Abstract
We assessed stage-specific trends in primary therapy and relative survival among adult follicular lymphoma (FL) patients diagnosed in the Netherlands between 1989–2016 (N = 12,372; median age, 62 years; and 21% stage I disease). Patients were stratified by disease stage and subsequently categorized into four calendar periods (1989–1995, 1996–2002, 2003–2008, and 2009–2016) and three age groups (18–60, 61–70, and >70 years). The use of radiotherapy in stage I FL remained relatively stable over time and across the three age groups (i.e., 66%, 54%, and 49% in 2009–2016, respectively). In stage II-IV FL, the start of chemotherapy within 12 months post-diagnosis decreased over time, indicating a broader application of a watch-and-wait approach. Relative survival improved considerably over time, especially since 2003 when rituximab was introduced in the Netherlands, and for stage III-IV FL patients and older age groups. Five-year relative survival for patients with stage I-II versus stage III-IV FL in the period 2009–2016 was 96% versus 90%, 93% versus 83%, and 92% versus 68% across the three age groups, respectively. Collectively, the improvement in survival since 2003 is accounted for by advances in FL management, particularly the implementation of rituximab. There remains, however, room for improvement among elderly stage III-IV FL patients.
Similar content being viewed by others
Introduction
Follicular lymphoma (FL) is the most common type of indolent non-Hodgkin lymphoma diagnosed in Western countries, with age-standardized incidence rates (ASRs) ranging from 2.2 to 6.2 per 100,000 person-years in a contemporary era [1,2,3,4]. The disease is frequently diagnosed after the sixth decade of life at an advanced stage (i.e., stage III-IV) and is considered incurable with current treatment modalities. The clinical behavior of FL is heterogeneous, ranging from a slow-growing disease with a propensity to spontaneous regression, to a more aggressive illness that relapses rapidly after initial treatment [5].
The introduction of rituximab dramatically changed the treatment paradigm of FL during the early-mid-2000s. The combination of rituximab with single- or combination chemotherapy set the stage for the current treatment of FL, in particular for patients with advanced-stage FL. Multiple randomized studies in the upfront [6,7,8] and salvage [9, 10] settings provided convincing evidence that, in patients with advanced-stage FL, the addition of rituximab to induction chemotherapy resulted in higher response rates, longer remission duration, and improved progression-free survival and overall survival, as compared to induction chemotherapy without rituximab.
Various population-based studies in FL have shown that, in several countries, the efficacy of rituximab with or without chemotherapy has translated into tangible benefits for patients with FL managed in routine clinical practice [3, 4, 11,12,13,14]. However, none of these studies have comprehensively assessed trends in incidence, primary therapy, and survival among patients with FL according to disease stage (limited- versus advanced-stage). Such a population-based analysis can illustrate how changes over time in treatment practices for both limited- and advanced-stage FL affects population-level survival. Therefore, we have performed a comprehensive, nationwide, population-based study spanning a 28-year period in the Netherlands to assess trends in incidence, primary therapy, and survival among adult patients with limited- and advanced-stage FL.
Patients and methods
The Netherlands Cancer Registry
The Netherlands Cancer Registry (NCR), which is managed by the Netherlands Comprehensive Cancer Organisation (IKNL), has a coverage of at least 95% of all newly diagnosed malignancies in the Netherlands since 1989 [15]. The NCR is notified of newly diagnosed malignancies by the Nationwide Network and Registry of Histopathology and Cytopathology, and the National Registry of Hospital Discharges (i.e., inpatient and outpatient discharges). Data on dates of birth and diagnosis, sex, disease topography and morphology, and primary therapy are routinely recorded in the NCR by trained registrars of IKNL through retrospective review of medical records. The NCR does not standardly ascertain detailed information on patient characteristics (e.g., performance score and comorbidities), prognostic factors (e.g., FL International Prognostic Index), methods of staging, and transformation/relapse rates. Details on the registration of primary therapy in the NCR are provided in another part of the patients and methods section. Topography (localization) and morphology are coded according to the International Classification of Diseases for Oncology. Data on vital statistics (i.e., alive, death, or emigration) are retrieved by establishing an annual linkage with the Nationwide Population Registries Network that holds these data for all residents in the Netherlands.
Study population
All patients diagnosed with FL grades 1-3B between January 1, 1989 and December 31, 2016 were selected from the NCR using the International Classification of Diseases for Oncology morphology codes 9693 (nodular, well-differentiated, lymphocytic malignant lymphoma) and 9697 (follicular, centroblastic type, malignant lymphoma) for patients diagnosed in 1989–2001 and 9690 (FL not otherwise specified), 9691 (FL grade II), 9695 (FL grade I), and 9698 (FL grade III) for patients diagnosed in 1989–2016 [16]. Patients with transformed FL at diagnosis and composite or discordant lymphomas were not included in our study. Further, we could not distinguish between grade 3A and 3B FL and contiguous and non-contiguous stage II FL since this information was not registered in the NCR before 2014. All patients were followed for survival from the date of diagnosis until death, emigration, or last follow-up (January 1, 2019), whichever occurred first. Patients below age 18 at diagnosis (n = 20) and patients diagnosed at autopsy (n = 26) were excluded from the study, except for the analysis of the overall incidence rate of FL. This manner of analysis complies with international standards for computing overall incidence rates.
According to the Central Committee on Research involving Human Subjects, this type of observational study does not require approval from an ethics committee in the Netherlands. The use of anonymous data for this study was approved by the Privacy Review Board of the NCR.
