Abstract
Classical Philadelphia chromosome-negative myeloproliferative neoplasms (MPN) are a heterogeneous group of hematopoietic malignancies including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). The JAK2V617F mutation plays a central role in these disorders and can be found in 90% of PV and ~50–60% of ET and PMF. Hypoxia-inducible factor 1 (HIF-1) is a master transcriptional regulator of the response to decreased oxygen levels. We demonstrate the impact of pharmacological inhibition and shRNA-mediated knockdown (KD) of HIF-1α in JAK2V617F-positive cells. Inhibition of HIF-1 binding to hypoxia response elements (HREs) with echinomycin, verified by ChIP, impaired growth and survival by inducing apoptosis and cell cycle arrest in Jak2V617F-positive 32D cells, but not Jak2WT controls. Echinomycin selectively abrogated clonogenic growth of JAK2V617F cells and decreased growth, survival, and colony formation of bone marrow and peripheral blood mononuclear cells and iPS cell-derived progenitor cells from JAK2V617F-positive patients, while cells from healthy donors were unaffected. We identified HIF-1 target genes involved in the Warburg effect as a possible underlying mechanism, with increased expression of Pdk1, Glut1, and others. That was underlined by transcriptome analysis of primary patient samples. Collectively, our data show that HIF-1 is a new potential therapeutic target in JAK2V617F-positive MPN.
Similar content being viewed by others
Introduction
Classical Philadelphia chromosome-negative myeloproliferative neoplasms (MPN), comprising polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), are a heterogeneous group of diseases [1]. Three so-called “driver”-mutations have been identified in their pathogenesis, including JAK2V617F, CALR, and MPL mutations [2,3,4]. JAK2V617F mutations are found in over 90% of PV and ~50–60% of ET and PMF patients leading to constitutive activation of JAK2 driving cytokine-independent hyperproliferation and accumulation of mature myeloid cells in the bone marrow (BM) and peripheral blood (PB) [5]. The JAK1/2 tyrosine-kinase inhibitor ruxolitinib can reduce clinical symptoms and splenomegaly, but largely fails to induce molecular remission and is not selective for mutated JAK2, also inhibiting wild-type JAK2 (JAK2WT) [6]. Newer JAK1 and/or JAK2 inhibitors such as fedratinib, pacritinib, and other drugs have been used in clinical trials, but none has shown the potential to cure MPN patients [7]. New therapeutic strategies need to be developed, possibly based on the combination of JAK2 inhibitors with molecules targeting downstream targets or JAK2-independent pathways [8].
Hypoxia-inducible factors (HIFs) are transcription factors regulating the expression of a large variety of target genes implicated in proliferation, survival, chemoresistance, and metabolism [9]. In normoxia, the HIF-1α subunit is hydroxylyzed by prolyl-hydroxylases (PHDs) initiating von-Hippel–Lindau (VHL)-mediated HIF-α ubiquitination and proteasomal degradation [10, 11]. Under hypoxic conditions, PHD activity decreases, whereby the α-subunit is stabilized, dimerizes with HIF-1β, and bind to hypoxia response elements (HRE). In situ tissue analysis of BM revealed enhanced HIF-1α expression in hematopoietic stem cells (HSCs) regardless of their localization indicating a regulation by oxygen-independent mechanisms [12].
Gene knockout (KO) studies in mice have begun to unveil the functional significance of HIF-1α and HIF-2α in HSCs, but results are controversial. One group found that HIF-1α-deficient HSCs showed impaired repopulating capacity under serial transplantation assays, other groups showed no impact of Hif-1α deletion on HSCs in a conditional KO model, neither in steady state nor in serial transplantation [13, 14]. Furthermore, deletion of Hif-1α and Hif-2α did not show any effect on self-renewal capacity of HSCs [15].
Recent studies showed evidence that maintenance of leukemic stem cells (LSCs) is enhanced by the hypoxic microenvironment within the BM niche [16]. Constitutive expression of HIF-1α was reported in leukemic cell lines, even under normoxic conditions [17]. Significant impact of HIF-1α deletion has been shown in some acute myeloid leukemia (AML) models and in chronic myeloid leukemia (CML), where deletion of HIF-1α is crucial for LSC maintenance [18, 19]. The germline loss-of-function mutation VHLR200W, which leads to a constitutive stabilization of HIFs, is responsible for Chuvash polycythemia, a benign condition involving proliferation of red blood cells similar to MPN [20]. Stabilization of HIF-2α, caused by a gain-of-function germline mutation in the HIF-2α gene (EPAS1), leads to familial erythrocytosis and germline mutations of PHD2 induce erythrocytosis and elevated EPO levels [21, 22].
