its autocrine role discussed above, HSPC secretion of ANGPT1 signals to TIE2-expressing endothelial cells [49]. Following irradiation, in vivo knockout of ANGPT1 in HSPCs and LEPR-positive stromal cells results in vascular leakiness, BM hypercellularity, and HSPC expansion [49]. In this way, HSPC-derived ANGPT1 serves to preserve stemness and an intact vasculature under stress. A similar role in remodeling the vascular niche following inflammatory stress extends to VEGF secreted by HSPCs [50]. Mice treated with IFN-α or an interferon mimetic experience a systemic inflammatory response with transient increases in endothelial cell proliferation and BM vasculature. These events are fueled by increased HSPC VEGF production and endothelial expression of the corresponding receptor VEGFR [50].
The bidirectional relationship between HSPCs and cellular components of the niche is not limited to the BM microenvironment. During development, definitive HSPCs appear to trigger changes in the nearby perivascular fetal niche. Migration of RUNX1-positive HSPCs into intermediate hematopoietic niches (zebrafish caudal hematopoietic tissue and murine fetal liver) has illustrated the endothelial and stromal remodeling that occurs with HSPC colonization [51]. Following extravasation into the niche, HSPCs trigger the formation of a multicellular sinusoidal pocket, in a process dubbed “endothelial cuddling”, which facilitates proper HSPC cell division. Direct HSPC contact with CXCL12-positive stromal cells may be required to orient HSPCs for this process [51]. Altogether, these findings reinforce a model of the HSPC niche during development and postnatal life as a highly adaptable operational unit built around bidirectional crosstalk.
HSPC signals facilitate cell-cell competition within the bone marrow microenvironment
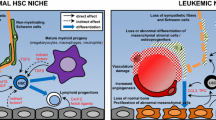
Beyond the reviewed evidence for autocrine and heterotypic signaling, there is emerging evidence for HSPC signaling as a mechanism underlying competition between hematopoietic cells for occupancy of the BM niche. Conceptually elegant studies of the Drosophila imaginal disc first shed light on non-cell autonomous events whereby cells that differ in their respective levels of fitness can confer neighbor elimination or force differentiation. By nature, the impact of differential cellular fitness requires the capacity of cells to sense and signal to neighboring cells, using many of the molecular mechanisms discussed above [52,53,54]. The possibility that cell competition between HSPCs in the BM exists has been experimentally demonstrated (Fig. 3) [52, 55].
Schematics of several proposed mechanisms of cell-cell competition in the bone marrow (BM) microenironment. a HSPC subpopulations compete based on relative p53 levels. HSPCs harboring higher levels of p53 induced by DNA damage (e.g., irradiation) are driven toward senescent-like phenotypes, and are outcompeted by HSPCs expressing relatively lower p53 levels. b In some situations, senescent cells in the BM induce paracrine senescence, at least in part by transfer of biologically active extracellular vesicles (EVs), allowing non-recipient HSPCs to outcompete senescent cells. c In the context of malignant leukemia cells, relative proportions of normal HSPCs and leukemic stem cells (LSCs) dictate the ultimate “winners” and “losers.” d Leukemia cells may also induce senescence in neighboring cells, including HSPCs and stromal cells, that in turn produce pro-mitogenic senescence-associated secretory phenotype (SASP) factors promoting leukemia cell growth.
p53-mediated cell competition
A landmark study showed that DNA damage following ionizing irradiation triggered HSPC competition and selective expansion of populations with relatively lower p53 levels [55]. Cells experiencing higher p53 levels as a result of experimental DNA damage underwent senescence-like changes, leaving them marked for gradual replacement by more highly proliferative cells [56]. In this experimental system, the mechanism of cell competition was manifested by selective growth and was based on relative differences between competitors, often revealed under stress, not homeostatic conditions [55, 57]. Interestingly, such differential p53-mediated fitness phenocopies events in models of the human BM disorder Diamond-Blackfan anemia (DBA) [58]. In DBA, increased nucleolar stress from disruption of ribosomal biogenesis can lead to an overall imbalance and relative excess of proteins RPL-5 and −11 that bind to MDM2. The resulting decrease in availability of MDM2 in turn slows p53 turnover, and promotes differential fitness.
Senescent cell contribution to competition
To communicate cellular fitness and impact competitor fates, the secretome of senescent cells encompasses a broad array of chemokines, cytokines, growth factors, and proteases, referred to in aggregate as senescence-associated secretory phenotype (SASP) [59, 60]. Individually and in combination, SASP factors stimulate the growth of neighboring cells, thereby promoting physiologic tissue regeneration. For example, one report showed that the trafficking of the JAK/STAT ligand, Unpaired-3, from senescent cells caused the expansion of more fit neighboring cells [61]. Conceivably, such pro-mitogenic paracrine stimulation may be important in the gradual replacement of senescent, damaged HSPCs by stem cells emerging from quiescence following injury. It is worth noting that SASP may, in some cases, alternatively promote the paracrine spread of a senescent bystander response to other HSPCs via secreted factors such as TGF-β family members, VEGF, CCL2, or CCL20 [62]. Finally, relative expression of adhesion molecules may provide another mechanism by which HSPCs contend for physical niche space, whereby higher levels of ROBO4, CXCR4, and integrin α4β7 offer a competitive advantage in homing, engraftment, and retention over cells expressing lower levels [63, 64]. Teleologically, HSPC signaling underlying cell competition provides a means of maximizing nutrient utilization and space, elimination of compromised cells, and potentially tissue-level tumor suppression during development and adult life.