Primary therapy
The NCR ascertains information on primary therapy that was initiated within 12 months after diagnosis. Of note, if relapse therapy was started within 12 months after diagnosis due to disease progression, this information was not standardly ascertained in the NCR. This also holds for patients who received primary therapy 12 months after diagnosis. These patients are registered in the NCR under the category of no anti-neoplastic therapy (including a watch-and-wait approach).
For the overall cohort (1989–2016), primary therapy was grouped into the following broad categories, namely (i) no anti-neoplastic therapy (including a watch-and-wait strategy) within 12 months after diagnosis, (ii) radiotherapy alone, (iii) chemotherapy without radiotherapy (with or without another modality), (iv) combined modality treatment (systemic therapy with radiotherapy), (v) and a variety of other less common therapies or unknown therapy.
In addition to the broad categories of therapy, the NCR registered information on the use of targeted immunotherapy as of January 1, 2007. Therefore, an assessment of nationwide trends in the use of (chemo)immunotherapy before that period was not possible. However, the uptake of rituximab into first-line treatment algorithms of FL before 2007 is presumed to be low, as rituximab was initially introduced for the treatment of relapsed or refractory FL. Also, information on the exact therapeutic regimen was registered in the NCR for patients diagnosed as of January 1, 2014. These regimens were defined as radiotherapy alone, rituximab with cyclophosphamide, vincristine, and prednisone (R-CVP), rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP21), rituximab with or without another systemic therapy, combined modality treatment (systemic therapy with radiotherapy), watch-and-wait approach, no anti-neoplastic therapy, and other or unknown therapy. Of note, a watch-and-wait approach involves closely monitoring asymptomatic patients without providing treatment. In contrast, the initial plan for patients who were classified as receiving no anti-neoplastic therapy is that they were not planned to receive anti-neoplastic therapy, even in the presence or development of symptoms. Reasons to withhold active treatment include the presence of comorbidities that hamper the safe application of anti-neoplastic therapy, refusal by the patient, and a short life expectancy due to causes related or unrelated to FL. In the Netherlands, R-CVP is still considered the standard of care for the initial therapy of FL, with a preference for R-CHOP in patients with a high FL International Prognostic Index. This recommendation relates to the absence of an overall survival benefit and concerns related to long-term toxicities with R-CHOP and bendamustine plus rituximab [17,18,19]. Also, rituximab monotherapy is not recommended for patients with asymptomatic FL. Instead, it is only recommended for patients with symptomatic stage I and non-contiguous stage II FL who are not eligible for curative therapy and unfit, symptomatic patients with contiguous stage II and stage III-IV FL. Therefore, bendamustine plus rituximab and rituximab monotherapy, given their low application as first-line treatment during the period 2014–2016, were not included as separate categories.
The distribution of primary therapy according to the four broad categories is presented for four calendar periods (1989–1995, 1996–2002, 2003–2008, and 2009–2016) and three age categories (18–60, 61–70, and >70 years). Further, the distribution of primary therapy is stratified by disease stage as per the Ann Arbor classification—that is, stage I, II, and III-IV. The calendar periods were defined based on the introduction of rituximab in the Netherlands. More specifically, the first two periods represent the pre-rituximab era, the third period the era in which rituximab was gradually implemented into routine clinical practice, and the fourth period presents the era in which rituximab-containing therapy was considered the standard first-line treatment for patients with advanced-stage FL in most centers and rituximab maintenance was gradually, but not universally, introduced into routine clinical practice.
Statistical analyses
Descriptive statistics were employed to present patient and treatment characteristics across the four calendar periods. These characteristics were also stratified by disease stage—that is, stage I-II (limited-stage) versus III-IV (advanced stage). The Pearson chi-square test was applied to compare categorical variables, and the Kruskal–Wallis test was applied to compare continuous variables.
Incidence rates of FL were calculated per 100,000 person-years using the annual mid-year population size that was obtained from Statistics Netherlands and age-standardized according to the European standard population. These rates were calculated overall and according to sex, age (15–59, 60–69, and ≥70 years), and calendar period, and stratified by disease stage. The age categories defined for incidence analysis slightly differ from the age categories defined earlier, since incidence rates can only be calculated for quinquennial years of age. Also, age-specific incidence rates were calculated per 10-year age groupings of 20–29 years to ≥90 years.
Relative survival (RS) was calculated to estimate disease-specific survival according to the complete approach [20]. RS is defined as the ratio of the observed patient survival (i.e., OS) to the expected survival of a comparable group in the general population, matched to the patients with respect to age, sex, and calendar period [21]. Expected survival was estimated by the Ederer II methodology using Dutch population life tables, stratified by age, sex, and calendar period [22]. Five- and 10-year RS rates (RSRs) with 95% confidence intervals (CIs) were calculated for four calendar periods and three age categories, stratified by disease stage. As cause of death information is unavailable in the NCR, we were unable to compute disease-specific survival. Therefore, we employed RS since it estimates disease-specific survival but does not require cause of death information. RS captures excess mortality—relative to the expected mortality in the general population—associated with an FL diagnosis, regardless of whether the excess mortality was directly or indirectly attributed to FL [21].
Generalized linear models were constructed that assumed a Poisson distribution for the observed number of deaths to model excess mortality over the calendar periods studied during the first five years after diagnosis. These models provide excess mortality rate ratios (EMRRs) with 95% CIs and were adjusted for years of follow-up, sex, age at diagnosis, and disease stage, and constructed separately for limited- and advanced-stage FL. The follow-up years were divided into 1-year time bands.