Gene expression analysis of granulocytes and CD34+ cells from JAK2V617F-positive patients demonstrated an upregulation of the PI3K/AKT pathway including mTOR, inducing translation of HIF-1α mRNA [23, 24]. In addition, JAK2V617F induced the accumulation of reactive oxygen species (ROS) in the HSC compartment in a Jak2V617F knock-in (KI) mouse model and in patients [25, 26]. ROS facilitate the stabilization of HIF-1α, even under normoxic conditions [27]. Ruxolitinib treatment inhibited cell growth and reduced the expression of HIF-1α and its target gene VEGF in JAK2V617F-positive HEL cells [28].
However, the role of HIFs in the maintenance and progression of JAK2V617F-positive MPN remains to be examined. We hypothesized that HIF signaling plays an important role in the maintenance of JAK2V617F-positive cells.
Materials and methods
Primary patient samples
Primary BM and blood samples from MPN patients were obtained from the Department of Hematology, Oncology, Hemostaseology, and Stem Cell Transplantation of the RWTH Aachen University in compliance with the local ethics committee (EK127/12) and blood samples from healthy donors were provided by the Department of Transfusion Medicine of the RWTH Aachen University (EK099/14), each after written informed consent. Mononuclear cells from BM and blood were isolated using density gradient centrifugation with Ficoll-Paque Premium (GE Healthcare, Chicago, Illinois, USA).
Cell lines
32D cells were purchased from the DSMZ and cultured in RPMI-1640 (Thermo Fisher Scientific, Waltham, Massachusetts, USA) supplemented with 10% FCS, 1% penicillin/streptomycin (Life technologies, Carlsbad, California, USA), and 10% WEHI3B supernatant, which served as an IL-3-source. Cultivation was performed under either normoxic or hypoxic (1% pO2) conditions at 37 °C with 5% CO2. For hypoxic experiments, a Tri-gas hypoxia chamber including hypoxic workspace was used.
Proliferation assay
32D cells were plated in triplicates in RPMI-1640 supplemented with 10% FCS, 1% penicillin/streptomycin. IL-3 (2 ng/ml; Immunotools) was added in experiments including Jak2WT-expressing cells. Cells were counted using a CASY-TTC cell-analyzer (OMNI Life Science, Bremen, Germany). Proliferation assays with primary human cells were performed in IMDM (Thermo Fisher Scientific) supplemented with 10% FCS, 1% penicillin/streptomycin, SCF (50 ng/ml), Flt3-L (50 ng/ml), IL-3 (10 ng/ml), and IL-6 (10 ng/ml; Immunotools, Friesoythe, Germany) at 37 °C and 5% CO2 and counted with a Neubauer hemocytometer.
Statistical analysis
Statistical evaluation of data and generation of graphs were performed with Prism 5 (GraphPad, San Diego, California, USA). For statistical hypothesis testing probability values were defined as *p < 0.05, **p < 0.01, ***p < 0.001, and data analyzed with t-test (two conditions) or ANOVA with Bonferroni posttest (≥3 conditions).
Additional methods (retroviral transduction, cell-cycle assays, apoptosis assays, methylcellulose colony formation assays, induced pluripotent stem (iPS) cells, RT-qPCR, cocultivation assays, SDS-PAGE, immunoblotting, MTT assays, DHE assays, ChIP assays, isolation of MNCs, CD34+ cell purification, and transcriptome analysis) including used reagents and formulas are described in the supplementary information (Supplementary Methods, Supplementary Tables 1–4).
Results
HIF-1α protein levels are elevated in Jak2V617F-expressing 32D cells
We retrovirally transduced the interleukin-3 (IL-3)-dependent murine BM cell line 32D with pMSCV-IRES-GFP-empty-vector (EV), pMSCV-IRES-GFP-Jak2WT, or pMSCV-IRES-GFP-Jak2V617F and sorted the GFP+ population. In contrast to EV- and Jak2WT-transduced cells, Jak2V617F-transduced cells showed IL-3-independent proliferation (Supplementary Fig. 1A). Upon cultivation under normoxic conditions, western blot (WB) analysis revealed higher HIF-1α protein levels in Jak2V617F compared with Jak2WT cells (Fig. 1a). We performed proliferation assays under hypoxic (1% pO2) and normoxic conditions and in accordance to earlier findings with the JAK2V617F-positive cell lines HEL and SET-2, we observed a hypoxia-induced inhibition of proliferation of Jak2V617F cells (Supplementary Fig. 1B). This results from the inhibition of SHP-2, which is indispensable for the autoactivation of JAK2V617F [29]. Concordantly, WB revealed a reduction of SHP-2, JAK2, and STAT1, but not STAT5, total and phosphorylated protein levels in our Jak2V617F cells (Supplementary Fig. 1C–E). Under normoxic conditions, HIF-1α protein was elevated in Jak2V617F cells (Supplementary Fig. 1F). Upon normoxic treatment with the HIF-1 inhibitor echinomycin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays revealed decreased mitochondrial activity of Jak2V617F but not Jak2WT cells at 1 nM. In contrast, no differences were detected under hypoxic conditions (Supplementary Fig. 2A, B).