Cell competition in hematopoietic malignancies
Not surprisingly, competitive cell interactions feature prominently in the context of hematopoietic malignancy where HSPCs and leukemia cells compete for the same physical niche in the BM. Seminal studies by several groups have recently upended the notion that physical overcrowding alone drives leukemic progression and hematopoietic suppression in the BM niche. Instead, niche occupancy depends in part on the proportional balance between healthy and malignant components, and direct crosstalk between the two populations contributes to a cellular tug of war in the niche [65,66,67]. Under dose escalation, healthy HSPCs can not only outcompete leukemic stem cell (LSC) growth in the niche and improve survival in animal models of AML, but alter the proliferative capacity of malignant bystander cells, consistent with the possibility of cell-cell signaling between the two populations [65, 68]. Conversely, when LSCs outcompete normal HSPCs for niche space, they show gains in chemoresistance compared to circulating leukemia cells, that potentially reflect the adhesive, immunosuppressive, and mitogenic properties acquired during cell-cell interactions in the BM niche [69].
Cell-cell competition may well reinforce the impact of genetic alterations in leukemia cells, where the loss of p53 tumor suppressor function could lead to the relative advantage over normal hematopoietic cells. Mechanistically, overexpression of the human proto-oncogene MYC, often activated in hematologic malignancies, may give rise to super-competitive leukemia cells [70]. Along with observations that AML cells can induce senescence in surrounding cells through secreted SASP factors [71], the crosstalk between malignant and non-malignant hematopoietic cells in the leukemic BM fits models and mechanisms of cell-cell competition, even as experimental confirmation in human is still lacking.
Extracellular vesicles modulate HSPC interactions in the niche
Leukemia-derived vesicles
One mode of crosstalk between leukemic cells and residual normal HSPCs is the cell-cell trafficking of EVs. Encompassing both secreted endosomal-derived exosomes and limiting cell membrane-derived microvesicles, EVs carry DNA, RNA, and protein cargo that can durably alter recipient cell behavior. Our group showed that selective microRNA (miRNA) species are highly abundant in EV populations from AML cells in culture, and in circulating vesicles from human patients or animals harboring AML xenografts [72,73,74]. Specifically, we showed that leukemia-derived EVs deliver select miRNAs, including miR-1246 and −1290, to HSPCs, leading to RAPTOR-mediated suppression of mTOR signaling and decreased protein synthesis while raising p53 levels, all core pathways operative in cell-cell competition [72, 75]. These findings support observations made by others that residual normal HSPCs in AML xenograft models are predominantly quiescent and generally more resistant to elimination [76]. Intriguingly, progenitor cells which are much less vulnerable to disruption of proteostasis, lose clonogenicity through RISC-mediated translational suppression of the transcription factor c-Myb by EV-contained miR-150 and −155 [74].
AML-derived EVs serve a broad array of proangiogenic, tumor-promoting, and hematopoietic-suppressive functions, and may be particularly important in maintaining LSC phenotype (Fig. 4) [72, 77]. For instance, impaired vacuolar protein sorting-associated protein 33B (VPS33B)-dependent vesicle secretion leads to loss of LSC quiescence, increased apoptosis, delayed onset of leukemogenesis, and increased survival in a murine MLL-AF9–transduced AML model [78]. Similar to its role in normal HSPCs, miR-126 promotes LSC quiescence and self-renewal through its modulation of PI3K/AKT/mTOR, thereby promoting chemotherapy resistance [79]. Given the role of EV-associated miR-126 in promoting angiogenesis, it is conceivable that LSC-derived EVs confer leukemia-supportive effects in the BM niche [80, 81]. Secretion of senescence-inducing EVs from LSCs may further suppress normal hematopoiesis in the niche, contributing to cell competition in favor of malignant and pre-malignant clones.
Interplay between autophagic activity, the endosomal sorting pathway, and membrane budding results in secreted extracellular vesicles (EVs) including multivesicular body (MVB)-derived exosomes and membrane-shed microvesicles. HSPC EVs rely in part on VPS33B-mediated secretion, and contain protein, lipid, and nucleic acid (DNA, mRNA, miRNA) cargo that exert both autocrine and paracrine effects in the niche. Stemness and growth factors associated with EVs likely provide self signals regulating HSPC renewal, quiescence, differentiation, and elimination of toxic intracellular material. Secreted EVs may also mediate cell-cell competition events in the niche by inducing senescence in neighboring HSPCs with lower fitness capacity. Endothelial cell uptake of HSPC EVs results in activation, chemoattraction, and angiognesis. While the imapct of HSPC EVs on leukemic cells remains unknown, leukemia-derived EVs can induce hematopoietic suppression, in part by transfer of small noncoding RNAs such as miR-1246 to HSPCs, resulting in reduced protein synthesis and HSPC quiescence. TPO thrombopoietin, ANGPTLs angiopoietin-like proteins, ANGPT1 angiopoietin-1, TGF-β transforming growth factor β, IFITM3 interferon induced transmembrane protein 3, VEGF vascular endothelial growth factor, KL Kit ligand, IGF-1 insulin-like growth factor 1, bFGF basic fibroblast growth factor, VPS33B vacuolar protein sorting-associated protein 33B, LSC leukemic stem cell.