A P value of less than 0.05 indicated statistical significance in all analyses. Statistical analyses were performed with STATA Statistical Software version 14.2 (StataCorp, College Station, TX).
Results
Patient characteristics
A total of 12,372 adult patients (51% males; median age, 62 years) were diagnosed with FL in the Netherlands between 1989 and 2016 and included in the study. The characteristics of these patients according to disease stage are presented in Table 1. The majority of patients were diagnosed with advanced-stage disease (62%), 35% had limited-stage disease, and the disease stage was unknown in 3%. The proportion of patients with an unknown disease stage decreased from 7% in 1989–1995 to 2% in 2009–2016 (P < 0.001).
Incidence of FL
Incidence rates of FL are presented overall and stratified by age, disease stage, and sex in Fig. 1a–c and Supplemental Table 1. The overall ASR of FL gradually increased from 1.92 per 100,000 person-years in 1989–1995 to 2.32 and 2.71 per 100,000 person-years in 1996–2002 and 2003–2008. Thereafter, the incidence remained nearly constant (i.e., 2.75 per 100,000 person-years in 2009–2016). There was a persistent, albeit slight, male predominance throughout the study period, which was consistent across all age and stage groups. Over time, the incidence increased more prominently for advanced-stage FL, as compared to limited-stage FL. This phenomenon was most pronounced among patients aged ≥60 years. Lastly, the age-specific incidence was considerably higher in older age groups and peaked in the 60–69 years age group in the most recent calendar period (2009–2016). This pattern was independent of sex and disease stage (Fig. 1d).
Panels a–c present age-specific incidence rates per 100,000 person-years of adult patients according to the calendar period of diagnosis and disease stage. These estimates are presented as values in Supplemental Table 1. Panel d presents age-specific incidence rates per 100,000 person-years according to quinquennial years of age for patients diagnosed during 2009–2016. Yearly incidence rates are presented in Supplemental Fig. 2. In that figure, it can be appreciated that the incidence increased until 2009 and stabilized thereafter.
Primary therapy of stage I FL
The distribution of primary therapy among adult patients with stage I FL is presented in Fig. 2a. Treatment patterns were comparatively similar over the calendar periods studied. The most commonly applied therapy was radiotherapy alone, with proportions of 66%, 54%, and 49% across the three age groups (18–60, 61–70, and >70 years) in the most recent calendar period (2009–2016), respectively. The respective proportions for the use of chemotherapy without radiotherapy were 7%, 8%, and 10% (Fig. 2a). Combined modality treatment was applied to 7%, 10%, and 4% across the three age groups in the most recent calendar period. Out of all patients that received chemotherapy without radiotherapy or combined modality treatment, 96%, 98%, and 94% comprised chemoimmunotherapy (data not shown). Lastly, the respective proportions of patients in whom no therapy was given within 12 months after diagnosis were 19%, 27%, and 36% in the three age groups.
Panels a, b, and c show the results for patients with stage I, II, and III-IV disease, respectively. Of note, among 79 (61%) of 130 patients who were classified as unknown or other therapy, it was unknown if patients were treated. The remaining patients received rituximab monotherapy (n = 37; 28%), rituximab combined with an unspecified therapy (n = 2; 1%), and unspecified therapies (n = 12; 9%). CT chemotherapy, RT radiotherapy, CMT combined modality treatment.
Primary therapy of stage II FL
The distribution of primary therapy among adult patients with stage II FL is presented in Fig. 2b. Consistent with findings in stage I disease, the use of radiotherapy was comparatively stable over the calendar periods studied. However, radiotherapy was less often applied—as compared to stage I disease—with proportions of 16%, 10%, and 14% across the three age groups in the most recent calendar period, respectively. Chemotherapy was the most frequently applied therapy among patients with stage II disease. However, its use decreased over time (P < 0.001). This decrease was followed by a parallel increase in the proportion of patients in whom no therapy was given within 12 months after diagnosis (P < 0.001). More specifically, the use of chemotherapy decreased from 62% to 37%, 49% to 37%, and 53% to 32% across the three age groups between 1989–1995 and 2009–2016, respectively. In the most recent calendar period, the respective proportions of chemoimmunotherapy use among chemotherapy-treated patients were 98%, 98%, and 96% (data not shown). Detailed data of patients diagnosed during 2014–2016 revealed that the majority of the chemotherapy-treated patients across the three age groups received R-CVP, followed by R-CHOP21 (Fig. 3). The proportion of patients in whom no therapy was given within 12 months after diagnosis increased from 10% to 43%, 17% to 49%, and 26% to 48% across the three age groups between 1989–1995 and 2009–2016, respectively. Data of patients diagnosed during 2014–2016 showed that the vast majority of patients who were classified as receiving no anti-neoplastic therapy were put on a watch-and-wait approach (i.e., 92%, 97%, and 96% across the three age groups; Fig. 3).