HIF-1α protein levels and ROS levels in Jak2V617F-expressing 32D cells. a Protein levels of HIF-1α were studied in 32D Jak2WT or Jak2V617F-expressing cells after 16 h cultivation under normoxic conditions without IL-3 by western blot analysis using α-Tubulin as loading control (densitometric evaluation, n = 3). b Reactive oxygen species (ROS) levels in Jak2WT or Jak2V617F-expressing 32D cells were evaluated after ruxolitinib or DMSO treatment using a dihydro-ethidium (DHE) assay after 16 h of cultivation under normoxic conditions without IL-3 (n = 3). c Data of DHE assay from b. MFI mean fluorescence intensity (n = 3)
As ROS facilitate the stabilization of HIF-1α, we measured ROS levels using a dihydroethidium (DHE) assay [27, 30]. Basal ROS levels were elevated in Jak2V617F compared with Jak2WT cells (Fig. 1b, c). In accordance, findings from other groups demonstrated that Jak2V617F-induced accumulation of ROS in the HSC compartment [25]. Upon treatment with ruxolitinib, ROS levels decreased in Jak2V617F cells. Ruxolitinib decreased HIF-1α protein levels, demonstrating that JAK2V617F signaling is responsible for the accumulation of HIF-1α (Supplementary Fig. 2C). Nuclear factor erythroid 2-related factor 2 (Nrf2) is the master regulator of the response to oxidative stress by inducing the antioxidant machinery [31]. Activation of Nrf2, which is aberrantly activated in many cancers including AML, was shown to effectively reduce ROS [32]. We hypothesized, that treatment with the Nrf2 activator dimethyl fumarate (DMF) reduced ROS and thereby HIF-1α protein levels [33]. In line, decreased HIF-1α protein and ROS levels after DMF treatment were observed (Supplementary Fig. 2D). Furthermore, DMF reduced viability and induced apoptosis in Jak2V617F, but not Jak2WT cells (Supplementary Fig. 2E, F).
Based on the inhibition of JAK2V617F under hypoxic conditions and the JAK2V617F-mediated stabilization of HIF-1α under normoxic conditions, further experiments were performed under normoxic conditions.
Pharmacological inhibition of HIF-1 by echinomycin induced apoptosis and cell cycle arrest in Jak2V617F-expressing 32D cells
To determine the impact of HIF-1 inhibition, we treated 32D Jak2WT or Jak2V617F cells with low doses of the HIF-1 inhibitor echinomycin that intercalates with DNA at HRE, inhibiting binding of HIF-1, but not HIF-2 [34]. Treatment resulted in reduced growth of Jak2V617F cells, whereas Jak2WT cells were affected to a lesser extent (Fig. 2a). Viability was solely reduced in Jak2V617F cells (Supplementary Fig. 2G). We transduced 32D cells with pMSCV-Jak2WT-IRES-BFP and performed a competitive cocultivation assay with Jak2V617F/GFP- and Jak2WT/BFP-expressing cells. While Jak2V617F cells grew significantly better compared with Jak2WT in the DMSO controls, the percentage of Jak2V617F cells was strongly reduced upon treatment with echinomycin (Fig. 2b).