EVs in physiologic hematopoiesis
An extensive review of EV biogenesis is beyond the scope of this report, but a number of studies speak to a much broader role vesicle trafficking plays in regulating physiologic cell-cell communication in the BM (Fig. 4) [82]. For example, mesenchymal cell- or platelet- derived vesicles containing adhesion and anti-apoptotic factors affect HSPC homing and survival [83,84,85]. However, little is known about the contribution of HSPC-derived vesicles to the function of the BM microenvironment. HSPC autocrine stemness factors including TPO and angiopoietin-like proteins (ANGPTLs) are actively recruited into VPS33B-dependent small EVs that maintain quiescence, survival, and repopulation following transplantation [78]. Other HSPC-stimulating ligands such as FLT3 ligand may also be present in membrane-bound form with functional extracellular domains [86], and conceivably secreted in association with membrane-encapsulated EVs. To date, ANGPT1, CXCR4, TGF-β, and numerous interleukin and leukocyte-specific integrin proteins have been identified in secreted vesicles [87]. The stem cell marker CD133 is also readily packaged and secreted in EVs [88], a process which aids in depletion of cellular CD133 levels and promotes HSPC differentiation. The importance of vesicle trafficking proteins (Nbea, Cadps2, and Gprasp2) for efficient hematopoietic engraftment and repopulation was recently reinforced in an in vivo shRNA screen targeting genes highly expressed in stem and progenitor cells [89].
As mentioned above, given their deep regulatory potential and frequent EV enrichment, small, non-coding RNAs have been of obvious interest to the field. During development, HSPC-derived EVs promote differentiation of embryonic stem cells into HSPCs in part by transfer of miR-126 that results in Notch inhibition [90]. In adult hematopoiesis, miR-126 appears to instead promote HSPC quiescence by limiting cell cycle progression through reduction of PI3K/AKT/mTOR activity [79, 91]. However, accumulation of miR-126-containing EVs in the BM in response to granulocyte colony-stimulating factor may drive mobilization and subsequent differentiation of HSPCs through downregulation of vascular cell adhesion molecule 1 expression on recipient HPSCs [92].
EVs secreted from HSPCs may also participate in heterotypic cell-to-cell communication in the HSPC niche. For instance, VEGF, KL, IGF-1, IL-8, and bFGF mRNAs in addition to miR-126 are enriched in small vesicles secreted from HSPCs and promote endothelial cell chemoattraction and angiogenic activity [80, 81, 93]. Packaging of other RNAs with known hematopoietic regulatory functions into EVs for cell transmission remain to be discovered.
EVs likely also play an important role in externalizing cellular content in order to retain stemness and maintain cellular integrity. For example, in conditions of heightened nuclear DNA damage, cells increase EV secretion. Inhibition of vesicle release leads to accumulation of cytoplasmic DNA and senescence or apoptosis [94]. Given the need for HSPCs to minimize genotoxic stress, EV elimination of harmful cytoplasmic DNA fragments is an attractive mechanism to guard HSPC pool integrity [95]. More broadly, EVs are critical for eliminating undesirable or toxic cell material from cells. Like autophagy and its role in HSPC self-renewal and maintenance, EV biogenesis and secretion may constitute an important mechanism of HSPC quality control [96, 97]. For instance, elimination of mitochondria in HSPCs is required to reduce reactive oxygen species and maintain quiescence; failure to do so results in cell cycle entry and differentiation [96]. Recent studies have demonstrated that mitochondrial material, including whole organelles, may be packaged into EVs for horizontal transfer. Mesenchymal stem cells in particular manage intracellular stress by packaging mitochondria into secreted microvesicles targeted to macrophages, thereby outsourcing mitophagy or mitochondrial recycling [98]. Additional investigation is needed to uncover whether analogous mechanisms are employed by HSPCs.
Recent evidence in other cellular models also suggests that EVs may serve as paracrine transmitters of a senescence phenotype in bystander cells through increasing expression of IL-8, integrin β3, and cell-cycle regulators CDKN2A and CDKN1A by transfer of interferon-induced transmembrane 3 (IFITM3) [99]. Proteomic profiling of senescent cell-derived EVs shows little overlap between known soluble factors secreted from cells, suggesting an EV- directed mechanism distinct from SASP that may be similarly employed by HSPCs.