The absolute number of patients within a specific stage and age group is shown in Supplemental Table 2. The group of rituximab with or without another modality (N = 57) included the following modalities: rituximab with chlorambucil (n = 25), rituximab with CHOP14 (n = 12), rituximab monotherapy (n = 8), rituximab with CP (n = 4), rituximab with bendamustine (n = 4), rituximab with FC (n = 1), rituximab with CEOP (n = 2), rituximab with miniCHOP (n = 1). The group of combined modality treatment (n = 31) included the following modalities: radiotherapy with R-CHOP21 (n = 22), radiotherapy with R-CVP (n = 6), radiotherapy with R-CHOP14 (n = 2), and radiotherapy with rituximab (n = 1). The group of other or unknown therapy (N = 7) included the following modalities: dexamethasone, cytarabine, and cisplatin (n = 1), chlorambucil (n = 3), CHOP21 (n = 1), CVP (n = 1), and unspecified therapy (n = 1). The reasons for refraining from anti-neoplastic therapy were only known in 43 (68%) of 63 patients and were as follows: the presence of comorbidities (n = 15), an insufficient functional status (n = 13), patient refusal (n = 13), and uncontrolled, advanced disease (n = 2). R-CVP rituximab with cyclophosphamide, vincristine, and prednisone, R-CHOP rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone, R rituximab, RT radiotherapy, CMT combined modality treatment, CP cyclophosphamide and prednisone, FC fludarabine and cyclophosphamide, CEOP cyclophosphamide, etoposide, vincristine, and prednisone.
Primary therapy of advanced-stage FL
The distribution of primary therapy among adult patients with advanced-stage FL is presented in Fig. 2c. Treatment patterns in these patients broadly mirrored those observed in patients with stage II disease. Radiotherapy alone and combined modality treatment were hardly applied among patients with advanced-stage disease. The vast majority of patients with advanced-stage disease were treated with chemotherapy. Its use within 12 months after diagnosis, however, decreased over time, following the broader application of an initial watch-and-wait approach. More specifically, the use of chemotherapy within 12 months after diagnosis decreased from 76%, 73%, and 67% in 1989–1995 to 54%, 55%, and 52% in 2009–2016 across the three age groups, respectively. In the most recent calendar period, the respective proportions of chemoimmunotherapy use in patients treated with chemotherapy or combined modality treatment were 98%, 98%, and 96% (data not shown). Detailed data of patients diagnosed during 2014–2016 showed that the vast majority of chemotherapy-treated patients across the three age groups received R-CVP, followed by R-CHOP21 (Fig. 3). The proportions for no initial anti-neoplastic therapy were 16%, 19%, and 28% in 1989–1995, as compared to 42%, 40%, and 41% in 2009–2016 across the three age groups. The vast majority of patients who were classified as receiving no anti-neoplastic therapy were put on a watch-and-wait approach (i.e., 98%, 95%, and 84% across the three age groups in 2014–2016, respectively; Fig. 3).
Relative survival of limited-stage FL
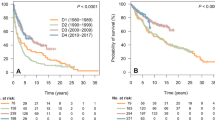
As shown in Fig. 4a–c, RS in adult patients with limited-stage FL improved significantly across all age groups, most notably in patients aged >70 years between 2003–2008 and 2009–2016. Of note, the survival of patients aged 18–60 and 61–70 years was already relatively high in earlier calendar periods. Five-year RS for patients aged >70 years increased to 91% (95% CI, 84%–97%) in the most recent calendar period. This is comparable to the 5-year RSRs of those aged 18–60 (96%; 95% CI, 93%–98%) and 61–70 years (93%; 95% CI, 88%–96%). Altogether, this implies that excess mortality within five years after diagnosis is low in patients with limited-stage FL with current diagnostic and treatment protocols. This conclusion is further supported by the fact that 5- and 10-year RSRs in the period 2003–2008 were comparable, especially for patients aged 18–60 and 61–70 years.
Panels a–c and d–f show the results for patients with limited-stage and advanced-stage FL, respectively. The tables present the projected 5- and 10-year relative survival rates (RSRs) with 95% confidence intervals (CIs), stratified by disease stage according to age at diagnosis and calendar period of diagnosis. P values for linear trends from the calendar period 1989–1995 to the calendar period 2009–2016 were statistically significant with P values of less than 0.001 for all age and stage groups.
The multivariable analysis confirmed an improvement of RS in 2009–2016, as compared to 1996–2002 (EMRR, 0.51; 95% CI 0.42–0.61; P < 0.001, Table 2). Also, there was an independent adverse prognostic effect of male sex, older age, and stage II disease.
Relative survival of advanced-stage FL
The survival improvement in advanced-stage FL across all age groups was generally more pronounced, as compared to limited-stage FL (Fig. 4d–f)—especially in the rituximab era. However, as compared to those with limited-stage FL, patients with advanced-stage FL had higher overall excess mortality that increased with older age.
The multivariable analysis confirmed an improvement of RS in 2009–2016, as compared to 1996–2002 (EMRR 0.39; 95% CI 0.35–0.43; P < 0.001; Table 2). Also, there was an independent adverse prognostic effect of male sex, older age, and stage IV disease.
Discussion
To the best of our knowledge, this comprehensive, nationwide, population-based study is the first to present outcomes on incidence, primary therapy, and RS of patients with FL across subgroups of age and disease stage. Our study showed that outcomes varied markedly over time across subgroups.
Incidence of FL
The overall ASR of FL in the Netherlands gradually increased over time—particularly among patients with advanced-stage FL aged ≥60 years—and has stabilized since 2009. Better diagnostic methods might explain this increase. Indeed, the proportion of patients with an unspecified lymphoma decreased over time (data not shown), particularly since 2001 when national quality monitoring programs for pathology practice became mandatory. Second, the widespread utilization of imaging techniques might have caused an increase in incidental findings of FL, particularly among older age groups [23,24,25,26]. The pronounced increase in advanced-stage FL can partially be accounted for by stage migration, as PET/CT can upstage 11–62% of patients with limited-stage FL to advanced-stage FL [27,28,29,30,31].