Pharmacological inhibition of HIF-1 in Jak2V617F-expressing 32D cells. a Effect of treatment with low doses of echinomycin (Ech., 1 nM) on cell number of Jak2WT- or Jak2V617F-expressing 32D cells was assessed by proliferation assays with 2 ng/ml IL-3 using a Casy cell counter (n = 3). b Competitive cocultivation assay of 32D Jak2WT (BFP+) and Jak2V617F (GFP+) expressing cells with 2 ng/ml IL-3. Cells of both cell lines (50,000 each) were combined and the percentage of the respective population was assessed using flow cytometry. Significances comparing Jak2WT (DMSO) vs. Jak2V617F (DMSO) and Jak2WT (echinomycin) vs. Jak2V617F (echinomycin) are indicated. c Apoptosis was measured using an Annexin-V and 7-AAD assay with flow cytometry. Cells were treated with echinomycin (1 nM) or DMSO for 24 h with IL-3 (2 ng/ml; n = 3). Apoptotic cells were defined as the sum of Annexin-V+/7-AAD− (early apoptotic) and Annexin-V+/7-AAD+ (late apoptotic) cells. d Cell cycle analysis of Jak2V617F-expressing cells after 24 h of treatment with echinomycin (1 nM) and DMSO controls with IL-3 (2 ng/ml). Cells were stained with an anti-BrdU antibody and 7-AAD and analyzed by flow cytometry. Gates in bivariate dot plots and 7-AAD histogram were defined according to the controls. Statistical analysis of cell cycle assay data was performed after fractionating cell populations into G0/G1, S, and G2/M phases according to gates in BrdU/7-AAD dot plots (Supplementary Fig. 3A, n = 3). e CFU assays of 200 Jak2WT- or Jak2V617F-expressing 32D cells/ml supplemented with echinomycin (1 nM) or DMSO as control and evaluated after 7 days (n = 3). f Chromatin immunoprecipitation (ChIP) assay of chromatin from Jak2V617F-expressing 32D cells after 4 h of treatment with echinomycin (1 nM) or DMSO controls. Chromatin-bound HIF-1α was immunoprecipitated with a HIF-1α targeting antibody. Binding of HIF-1 to HREs in the promoter region of the HIF-1 target genes Vegf, Aldoa, and Ldha was assessed by RT-qPCR (n = 3)
We observed an induction of apoptosis in Jak2V617F cells upon treatment with echinomycin, but not in Jak2WT cells (Fig. 2c). Jak2V617F showed enhanced apoptosis in the DMSO controls, which can be explained by oncogene induced replicative stress and ROS production [25, 35, 36]. Furthermore, we identified cell cycle arrest in G0/G1 phase (Fig. 2d, Supplementary Fig. 3A). This effect was only observed in Jak2V617F-expressing cells, but not Jak2WT controls. Using RT-qPCR, cell cycle arrest was verified by a strong upregulation of the negative cell cycle regulators p16Ink4a and p21Waf1/Cip1, which are involved in cell cycle arrest in the G1 phase (Supplementary Fig. 3B). To assess colony formation ability, Jak2WT and Jak2V617F 32D cells were plated in semisolid media with echinomycin. After 7 days of treatment, 98% less colonies had formed from Jak2V617F cells, while the effect on Jak2WT cells was far smaller (Fig. 2e).
Echinomycin treatment specifically inhibited binding of HIF-1α to HREs
To test whether echinomycin inhibited HIF-1 DNA binding, we performed chromatin immunoprecipitation (ChIP) with an antibody targeting HIF-1α followed by RT-qPCR of the promotor regions of the HIF-1 target genes vascular endothelial growth factor (Vegf), aldolase A (Aldoa), and lactate dehydrogenase A (Ldha) in Jak2V617F cells. ChIP-RT-qPCR data demonstrated that echinomycin significantly reduced binding of HIF-1α to HREs of all three genes (Fig. 2f). To show that these effects were specific, we performed RT-qPCR on the promoter regions of Stat3 and Cd44, which do not feature HREs in their promoter regions. No differences in the precipitation of these regions were detected (Supplementary Fig. 3C).
As ruxolitinib represents a standard in the treatment of MPN, we determined whether a combinational use of ruxolitinib and echinomycin produced synergistic effects. Jak2V617F cells were cultured with echinomycin (200 pM), ruxolitinib (100 nM), or both drugs. While both drugs alone reduced growth, their combination enhanced the effects in an additive fashion (Supplementary Fig. 3D).
Jak2V617F-mutated cells are characterized by a constitutive phosphorylation of STAT1/5 leading to higher proliferation [37, 38]. The antiproliferative effects of HIF-1α deprivation are unlikely to originate from interference with JAK2 signaling, as, in contrast to treatment with ruxolitinib, echinomycin did not reduce levels of pY-STAT1 and pY-STAT5 (Supplementary Fig. 3E).