Perspective and future directions
While most attention has focused on HSPCs as epigenetically programmed units, it is clear that the cell-autonomous activity of HSPCs does not stop at the cell membrane, but also involves secretory pathways to self-communicate and actively remodel the microenvironment. These cellular activities are bound to impact self-renewal, differentiation, and clonal expansion under homeostatic conditions, and in the context of aging, stress, and malignancy. Importantly, emerging differences between heterogeneous populations of hematopoietic stem and multipotent progenitor cells have recently challenged the traditional perspective of compartmentalized, hierarchal hematopoietic development [1]. Variance in self-renewal capacity, differential mature lineage output, and ability to secrete and respond to cytokine stimuli may explain how distinct HSPC subsets propagate clonal trajectories during serial transplantation [1, 100]. Given the emerging evidence of early lineage demarcation and shared cellular transcriptional networks at various stages of HSPC development, categorical distinctions between stem and progenitor cell populations may be blurred. HSPC subpopulations also likely engage in direct crosstalk as they orchestrate lineage-commitment and maturation, though subset-specific communication remains to be determined.
The available evidence suggests that a better understanding of HSPC cell interaction and the mechanisms underlying cell-cell competition in the niche may have broad implications in advancing knowledge of age-associated changes in immune function, referred to as senescent immune remodeling (SIR) [101]. Interestingly as mice and humans age, a bias of HSPC differentiation toward the myeloid lineage and a relative reduction in common lymphoid progenitor cell differentiation is generally apparent [102]. An overall reduction in the size of the HSPC pool is concomitantly noted [103]. In part, we hypothesize that SIR may rely upon cell-cell competition amongst HSPCs harboring intrinsic myeloid or lymphoid bias, such that populations of disadvantaged cells make room for, and even support, the growth of other HSPCs. The resulting restriction in clonal diversity of HSPCs may in turn drive clonal hematopoiesis of indeterminate potential and potential pre-malignant states [104]. Accordingly, HSPC signals may also play a role in the remarkable stability of epigenetically programmed clonal trajectories that transfer fundamental traits such as regenerative capacity and time to exhaustion in models of murine serial recipient transplantation [40, 105].
It is conceivable that harnessing the mechanisms and mediators underlying HSPC-niche crosstalk could be exploited for therapeutic gain. Further insight into autocrine and paracrine signals employed by HSPCs in processes of self-renewal and divisional symmetry could facilitate improvements in laboratory HSPC expansion, perhaps through manipulation of important regulators of repopulation activity. Beyond the research implications, ex vivo HSPC expansion could be used to overcome the numerical limitations in allogeneic transplant grafts or autologous gene therapy products.
Finally, the roles of EVs in mediating BM cell-cell interactions is an emerging topic of great interest. Selective manipulation of HSPC- or LSC-derived EV cargo secretion may confer potential therapeutic strategies for de novo hematologic malignancies or relapse conditions. It is also increasingly recognized that suppression of normal hematopoiesis by leukemic cells involves secretion of soluble or vesicle-associated modulatory factors. Adjuvant treatments that improve the resilience of HSPCs and contribute to resetting physiologic conditions in a leukemic niche have the potential to profoundly impact outcomes of leukemia treatment. Future studies further elucidating the active roles HSPCs employ in shaping their BM niche will certainly advance hematopoiesis and stem cell research and inform therapeutic strategies.
References
Laurenti E, Göttgens B. From haematopoietic stem cells to complex differentiation landscapes. Nature. 2018;553:418–26.
Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci USA. 1999;96:3120–5.
Beerman I, Seita J, Inlay MA, Weissman IL, Rossi DJ. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell. 2014;15:37–50. 2014/05/08 ed.
Frisch BJ. The hematopoietic stem cell niche: what’s so special about bone? Bone. 2018/05/17 ed. 2019;119:8–12.
Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–64.
Yu VW, Scadden DT. Hematopoietic Stem Cell and Its Bone Marrow Niche. Curr Top Dev Biol. 2016;118:21–44. 2016/03/21 ed.
Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–61.
Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685–97. 2007/11/20 ed.
Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–88.
Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y, et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med. 2014;20:1315–20. 2014/10/19 ed.
Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–28.
Parekh C, Crooks GM. Critical differences in hematopoiesis and lymphoid development between humans and mice. J Clin Immunol. 2013;33:711–5. 2012/12/30 ed.
Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, Kowalska MA, et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 2001;97:3075–85.
Janowska-Wieczorek A, Majka M, Ratajczak J, Ratajczak MZ. Autocrine/paracrine mechanisms in human hematopoiesis. Stem Cells. 2001;19:99–107.
Ratajczak MZ, Kuczynski WI, Sokol DL, Moore JS, Pletcher CH, Gewirtz AM. Expression and physiologic significance of Kit ligand and stem cell tyrosine kinase-1 receptor ligand in normal human CD34+, c-Kit+ marrow cells. Blood. 1995;86:2161–7.
Behringer D, Kresin V, Henschler R, Mertelsmann R, Lindemann A. Cytokine and chemokine production by CD34+ haemopoietic progenitor cells: detection in single cells. Br J Haematol. 1997;97:9–14.