The overall ASR of FL in the Netherlands and trends herein were mainly comparable to most [1, 4, 32,33,34], but not all [3, 32], previous studies. Differences in incidence rates across countries have been noted in a few studies [32, 34]. These disparities might be attributable to differences in race, as well as imaging techniques and registration practices [1, 32, 35].
Primary therapy of FL
In line with European clinical practice guidelines [36], radiotherapy alone was most commonly applied in stage I FL in the Netherlands. Radiotherapy with a dose of 24 Gy is highly effective and potentially curative in this patient population [37, 38]. However, 17–27% of stage I patients received a watch-and-wait approach in our study (2014–2016). Generally, factors associated with not providing radiotherapy were an FL localization in the abdomen, a higher grade of FL, and a higher risk profile according to the FL International Prognostic Index (2014–2016; data not shown). The use of radiotherapy in the US is lower than practices in the Netherlands. According to the authors of previous studies, radiotherapy appears to be underutilized in patients with stage I FL in the US [39,40,41]. Conversely, the use of radiotherapy in Australia and Canada is somewhat higher than practices in the US and the Netherlands [42]. Altogether, apart from the earlier mentioned factors, it remains unknown whether reasons to have withheld radiotherapy in stage I FL patients in the Netherlands were valid [43, 44]. Recent randomized trials showed that the use of chemoimmunotherapy following involved-field radiotherapy improved PFS as compared with involved-field radiotherapy alone [42, 45, 46]. However, these studies did not demonstrate an improvement in OS [42, 45, 46]. Therefore, it remains a subject of ongoing controversy how to best manage patients with limited-stage FL [36, 47].
Chemotherapy was the treatment of choice in the Netherlands for patients with stage II-IV FL. This choice is generally consistent with clinical practice guidelines [36]. Most chemotherapy-treated patients diagnosed between 2014–2016 received R-CVP. Although R-CVP is associated with a higher risk of progression, as compared to R-CHOP and bendamustine plus rituximab, all regimens produce a similar overall survival. Also, R-CVP has a more favorable toxicity profile [48]. Collectively, the choice of a particular regimen depends on the delicate balance between treatment tolerability, long-term toxicity, and disease control.
The indolent natural history of FL prompted cooperative trial groups in the 1990s to assess the value of a watch-and-wait approach over the early initiation of therapy in asymptomatic patients with advanced-stage FL. These trials revealed that the early initiation of therapy did not improve overall survival, as compared to a watch-and-wait approach [43, 49,50,51]. As a result, the use of chemotherapy within 12 months after diagnosis decreased over time in the Netherlands in patients with advanced-stage FL, probably following a broader institution of a watch-and-wait approach.
Relative survival of FL
RS of patients with FL in the Netherlands improved considerably over time across all age and stage groups. This observation is consistent with findings from prior randomized trials [6,7,8,9,10] and population-based studies [3, 4, 11,12,13,14]. However, we extended on prior findings by including patients diagnosed in contemporary clinical practice with well-established lymphoma management. Our study supports the general notion that advances in the management of FL—especially the introduction of rituximab—are key factors contributing to the improved survival in FL over time. These advances merit brief acknowledgment.
First, rituximab was introduced in the Netherlands in the early-mid-2000s. The overall impact of these introductions was, in part, reflected by the substantial improvement in RSRs from 1996–2002 to 2003–2008. Secondly, the improvement in RSRs was more profound for patients with advanced-stage FL than limited-stage FL. Indeed, for patients with advanced-stage FL, rituximab-containing chemotherapy has prevailed as the standard of care since its introduction in the early-mid-2000s [36]. Of note, the improvement in RSRs for patients with limited-stage FL, especially in the elderly, could also be explained by advances in supportive care and the incorporation of rituximab into treatment algorithms in the relapsed/refractory setting [9, 10]. Third, the improvement in RSRs was most pronounced among older age groups. Advances in supportive care and treatment most probably have improved the tolerability of treatment among older, often comorbid patients. Examples are the introduction of granulocyte colony-stimulating factor [52, 53] and less intensive regimens with a comparatively low toxicity profile [54,55,56,57]. These advances might have changed the attitude of physicians towards providing treatment to older patients.
An encouraging and novel finding in our study is that the strong association between survival and age in earlier calendar periods virtually diminished in the most recent calendar period among patients with limited-stage FL. Among these patients, excess mortality within five years after diagnosis is comparatively low (<10%). In contrast, patients with advanced-stage FL continue to experience considerable excess mortality, which increases with older age. As most patients are diagnosed with advanced-stage FL, further advances in the management of these patients across various lines of therapy are needed. More recently, new therapeutic options have become available for the management of FL, such as with Bruton tyrosine kinase inhibitors, PI3 kinase inhibitors, and novel immunotherapies (e.g., CD19 CAR-T cell immunotherapy) [58,59,60,61].
It should be noted that upward stage migration resulting from PET/CT staging—which was introduced in the Netherlands in 2003—might also have artificially improved survival for advanced-stage FL. However, RS also improved over time when considering the total cohort where all stages were combined (Supplemental Fig. 1). This finding suggests that stage migration did not primarily affect the improvement in survival.
Overall, RSRs in the current study were generally comparable to RSRs across different countries, albeit slight survival disparities exist across countries in the rituximab-era [2,3,4, 11,12,13,14, 62]. These disparities may be caused by persisting inequalities in the provision of care. For instance, costs of and limited access to rituximab are barriers for its use to manage patients with non-Hodgkin lymphoma in resource-limited countries [12, 63]. The availability of rituximab biosimilar as from 2018 might, however, increase survival rates in countries with a comparatively low survival rate.