HIF-1α KD impaired proliferation of Jak2V617F 32D cells by inducing apoptosis and cell cycle arrest
In addition, we undertook a genetic approach to test whether the impact of echinomycin on Jak2V617F-positive cells is caused by specific inhibition of HIF-1. We knocked down Hif-1α expression using specific shRNAs and analyzed Hif-1α mRNA and HIF-1α protein levels revealing a knockdown (KD) efficiency of 70% (Supplementary Fig. 4A, B). In contrast to our pharmacological approach, no differences in growth between shRNA-transduced and control cells were observed (Supplementary Fig. 4C). However, several groups reported that suppression of one HIF-α subunit induces a compensatory increase of the other subunit [39, 40]. We analyzed HIF-2α protein levels, revealing elevated levels in shHIF-1α-expressing cells compared with scrambled controls (Supplementary Fig. 4D). In contrast to echinomycin treated cells, in which HIF-1 is fully inhibited upon start of treatment, HIF-1α is downregulated by 70% in the shHIF-1α clones. We hypothesized that remaining HIF-1α protein levels together with compensatory HIF-2α upregulation rescue the phenotype.
In accordance with this hypothesis, the growth of shHIF-1α cells, but not scrambled controls, was notably decreased after treatment with HIF-2 antagonist 2 (H2A2; Sigma; Fig. 3a) [41] and apoptosis was induced in shHIF-1α Jak2V617F cells (Fig. 3b). In shHIF-1α Jak2WT cells, apoptosis was induced only marginally (Supplementary Fig. 4E). Moreover, p16Ink4a expression was remarkably induced in the shHIF-1α Jak2V617F-positive cells (Fig. 3c). Taken together, these data corroborate our data on pharmacologic HIF-1 inhibition.
Effect of HIF-1α knockdown on Jak2V617F-expressing 32D cells. a Proliferation assay of 32D Jak2V617F-expressing cells transduced with shHIF-1α or scrambled controls in combination with HIF-2 antagonist 2 (H2A2; 10 µM; n = 3). b Apoptosis was measured using Annexin-V-APC and 7-AAD assay via flow cytometry. Cells were treated with H2A2 for 24 h (10 µM; n = 3). Apoptotic cells were defined as the sum of Annexin-V+/7-AAD− (early apoptotic) and Annexin-V+/7-AAD+ (late apoptotic) cells. c mRNA levels of negative cell cycle regulator p16Ink4a in Jak2V617F-positive 32D cells expressing either shHIF-1α shRNA or scrambled control after 24 h of treatment with H2A2 (10 µM; n = 3)
Silencing HIF-1α reduced the sensitivity of the cells to echinomycin, further supporting the specificity of the drug (Supplementary Fig. 4F). We measured apoptosis without H2A2 and observed higher levels in shHIF-1α than scrambled control cells. However, the effect was marginal compared with experiments including H2A2 (Supplementary Fig. 4G). To test the impact of HIF-2 inhibition alone, Jak2V617F or Jak2WT cells without KD of HIF-1α were subjected to MTT assays with H2A2 under normoxic and hypoxic conditions. No effect was observed, suggesting that targeting HIF-2α is effective only in the case of its compensatory upregulation induced by HIF-1α ablation (Supplementary Fig. 5A). ChIP experiments demonstrated that KD of HIF-1α impaired HIF-1 binding to HREs in the promoter regions of Aldoa and Ldha (Supplementary Fig. 5B), whereas no enrichment of Stat3 and Cd44 was detected (Supplementary Fig. 5C). We tested, whether HIF-1 inhibition with echinomycin induced HIF-2α in a similar compensatory manner. While HIF-2α protein was detectable and gradually increasing between 48 and 96 h of cultivation up to a level of shHIF-1α transduced cells, no increase by echinomycin was found (Supplementary Fig. 5D). In the opposite, HIF-2α protein levels were higher in the control cells after 24 and 72 h, presumably by insufficient oxygenation of the growth media due to higher proliferation.
Echinomycin reduced cell number and viability in BMMCs, PBMCs, and iPS cell-derived progenitor cells from JAK2V617F-positive MPN patients
To examine the effectiveness of HIF-1 inhibition by echinomycin on primary cells from JAK2V617F-positive MPN patients (Table 1 and Supplementary Table 5), BM and PB mononuclear cells (BMMCs/PBMCs) were treated. Cell number and viability of BMMCs and PBMCs from JAK2V617F-positive MPN patients were decreased in a dose dependent manner (Fig. 4). In contrast, no impact of echinomycin was observed on G-CSF mobilized CD34+ cells from healthy controls (HCs). Accordingly, no detrimental effect of echinomycin was observed on PBMCs from untreated HCs (Supplementary Fig. 6A, B). PMF patient cells showed a stronger response to echinomycin than those from PV patients (Supplementary Fig. 6C). We observed a correlation between the viability reduction and allele burden (p = 0.028, R2 = 0.512, Supplementary Fig. 6D). To investigate whether echinomycin impaired JAK2V617F-negative MPN cells, we treated BMMCs from three ET patients (two triple-negative, one MPLW515L). In all samples, cell number and viability were reduced by treatment (Supplementary Fig. 6E).