Kim H, Whartenby KA, Georgantas RW, Wingard J, Civin CI. Human CD34+ hematopoietic stem/progenitor cells express high levels of FLIP and are resistant to Fas-mediated apoptosis. Stem Cells. 2002;20:174–82.
Ratajczak MZ, Bujko K, Cymer M, Thapa A, Adamiak M, Ratajczak J, et al. The Nlrp3 inflammasome as a “rising star” in studies of normal and malignant hematopoiesis. Leukemia. 2020;34:1512–23.
Gerber H-P, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–8.
Kirito K, Fox N, Komatsu N, Kaushansky K. Thrombopoietin enhances expression of vascular endothelial growth factor (VEGF) in primitive hematopoietic cells through induction of HIF-1alpha. Blood. 2005;105:4258–63. 2005/02/10 ed.
Browder TM, Abrams JS, Wong PM, Nienhuis AW. Mechanism of autocrine stimulation in hematopoietic cells producing interleukin-3 after retrovirus-mediated gene transfer. Mol Cell Biol. 1989;9:204–13.
Müller E, Wang W, Qiao W, Bornhäuser M, Zandstra PW, Werner C, et al. Distinguishing autocrine and paracrine signals in hematopoietic stem cell culture using a biofunctional microcavity platform. Sci Rep. 2016/08/18 ed. 2016;6:31951.
Qiao W, Wang W, Laurenti E, Turinsky AL, Wodak SJ, Bader GD, et al. Intercellular network structure and regulatory motifs in the human hematopoietic system. Mol Syst Biol. 2014;10:741. 2014/07/15 ed.
Han W, Yu Y, Liu XY. Local signals in stem cell-based bone marrow regeneration. Cell Res. 2006;16:189–95.
Sitnicka E, Ruscetti FW, Priestley GV, Wolf NS, Bartelmez SH. Transforming growth factor beta 1 directly and reversibly inhibits the initial cell divisions of long-term repopulating hematopoietic stem cells. Blood. 1996;88:82–8.
Langer JC, Henckaerts E, Orenstein J, Snoeck HW. Quantitative trait analysis reveals transforming growth factor-beta2 as a positive regulator of early hematopoietic progenitor and stem cell function. J Exp Med. 2004;199:5–14.
Ruscetti FW, Akel S, Bartelmez SH. Autocrine transforming growth factor- β regulation of hematopoiesis: many outcomes that depend on the context. Oncogene. 2005;24:5751–63.
Larsson J, Blank U, Helgadottir H, Björnsson JM, Ehinger M, Goumans MJ, et al. TGF-beta signaling-deficient hematopoietic stem cells have normal self-renewal and regenerative ability in vivo despite increased proliferative capacity in vitro. Blood. 2003;102:3129–35. 2003/07/03 ed.
Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6:265–78.
Konrad L, Scheiber JA, Völck-Badouin E, Keilani MM, Laible L, Brandt H, et al. Alternative splicing of TGF-betas and their high-affinity receptors T beta RI, T beta RII and T beta RIII (betaglycan) reveal new variants in human prostatic cells. BMC Genom. 2007;8:318. 2007/09/11 ed.
Lemischka IR, Pritsker M. Alternative splicing increases complexity of stem cell transcriptome. Cell Cycle. 2006;5:347–51. 2006/02/15 ed.
Ficara F, Murphy MJ, Lin M, Cleary ML. Pbx1 regulates self-renewal of long-term hematopoietic stem cells by maintaining their quiescence. Cell Stem Cell. 2008;2:484–96.
Tipping AJ, Pina C, Castor A, Hong D, Rodrigues NP, Lazzari L, et al. High GATA-2 expression inhibits human hematopoietic stem and progenitor cell function by effects on cell cycle. Blood. 2009;113:2661–72. 2009/01/23 ed.
Li J. Quiescence regulators for hematopoietic stem cell. Exp Hematol. 2011;39:511–20. 2011/02/01 ed.
Lacombe J, Krosl G, Tremblay M, Gerby B, Martin R, Aplan PD, et al. Genetic interaction between Kit and Scl. Blood. 2013;122:1150–61. 2013/07/08 ed.
Benyoucef A, Calvo J, Renou L, Arcangeli ML, van den Heuvel A, Amsellem S, et al. The SCL/TAL1 Transcription Factor Represses the Stress Protein DDiT4/REDD1 in Human Hematopoietic Stem/Progenitor Cells. Stem Cells. 2015;33:2268–79. 2015/05/25 ed.
Wilson A, Murphy MJ, Oskarsson T, Kaloulis K, Bettess MD, Oser GM, et al. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004;18:2747–63.
Doğaner BA, Yan LKQ, Youk H. Autocrine signaling and quorum sensing: extreme ends of a common spectrum. Trends Cell Biol. 2016;26:262–71. 2015/12/05 ed.
Cabezas-Wallscheid N, Klimmeck D, Hansson J, Lipka DB, Reyes A, Wang Q, et al. Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis. Cell Stem Cell. 2014;15:507–22. 2014/08/21 ed.