Strengths and limitations
The strengths of our study include the use of a long-running and well-established nationwide population-based cancer registry with comprehensive data available for individual patients. The use of such a registry allowed analysis according to disease stage. Limitations mainly pertain to the lack of detailed data throughout most of the registry (1989–2013) on prognostic factors, therapy, the subdivision of grade 3 FL into grade 3A and 3B and stage II FL into contiguous and non-contiguous disease, as well as transformation/relapse rates and therapy beyond 12 months after diagnosis. Consequently, current controversies can not be addressed with the current data, such as the comparative effectiveness of R-CVP and R-CHOP and the advantage of rituximab alone or combined modality treatment over radiotherapy alone in non-bulky limited-stage FL. These controversies can be addressed with extended follow-up activities through retrospective medical records review. Also, it merits brief acknowledgment that the cases ascertained in the NCR were not submitted for central pathology review. However, for diagnostic purposes, central pathology review is covered for all lymphoma patients in the Netherlands by regional hematopathology expert panels. Therefore, in conjunction with retrospective medical records review, a large number of patients in this population-based series, and the diagnostic criteria for FL remaining virtually unchanged over the study period [64,65,66,67,68], we feel that a potential misclassification of FL would have only marginally biased our results.
Summary
In this nationwide, population-based study, survival among patients with FL improved considerably over time, particularly in patients with advanced-stage FL and older age groups. A novel and encouraging finding is that the vast majority of patients with limited-stage FL diagnosed in a contemporary era rarely experience excess mortality within five years after diagnosis. The improvements in survival over time are likely caused by advances in the management of FL, especially the introduction of rituximab in the early-mid-2000s. However, as patients with advanced-stage FL, especially those above age >70 years, continue to experience substantial excess mortality in contemporary clinical practice, further therapeutic advances in the upfront and salvage settings are needed to reduce short- and long-term excess mortality.
References
Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66:443–59.
Smith A, Crouch S, Lax S, Li J, Painter D, Howell D, et al. Lymphoma incidence, survival and prevalence 2004–2014: sub-type analyses from the UK’s Haematological Malignancy Research Network. Br J Cancer. 2015;112:1575.
Ye X, Mahmud S, Skrabek P, Lix L, Johnston JB. Long-term time trends in incidence, survival and mortality of lymphomas by subtype among adults in Manitoba, Canada: a population-based study using cancer registry data. BMJ Open. 2017;7:e015106.
Junlen HR, Peterson S, Kimby E, Lockmer S, Linden O, Nilsson-Ehle H, et al. Follicular lymphoma in Sweden: nationwide improved survival in the rituximab era, particularly in elderly women: a Swedish Lymphoma Registry study. Leukemia. 2015;29:668–76.
Becnel MR, Nastoupil LJ. Follicular lymphoma: past, present, and future. Curr Treat Options Oncol. 2018;19:32.
Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–32.
Herold M, Haas A, Srock S, Neser S, Al-Ali KH, Neubauer A, et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group Hematology and Oncology Study. J Clin Oncol. 2007;25:1986–92.
Marcus R, Imrie K, Solal-Celigny P, Catalano JV, Dmoszynska A, Raposo JC, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26:4579–86.
Forstpointner R, Dreyling M, Repp R, Hermann S, Hanel A, Metzner B, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104:3064–71.
van Oers MH, Klasa R, Marcus RE, Wolf M, Kimby E, Gascoyne RD, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108:3295–301.
Sant M, Minicozzi P, Mounier M, Anderson LA, Brenner H, Holleczek B, et al. Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study. Lancet Oncol. 2014;15:931–42.
De Angelis R, Minicozzi P, Sant M, Dal Maso L, Brewster DH, Osca-Gelis G, et al. Survival variations by country and age for lymphoid and myeloid malignancies in Europe 2000-2007: Results of EUROCARE-5 population-based study. Eur J Cancer. 2015;51:2254–68.
Keegan THM, McClure LA, Foran JM, Clarke CA. Improvements in survival after follicular lymphoma by race/ethnicity and socioeconomic status: a population-based study. J Clin Oncol. 2009;27:3044–51.
Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol. 2015;90:790–5.
Schouten LJ, Hoppener P, van den Brandt PA, Knottnerus JA, Jager JJ. Completeness of cancer registration in Limburg, The Netherlands. Int J Epidemiol. 1993;22:369–76.
Fritz AP, Jack CA. International classification of diseases for oncology. 3 edn. Geneva: World Health Organisation; 2000.
Hiddemann W, Barbui AM, Canales MA, Cannell PK, Collins GP, Dürig J, et al. Immunochemotherapy with obinutuzumab or rituximab for previously untreated follicular lymphoma in the GALLIUM study: influence of chemotherapy on efficacy and safety. J Clin Oncol. 2018;36:2395–404.
Flinn IW, van der Jagt R, Kahl B, Wood P, Hawkins T, MacDonald D, et al. First-line treatment of patients with indolent non-Hodgkin lymphoma or mantle-cell lymphoma with bendamustine plus rituximab versus R-CHOP or R-CVP: results of the BRIGHT 5-year follow-up study. J Clin Oncol. 2019;37:984–91.
Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grünhagen U, Losem C, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–10.
Henson DE, Ries LA. The relative survival rate. Cancer. 1995;76:1687–8.
Dickman PW, Coviello E. Estimating and modeling relative survival. Stata J. 2015;15:186–215.
Ederer F, Heise H. Instructions to IBM 650 Programmers in Processing Survival Computations. Methodological Note No. 10. Bethesda, MD: National Cancer Institute; 1959.