HIF-1 inhibition with echinomycin reduced growth and cell survival in mononuclear cells from JAK2V617F-positive MPN patients. Effect of echinomycin (Ech., 1 and 5 nM) or DMSO on cell count and viability of bone marrow mononuclear cells (BMMCs) from eight JAK2V617F-positive MPN patients (four PV, three PMF, one preMF), peripheral blood mononuclear cells (PBMCs) from eight JAK2V617F-positive MPN patients (seven PV, one PMF) and G-CSF mobilized CD34+ cells from three healthy donors. Cell number and viability were examined by trypan blue staining over 10 days after an initial treatment with the indicated concentrations. Data points were nudged to increase readability. Patient data are given in Table 1 and Supplementary Table 5
In colony formation assays, G-CSF mobilized CD34+ cells from healthy donors showed an increased colony formation after echinomycin treatment, in contrast to ruxolitinib treatment. Instead, echinomycin exhibited inhibitory effects on colony formation on cells from JAK2V617F-positive patients. In the combinational treatment, echinomycin enhanced the effect of ruxolitinib in patients. In contrast, the detrimental effect of ruxolitinib on healthy donor cells was partially reversed by echinomycin treatment. As echinomycin did not impair colony formation of CD34+ HC cells underlining the nontoxicity of the drug at these concentrations, we tested the impact on CD34+ enriched MPN patient cells as well resulting in lower colony formation (Supplementary Fig. 6F). These findings further underline the specificity of echinomycin on JAK2V617F mutant cells.
To further extend our data on primary patient samples, we treated isogenic iPS cell-derived progenitor cells from JAK2V617F-positive PV patients carrying JAK2WT and/or JAK2V617F mutation. We observed decreases in the viable cell number of JAK2V617F mutated cells by echinomycin, but not JAK2WT cells (Fig. 5b). The growth advantage of JAK2V617F cells compared with JAK2WT cells upon treatment with DMSO might be caused by oncogene signaling. Investigation of apoptosis uncovered a prominent increase of apoptosis in JAK2V617F-mutated, but not JAK2WT cells (Fig. 5c). Thus, HIF-1 is required for the survival of patient-derived cells harboring the JAK2V617F mutation.
HIF-1 inhibition with echinomycin impaired colony formation of primary cells from MPN patients and reduced cell number and viability of iPS cell-derived progenitor cells from JAK2V617F-positive MPN patients. a CFU assays of PBMCs and BMMCs from JAK2V617F-positive MPN patients or G-CSF mobilized CD34+ cells from healthy donors supplemented with echinomycin (2 nM), ruxolitinib (200 nM), or both drugs combined (n = 3). CFU assays were performed with 20,000 BMMCs/ml or 106 PBMCs or 1000 G-CSF mobilized CD34+ cells after Ficoll density gradient centrifugation in presence of SCF, IL-3, GM-CSF, and EPO. CFUs were assessed after 7 days of treatment. b Proliferation assay of JAK2WT/WT (n = 2), JAK2WT/V617F (n = 3), or JAK2V617F/V617F (n = 2) iPS cell-derived progenitor cells from JAK2V617F-positive PV patients after initial treatment with echinomycin (Ech., 1 nM) or DMSO. c Apoptosis assay of JAK2WT/V617F or JAK2V617F/V617F mutated iPS cell-derived progenitor cells from a JAK2V617F-positive PV patient after 9 days of treatment with echinomycin (Ech., 1 or 5 nM) or DMSO using AnnexinV-APC/7-AAD (data of one experiment shown). Cells were analyzed in triplicates using flow cytometry
HIF-1 signaling regulates genes implicated in aerobic glycolysis
In order to determine the molecular mechanisms underlying HIF-1α inhibition or KD, we examined the potential involvement of deregulated cellular energetics. Proliferating cells do not only require energy, but also metabolic intermediate products that allow the generation of nucleotides, amino acids, and fatty acids in an effort to increase biomass and assemble new cells [42]. While the breakdown of glucose by glycolysis, citric acid cycle and oxidative phosphorylation yields a high amount of energy, no intermediates are generated. In contrast, the switch to enhanced glycolysis yields less energy, but also glycolytic