Yu VWC, Yusuf RZ, Oki T, Wu J, Saez B, Wang X, et al. Epigenetic Memory Underlies Cell-Autonomous Heterogeneous Behavior of Hematopoietic Stem Cells. Cell. 2016;167:1310–.e17.
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–6.
Taichman RS, Reilly MJ, Verma RS, Emerson SG. Augmented production of interleukin-6 by normal human osteoblasts in response to CD34+ hematopoietic bone marrow cells in vitro. Blood. 1997;89:1165–72.
Gillette JM, Larochelle A, Dunbar CE, Lippincott-Schwartz J. Intercellular transfer to signalling endosomes regulates an ex vivo bone marrow niche. Nat Cell Biol. 2009;11:303–11.
Jung Y, Song J, Shiozawa Y, Wang J, Wang Z, Williams B, et al. Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche. Stem Cells. 2008;26:2042–51.
Lymperi S, Ersek A, Ferraro F, Dazzi F, Horwood NJ. Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood. 2011;117:1540–9.
Miyamoto K, Yoshida S, Kawasumi M, Hashimoto K, Kimura T, Sato Y, et al. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J Exp Med. 2011;208:2175–81.
Dürig J, Rosenthal C, Halfmeyer K, Wiemann M, Novotny J, Bingmann D, et al. Intercellular communication between bone marrow stromal cells and CD34+ haematopoietic progenitor cells is mediated by connexin 43-type gap junctions. Br J Haematol. 2000;111:416–25.
Taniguchi Ishikawa E, Gonzalez-Nieto D, Ghiaur G, Dunn SK, Ficker AM, Murali B, et al. Connexin-43 prevents hematopoietic stem cell senescence through transfer of reactive oxygen species to bone marrow stromal cells. Proc Natl Acad Sci USA. 2012;109:9071–6. 2012/05/18 ed.
Zhou BO, Ding L, Morrison SJ. Hematopoietic stem and progenitor cells regulate the regeneration of their niche by secreting Angiopoietin-1. Elife. 2015;4:e05521. 2015/03/30 ed.
Prendergast ÁM, Kuck A, van Essen M, Haas S, Blaszkiewicz S, Essers MAG. IFNα-mediated remodeling of endothelial cells in the bone marrow niche. Haematologica. 2017;102:445–53.
Tamplin OJ, Durand EM, Carr LA, Childs SJ, Hagedorn EJ, Li P, et al. Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell. 2015;160:241–52.
Amoyel M, Bach EA. Cell competition: how to eliminate your neighbours. Development. 2014;141:988–1000.
Ellis SJ, Gomez NC, Levorse J, Mertz AF, Ge Y, Fuchs E. Distinct modes of cell competition shape mammalian tissue morphogenesis. Nature. 2019;569:497–502.
Morata G, Ripoll P. Minutes: mutants of drosophila autonomously affecting cell division rate. Dev Biol. 1975;42:211–21.
Bondar T, Medzhitov R. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell. 2010;6:309–22.
Zhang G, Xie Y, Zhou Y, Xiang C, Chen L, Zhang C, et al. p53 pathway is involved in cell competition during mouse embryogenesis. Proc Natl Acad Sci USA. 2017;114:498–503. 2017/01/03 ed.
Marusyk A, Porter CC, Zaberezhnyy V, DeGregori J. Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol. 2010;8:e1000324.
Baker NE, Kiparaki M, Khan C. A potential link between p53, cell competition and ribosomopathy in mammals and in Drosophila. Dev Biol. 2019;446:17–9. 2018/12/02 ed.
Neves J, Demaria M, Campisi J, Jasper H. Of flies, mice, and men: evolutionarily conserved tissue damage responses and aging. Dev Cell. 2015;32:9–18.
Ito T, Igaki T. Dissecting cellular senescence and SASP in Drosophila. Inflamm Regen. 2016;36:25.
Kolahgar G, Suijkerbuijk SJ, Kucinski I, Poirier EZ, Mansour S, Simons BD, et al. Cell Competition Modifies Adult Stem Cell and Tissue Population Dynamics in a JAK-STAT-Dependent Manner. Dev Cell. 2015;34:297–309. 2015/07/23 ed.
Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15:978–90.
Murakami JL, Xu B, Franco CB, Hu X, Galli SJ, Weissman IL, et al. Evidence that β7 Integrin Regulates Hematopoietic Stem Cell Homing and Engraftment Through Interaction with MAdCAM-1. Stem Cells Dev. 2016;25:18–26. 2015/11/05 ed.
Smith-Berdan S, Nguyen A, Hassanein D, Zimmer M, Ugarte F, Ciriza J, et al. Robo4 Cooperates with Cxcr4 to Specify Hematopoietic Stem Cell Localization to Bone Marrow Niches. Cell Stem Cell. 2011;8:72–83.
Boyd AL, Campbell CJV, Hopkins CI, Fiebig-Comyn A, Russell J, Ulemek J, et al. Niche displacement of human leukemic stem cells uniquely allows their competitive replacement with healthy HSPCs. J Exp Med. 2014;211:1925–35.