Smith-Bindman R, Kwan ML, Marlow EC, Theis MK, Bolch W, Cheng SY, et al. Trends in use of medical imaging in US Health Care Systems and in Ontario, Canada, 2000–2016. JAMA. 2019;322:843–56.
Smith-Bindman R, Miglioretti DL, Johnson E, Lee C, Feigelson HS, Flynn M, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996–2010. JAMA. 2012;307:2400–9.
Carbone A, Roulland S, Gloghini A, Younes A, von Keudell G, Lopez-Guillermo A, et al. Follicular lymphoma. Nat Rev Dis Prim. 2019;5:83.
Orme NM, Fletcher JG, Siddiki HA, Harmsen WS, O’Byrne MM, Port JD, et al. Incidental findings in imaging research: evaluating incidence, benefit, and burden. Arch Intern Med. 2010;170:1525–32.
Wirth A, Foo M, Seymour JF, Macmanus MP, Hicks RJ. Impact of [18f] fluorodeoxyglucose positron emission tomography on staging and management of early-stage follicular non-hodgkin lymphoma. Int J Radiat Oncol, Biol, Phys. 2008;71:213–9.
Luminari S, Biasoli I, Arcaini L, Versari A, Rusconi C, Merli F, et al. The use of FDG-PET in the initial staging of 142 patients with follicular lymphoma: a retrospective study from the FOLL05 randomized trial of the Fondazione Italiana Linfomi. Ann Oncol. 2013;24:2108–12.
Scott AM, Gunawardana DH, Wong J, Kirkwood I, Hicks RJ, Ho Shon I, et al. Positron emission tomography changes management, improves prognostic stratification and is superior to gallium scintigraphy in patients with low-grade lymphoma: results of a multicentre prospective study. Eur J Nucl Med Mol imaging. 2009;36:347–53.
Le Dortz L, De Guibert S, Bayat S, Devillers A, Houot R, Rolland Y, et al. Diagnostic and prognostic impact of 18F-FDG PET/CT in follicular lymphoma. Eur J Nucl Med Mol imaging. 2010;37:2307–14.
Karam M, Novak L, Cyriac J, Ali A, Nazeer T, Nugent F. Role of fluorine-18 fluoro-deoxyglucose positron emission tomography scan in the evaluation and follow-up of patients with low-grade lymphomas. Cancer. 2006;107:175–83.
Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116:3724–34.
Smith A, Crouch S, Lax S, Li J, Painter D, Howell D, et al. Lymphoma incidence, survival and prevalence 2004-2014: sub-type analyses from the UK’s Haematological Malignancy Research Network. Br J Cancer. 2015;112:1575–84.
Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–76.
Clarke CA, Glaser SL, Gomez SL, Wang SS, Keegan TH, Yang J, et al. Lymphoid malignancies in U.S. Asians: incidence rate differences by birthplace and acculturation. Cancer Epidemiol Biomark Prev. 2011;20:1064–77.
Dreyling M, Ghielmini M, Marcus R, Salles G, Vitolo U, Ladetto M. Newly diagnosed and relapsed follicular lymphoma: ESMO clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:iii76–82.
Lowry L, Smith P, Qian W, Falk S, Benstead K, Illidge T, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother Oncol. 2011;100:86–92.
Hoskin PJ, Kirkwood AA, Popova B, Smith P, Robinson M, Gallop-Evans E, et al. 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): a randomised phase 3 non-inferiority trial. Lancet Oncol. 2014;15:457–63.
Friedberg JW, Taylor MD, Cerhan JR, Flowers CR, Dillon H, Farber CM, et al. Follicular lymphoma in the United States: first report of the national LymphoCare study. J Clin Oncol. 2009;27:1202–8.
Pugh TJ, Ballonoff A, Newman F, Rabinovitch R. Improved survival in patients with early stage low-grade follicular lymphoma treated with radiation: a surveillance, epidemiology, and end results database analysis. Cancer. 2010;116:3843–51.
Vargo JA, Gill BS, Balasubramani GK, Beriwal S. What is the optimal management of early-stage low-grade follicular lymphoma in the modern era? Cancer. 2015;121:3325–34.
Tobin JWD, Rule G, Colvin K, Calvente L, Hodgson D, Bell S, et al. Outcomes of stage I/II follicular lymphoma in the PET era: an international study from the Australian Lymphoma Alliance. Blood Adv. 2019;3:2804–11.
Solal-Céligny P, Bellei M, Marcheselli L, Pesce EA, Pileri S, McLaughlin P, et al. Watchful waiting in low-tumor burden follicular lymphoma in the rituximab era: results of an F2-study database. J Clin Oncol. 2012;30:3848–53.
Friedberg JW, Byrtek M, Link BK, Flowers C, Taylor M, Hainsworth J, et al. Effectiveness of first-line management strategies for stage I follicular lymphoma: analysis of the National LymphoCare Study. J Clin Oncol. 2012;30:3368–75.
MacManus M, Fisher R, Roos D, O’Brien P, Macann A, Davis S, et al. Randomized trial of systemic therapy after involved-field radiotherapy in patients with early-stage follicular lymphoma: TROG 99.03. J Clin Oncol. 2018;36:2918–25.
Lo A, Campbell BA, Pickles T, Aquino-Parsons C, Sehn LH, Connors J, et al. Long-term outcomes for patients with limited-stage follicular lymphoma: update of a population-based study. Blood. 2020;136:1006–10.
National Comprehensive Cancer Network. Clinical practice guidelines in oncology: B-cell lymphomas (Version 2.2020). https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf.