intermediates, serving as a substrate for several anabolic reactions. A large majority of cancers are characterized by an increased rate of glycolysis with an attenuation of oxidative phosphorylation [43]. This “Warburg effect” is mainly mediated via HIF-1 by the upregulation of lactate dehydrogenase A (LDHA), glucose transporters (such as GLUT1 or GLUT3), phosphofructokinase/fructose-bisphosphatase 3 (PFKFB3), and pyruvate dehydrogenase kinase 1 (PDK1). While LDHA catalyzes the conversion of pyruvate to lactate to regenerate NAD+, which is essential to maintain glycolysis, GLUT1 and GLUT3 facilitate glucose uptake into the cell. PDK1 phosphorylates pyruvate dehydrogenase, thereby inhibiting the conversion of pyruvate to acetyl-CoA and shunting the citric acid cycle. PFKFB3 enhances aerobic glycolysis by activation of phosphofructokinase (PFK) and is a regulator of cyclin-dependent kinase 1, underlining its importance for cell proliferation and survival of cancer cells [44]. We analyzed the expression of these genes and found elevated levels of Pdk1, Ldha, Pfkfb3, Glut1, and Glut3 in Jak2V617F cells compared with Jak2WT cells, which were decreased by treatment with echinomycin (Fig. 6a). In HIF-1α KD cells, Pdk1, Ldha, Glut1, and Glut3 were significantly downregulated upon additional inhibition of HIF-2 (Fig. 6b). In contrast, the expression of Glut10 was increased, showing that HIF-1 inhibition or KD did not lead to a general decrease in glucose transporter expression (Supplementary Fig. 6G).
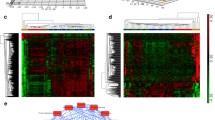
HIF-1 target genes implicated in inducing the metabolic switch to aerobic glycolysis are differentially regulated in Jak2V617F cells. mRNA levels of HIF-1 target genes Pdk1, Ldha, Pfkfb3, Glut1, and Glut3 in a 32D Jak2WT or Jak2V617F cells after 4 h of treatment with echinomycin (Ech., 1 nM; n = 3) or b 32D Jak2V617F cells expressing either shHIF-1α shRNA or scrambled control after 24 h of treatment with H2A2 (10 µM; n = 3). c Heat map for unsupervised hierarchical clustering of HIF-1 target genes in the CD34+ population of JAK2V617F vs. healthy controls (n = 19 vs. 6, respectively). Genes were selected according to ChIP-Seq analysis by Schödel et al. showing a peak height of >40 (Supplementary Methods)
We performed microarray gene expression analysis of HIF-1 target genes using CD34+ BMMCs and PBMCs cells derived from 19 JAK2V617F positive MPN patients (two ET, two pPV-MF, six PMF, and nine PV) vs. 6 HCs. We found that several HIF-1 target genes including genes that are involved in glucose metabolism, such as SLC2A3 (GLUT3), PFKFB3, PFKFB4, ALDOC, FUT11, RDH11, and MAX were upregulated in JAK2V617F positive MPN patients (Fig. 6c) [45,46,47]. Together, our data suggest that HIF-1 signaling is involved in the regulation of genes responsible for the metabolic switch to aerobic glycolysis.
Discussion
In this report, we investigated the requirement of HIF-1 in JAK2V617F-positive MPN. In line with findings from another group, we showed that JAK2V617F signaling is suppressed under hypoxic conditions (1% O2) [29]. However, we are the first to demonstrate that HIF-1α protein levels are elevated in Jak2V617F-positive 32D cells under normoxic conditions and reduced by ruxolitinib treatment. Albeit normoxic conditions are not physiologic, making the in vivo translation difficult, severe hypoxia of 1% O2, and below is only obtained in the most hypoxic regions of the BM. Several groups reported that oncogenes are able to stabilize HIF-1α in an oxygen-independent manner [48, 49]. While under normoxic conditions, HIF-1α is difficult to detect in Jak2WT cells (the faint band might be explained by residual IL-3), high protein levels were found in cells expressing Jak2V617F in association with elevated ROS levels, corresponding to findings in a murine Jak2V617F KI model [25, 50]. ROS are postulated to facilitate the stabilization of HIF-1α protein [27]. Treatment with the antioxidant Nrf2 activator DMF reduced HIF-1α protein levels indicating that HIF-1α is induced via ROS. Further, DMF reduced viability and induced apoptosis in Jak2V617F cells.