Boyd AL, Reid JC, Salci KR, Aslostovar L, Benoit YD, Shapovalova Z, et al. Acute myeloid leukaemia disrupts endogenous myelo-erythropoiesis by compromising the adipocyte bone marrow niche. Nat Cell Biol. 2017;19:1336–47. 2017/10/16 ed.
Wagstaff L, Kolahgar G, Piddini E. Competitive cell interactions in cancer: a cellular tug of war. Trends Cell Biol. 2013;23:160–7. 2012/12/04 ed.
Glait-Santar C, Desmond R, Feng X, Bat T, Chen J, Heuston E, et al. Functional Niche Competition Between Normal Hematopoietic Stem and Progenitor Cells and Myeloid Leukemia Cells. Stem Cells. 2015;33:3635–42.
Tabe Y, Konopleva M. Leukemia Stem Cells Microenvironment. Adv Exp Med Biol. 2017;1041:19–32.
Moreno E, Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117:117–29.
Abdul-Aziz AM, Sun Y, Hellmich C, Marlein CR, Mistry J, Forde E, et al. Acute myeloid leukemia induces protumoral p16INK4a-driven senescence in the bone marrow microenvironment. Blood. 2019;133:446–56. 2018/11/06 ed.
Abdelhamed S, Butler JT, Doron B, Halse A, Nemecek E, Wilmarth PA, et al. Extracellular vesicles impose quiescence on residual hematopoietic stem cells in the leukemic niche. EMBO Rep [Internet]. 2019;20. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6607014/.
Caivano A, La Rocca F, Simeon V, Girasole M, Dinarelli S, Laurenzana I, et al. MicroRNA-155 in serum-derived extracellular vesicles as a potential biomarker for hematologic malignancies - a short report. Cell Oncol (Dordr). 2017;40:97–103. 2016/10/19 ed.
Hornick NI, Doron B, Abdelhamed S, Huan J, Harrington CA, Shen R, et al. AML suppresses hematopoiesis by releasing exosomes that contain microRNAs targeting c-MYB. Sci Signal. 2016/09/06 ed. 2016;9:ra88.
Waclawiczek A, Hamilton A, Rouault-Pierre K, Abarrategi A, Albornoz MG, Miraki-Moud F, et al. Mesenchymal niche remodeling impairs hematopoiesis via stanniocalcin 1 in acute myeloid leukemia. J Clin Investig. [Internet]. 2020/05/04 ed. 2020. https://www.ncbi.nlm.nih.gov/pubmed/32364536.
Akinduro O, Weber TS, Ang H, Haltalli MLR, Ruivo N, Duarte D, et al. Proliferation dynamics of acute myeloid leukaemia and haematopoietic progenitors competing for bone marrow space. Nat Commun. 2;9:519. 2018/02/06 ed.
Pando A, Reagan JL, Quesenberry P. Fast LD Extracellular vesicles in leukemia. Leuk Res. 2018;64:52–60. 2017/11/22 ed.
Gu H, Chen C, Hao X, Wang C, Zhang X, Li Z, et al. Sorting protein VPS33B regulates exosomal autocrine signaling to mediate hematopoiesis and leukemogenesis. J Clin Investig. 2016;126:4537–53.
Lechman ER, Gentner B, Ng SWK, Schoof EM, van Galen P, Kennedy JA, et al. miR-126 Regulates Distinct Self-Renewal Outcomes in Normal and Malignant Hematopoietic Stem Cells. Cancer Cell. 2016;29:602–6.
Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724–8. 2011/08/11 ed.
Mathiyalagan P, Liang Y, Kim D, Misener S, Thorne T, Kamide CE, et al. Angiogenic Mechanisms of Human CD34. Circ Res. 2017;120:1466–76. 2017/03/15 ed.
Butler JT, Abdelhamed S, Kurre P. Extracellular vesicles in the hematopoietic microenvironment. Haematologica. 2018;103:382–94.
Stik G, Crequit S, Petit L, Durant J, Charbord P, Jaffredo T, et al. Extracellular vesicles of stromal origin target and support hematopoietic stem and progenitor cells. J Cell Biol. 2017/06/19 ed. 2017;216:2217–30.
De Luca L, Trino S, Laurenzana I, Simeon V, Calice G, Raimondo S, et al. MiRNAs and piRNAs from bone marrow mesenchymal stem cell extracellular vesicles induce cell survival and inhibit cell differentiation of cord blood hematopoietic stem cells: a new insight in transplantation. Oncotarget. 2016;7:6676–92.
Janowska-Wieczorek A, Majka M, Kijowski J, Baj-Krzyworzeka M, Reca R, Turner AR, et al. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood. 2001;98:3143–9.
Lyman SD, James L, Escobar S, Downey H, de Vries P, Brasel K, et al. Identification of soluble and membrane-bound isoforms of the murine flt3 ligand generated by alternative splicing of mRNAs. Oncogene. 1995;10:149–57.
Hurwitz SN, Rider MA, Bundy JL, Liu X, Singh RK, Meckes DG. Proteomic profiling of NCI-60 extracellular vesicles uncovers common protein cargo and cancer type-specific biomarkers. Oncotarget. 2016;7:86999–7015.