Itchaki G, Gafter-Gvili A, Lahav M, Vidal L, Raanani P, Shpilberg O, et al. Anthracycline-containing regimens for treatment of follicular lymphoma in adults. Cochrane Database Syst Rev. 2013;7:Cd008909.
Advani R, Rosenberg SA, Horning SJ. Stage I and II follicular non-Hodgkin’s lymphoma: long-term follow-up of no initial therapy. J Clin Oncol. 2004;22:1454–9.
Ardeshna KM, Qian W, Smith P, Braganca N, Lowry L, Patrick P, et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: an open-label randomised phase 3 trial. Lancet Oncol. 2014;15:424–35.
El-Galaly TC, Bilgrau AE, de Nully Brown P, Mylam KJ, Ahmad SA, Pedersen LM, et al. A population-based study of prognosis in advanced stage follicular lymphoma managed by watch and wait. Br J Haematol. 2015;169:435–44.
Vitolo U, Angrili F, DeCosta L, Wetten S, Federico M. G-CSF use in patients receiving first-line chemotherapy for non-Hodgkin’s lymphoma (NHL) and granulocyte-colony stimulating factors (G-CSF) as observed in clinical practice in Italy. Med Oncol. 2016;33:139.
Lyman GH, Dale DC, Culakova E, Poniewierski MS, Wolff DA, Kuderer NM, et al. The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol. 2013;24:2475–84.
Huang HH, Wen YC, Chen HM, Hsiao FY, Ko BS. Rituximab maintenance improves overall survival in follicular lymphoma: a retrospective nationwide real-world analysis from Taiwan Cancer Registry Database. Cancer Med. 2018;7:3582–91.
Martinelli G, Schmitz SF, Utiger U, Cerny T, Hess U, Bassi S, et al. Long-term follow-up of patients with follicular lymphoma receiving single-agent rituximab at two different schedules in trial SAKK 35/98. J Clin Oncol. 2010;28:4480–4.
Kahl BS, Hong F, Williams ME, Gascoyne RD, Wagner LI, Krauss JC, et al. Rituximab extended schedule or re-treatment trial for low-tumor burden follicular lymphoma: eastern cooperative oncology group protocol e4402. J Clin Oncol. 2014;32:3096–102.
Martinelli G, Montoro J, Vanazzi A, Andreola G, Liptrott S, Radice D, et al. Chlorambucil–rituximab as first-line therapy in patients affected by follicular non-Hodgkin’s lymphoma: a retrospective single-centre study. Hematological Oncol. 2015;33:129–35.
Dreyling M, Santoro A, Mollica L, Leppä S, Follows GA, Lenz G, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol: Off J Am Soc Clin Oncol. 2017;35:3898–905.
Flinn IW, Miller CB, Ardeshna KM, Tetreault S, Assouline SE, Mayer J, et al. DYNAMO: a phase II study of duvelisib (IPI-145) in patients with refractory indolent non-Hodgkin lymphoma. J Clin Oncol. 2019;37:912–22.
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl J Med. 2017;377:2545–54.
Hirayama AV, Gauthier J, Hay KA, Voutsinas JM, Wu Q, Pender BS, et al. High rate of durable complete remission in follicular lymphoma after CD19 CAR-T cell immunotherapy. Blood. 2019;134:636–40.
Tan D, Horning SJ, Hoppe RT, Levy R, Rosenberg SA, Sigal BM, et al. Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: the Stanford University experience. Blood. 2013;122:981–7.
Baer IiWH, Maini A, Jacobs I. Barriers to the access and use of rituximab in patients with non-Hodgkin’s lymphoma and chronic lymphocytic leukemia: a physician survey. Pharm. 2014;7:530–44.
Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–92.
Lennert K, Feller AC. Histopathology of Non-Hodgkin’s lymphomas. Berlin, Heidelberg: Springer-Verlag; 1992.
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90.
Norris D, Stone J WHO classification of tumours of haematopoietic and lymphoid tissues. Geneva: WHO. 2008:22-3.
Hossfeld D. World Health Organization classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. Ann Oncol. 2002;13:490.
Acknowledgements
The authors would like to thank the registration clerks of the Netherlands Cancer Registry (NCR) for their dedicated data collection. The nationwide population-based NCR is maintained and hosted by the Netherlands Comprehensive Cancer Organisation (IKNL).
Author information
Authors and Affiliations
Contributions
AGD and PJL designed the study; MAWD analyzed the data; OV collected the data; MAWD wrote the manuscript with contributions from all authors, who also interpreted the data, and read, commented on, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MJK has received research and travel support, as well as honoraria for presentations from Roche. The remaining authors have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Dinnessen, M.A.W., van der Poel, M.W.M., Tonino, S.H. et al. Stage-specific trends in primary therapy and survival in follicular lymphoma: a nationwide population-based analysis in the Netherlands, 1989–2016. Leukemia 35, 1683–1695 (2021). https://doi.org/10.1038/s41375-020-01048-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-01048-6
- Springer Nature Limited
This article is cited by
-
Causes of death of patients with follicular lymphoma in the Netherlands by stage and age groups: a population-based study in the pre- and post-rituximab era
Leukemia (2022)
-
Time trends in primary therapy and relative survival of diffuse large B-cell lymphoma by stage: a nationwide, population-based study in the Netherlands, 1989–2018
Blood Cancer Journal (2022)
-
Risk of second primary malignancies in patients with follicular lymphoma: a population-based study in the Netherlands, 1989-2018
Blood Cancer Journal (2021)