Bauer N, Wilsch-Bräuninger M, Karbanová J, Fonseca AV, Strauss D, Freund D, et al. Haematopoietic stem cell differentiation promotes the release of prominin-1/CD133-containing membrane vesicles-a role of the endocytic-exocytic pathway. EMBO Mol Med. 2011;3:398–409. 2011/05/18 ed.
Holmfeldt P, Ganuza M, Marathe H, He B, Hall T, Kang G, et al. Functional screen identifies regulators of murine hematopoietic stem cell repopulation. J Exp Med. 2016;213:433–49.
Liao F, Tan L, Liu H, Wang J, Ma X, Zhao B, et al. Hematopoietic stem cell-derived exosomes promote hematopoietic differentiation of mouse embryonic stem cells in vitro via inhibiting the miR126/Notch1 pathway. Acta Pharm Sin. 2018;39:552–60.
Lechman ER, Gentner B, van Galen P, Giustacchini A, Saini M, Boccalatte FE, et al. Attenuation of miR-126 activity expands HSC in vivo without exhaustion. Cell Stem Cell. 2012;11:799–811. 2012/11/08 ed.
Salvucci O, Jiang K, Gasperini P, Maric D, Zhu J, Sakakibara S, et al. MicroRNA126 contributes to granulocyte colony-stimulating factor-induced hematopoietic progenitor cell mobilization by reducing the expression of vascular cell adhesion molecule 1. Haematologica. 2012;97:818–26. 2012/01/22 ed.
Ratajczak J, Kucia M, Mierzejewska K, Marlicz W, Pietrzkowski Z, Wojakowski W, et al. Paracrine proangiopoietic effects of human umbilical cord blood-derived purified CD133+ cells-implications for stem cell therapies in regenerative medicine. Stem Cells Dev. 2013;22:422–30. 2012/11/05 ed.
Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun. 2017/05/16 ed. 2017;8:15287.
Mohrin M, Bourke E, Alexander D, Warr MR, Barry-Holson K, Le Beau MM, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7:174–85. 2010/07/08 ed.
Koschade SE, Brandts CH. Selective Autophagy in Normal and Malignant Hematopoiesis. J Mol Biol. 2020;432:261–82. 2019/06/28 ed.
Xu J, Camfield R, Gorski SM. The interplay between exosomes and autophagy - partners in crime. J Cell Sci. [Internet]. 2018;131. 2018/08/03 ed. https://www.ncbi.nlm.nih.gov/pubmed/30076239.
Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. 2015/10/07 ed.
Borghesan M, Fafián-Labora J, Eleftheriadou O, Carpintero-Fernández P, Paez-Ribes M, Vizcay-Barrena G, et al. Small Extracellular Vesicles Are Key Regulators of Non-cell Autonomous Intercellular Communication in Senescence via the Interferon Protein IFITM3. Cell Rep. 2019;27:3956–71.e6.
Zhao JL, Ma C, O’Connell RM, Mehta A, DiLoreto R, Heath JR, et al. Conversion of danger signals into cytokine signals by hematopoietic stem and progenitor cells for regulation of stress-induced hematopoiesis. Cell Stem Cell. 2014;14:445–59.
Denkinger MD, Leins H, Schirmbeck R, Florian MC, Geiger H. HSC Aging and Senescent Immune Remodeling. Trends Immunol. 2015;36:815–24.
Dykstra B, Olthof S, Schreuder J, Ritsema M, de Haan G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J Exp Med. 2011;208:2691–703. 2011/11/21 ed.
Kim MJ, Kim MH, Kim SA, Chang JS. Age-related Deterioration of Hematopoietic Stem Cells. Int J Stem Cells. 2008;1:55–63.
Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16. 2015/04/30 ed.
Sieburg HB, Rezner BD, Muller-Sieburg CE. Predicting clonal self-renewal and extinction of hematopoietic stem cells. Proc Natl Acad Sci USA. 2011;108:4370–5. 2011/02/28 ed.
Acknowledgements
We thank Dr. Peter S. Klein for careful review of the paper and editorial contributions. We also acknowledge many colleagues whose relevant research in the field we were not able to cite due to space limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hurwitz, S.N., Jung, S.K. & Kurre, P. Hematopoietic stem and progenitor cell signaling in the niche. Leukemia 34, 3136–3148 (2020). https://doi.org/10.1038/s41375-020-01062-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-01062-8
- Springer Nature Limited
This article is cited by
-
Trim47 prevents hematopoietic stem cell exhaustion during stress by regulating MAVS-mediated innate immune pathway
Nature Communications (2024)
-
Aged bone marrow macrophages drive systemic aging and age-related dysfunction via extracellular vesicle-mediated induction of paracrine senescence
Nature Aging (2024)
-
Influential factors for optimizing and strengthening mesenchymal stem cells and hematopoietic stem cells co-culture
Molecular Biology Reports (2024)
-
The Endometrial Stem/Progenitor Cells and Their Niches
Stem Cell Reviews and Reports (2024)
-
New cell sources for CAR-based immunotherapy
Biomarker Research (2023)