Targeting HIF-1 is emerging as a therapeutic strategy in a variety of malignancies. Besides newer small molecules, some drugs that are already approved for other diseases show HIF-1 inhibitory activity, like the cardiac glycosides (e.g., digoxin) and the proteasome inhibitor bortezomib, or stabilize HIF-1α through nitric oxide increase by molsidomine [51,52,53]. We tested the impact of the peptide antibiotic echinomycin that specifically inhibits HIF-1 and is evaluated in preclinical stage in AML [18, 54]. Interestingly, pharmacological inhibition of HIF-1 with echinomycin, strongly reduced proliferation specifically of 32D Jak2V617F cells at low doses of 1 nM [18, 54]. In contrast to ruxolitinib treatment, echinomycin did not lead to a decrease in pSTAT1 and pSTAT5 levels, which indicates a different and new potential therapeutic strategy.
In addition to that, we uncovered the induction of apoptosis in Jak2V617F, but not Jak2WT cells. Accordingly, Hif-1α KO induced apoptosis in BCR-ABL transduced LSK cells in CML [19]. Further, we encountered a cell cycle arrest in G0/G1 phase, accompanied by the upregulation of negative cell cycle regulators p16Ink4a and p21Kip1/Waf1. This effect was also reported in echinomycin-treated CML cells characterized by the exhaustion of LSCs and their incapability to reconstitute the disease upon serial transplantation [19]. Both cyclin-dependent kinase inhibitors constitute markers of senescence and are involved in the regulation of the critical G1 to S phase transition [13, 55]. Hif-1α deficient LSK cells exhibit elevated levels of p16Ink4a and KD of HIF-1α promoted the expression of p21Kip1/Waf1 in mesenchymal stem cells [13, 56]. These findings suggest an essential role of HIF signaling in JAK2V617F-positive MPNs.
In accordance with echinomycin treatment, KD of Hif-1α in combination with HIF-2 inhibition resulted in diminished growth. The growth-inhibitory effect of the KD was attenuated compared with the effect of echinomycin treatment, which can be explained with the KD efficiency resulting in ~30% functional protein. Consistent with the findings from echinomycin treatments, induction of apoptosis and p16Ink4a mRNA levels were observed in the shHIF-1α KD cells. While HIF-2 inhibition alone did affect neither Jak2WT nor Jak2V617F cells, inhibition of HIF-2 was required to observe the effects of Hif-1α KD due to the constitutive compensatory upregulation of HIF-2α [39, 40]. Without inhibition of HIF-2 an increase of apoptosis was observed, but to a minor extent compared with H2A2 treated cells, suggesting that the effects of HIF-1α KD are mainly compensated by HIF-2α.
We were able to demonstrate that echinomycin and KD of Hif-1α strongly interfered with binding of HIF-1α to the promoter regions of HIF-1 target genes, featuring HREs. In contrast, no enrichment of the control genes Cd44 and Stat3 was encountered. These findings indicate that echinomycin exerts its effects by the prevention of HIF-1α binding to HREs. In contrast to scrambled controls, HIF-1α KD cells lost their susceptibility to echinomycin suggesting that the effects induced by echinomycin treatment are not driven by off-target effects, but specifically by HIF-1 signaling, as HIF-1α expression is required for the sensitivity to the drug [18].
In a translational approach, we tested the impact of echinomycin on growth and viability of mononuclear cells from JAK2V617F-positive MPN patients. Echinomycin was well tolerated by HCs, whereas growth and viability of patient cells was significantly reduced. As normal PBMCs constitute a large percentage of cells, which are fully differentiated and are unable to proliferate or sustain, we included G-CSF mobilized CD34+ HC cells in addition to normal PBMCs. We further investigated the self-renewal capacity of these cells. Ruxolitinib impaired colony formation in patient and control cells, presumably by impeding cytokine-signaling through inhibition of both, JAK2WT and JAK2V617F cells. In contrast, low dose treatment with echinomycin reduced colony formation in JAK2V617F positive patient cells only, whereas colony formation of CD34+ cells from healthy donors was even increased. The combination of both inhibitors led to an enhanced impairment of colony formation in patient cells, whereas the ruxolitinib-induced defective colony formation was partially reversed by combination with echinomycin. These findings indicate the potential of echinomycin, alone and in combination with ruxolitinib, to selectively target JAK2V617F positive cells. Despite the ruxolitinib-induced reduction of HIF-1α protein levels, our data from 32D cells and primary patients indicate, that JAK2V617F-positive cells are addicted to remaining HIF-1α protein suggesting the efficacy of combinational targeting of JAK2 and HIF-1.
The eradication of LSCs without impacting self-renewal and differentiation of normal HSCs was demonstrated for echinomycin in a murine model of relapsed AML [54]. Patient and disease specific iPS cells represent an excellent means for drug screening, since isogenic cells with and without JAK2V617F mutation are readily obtained from the very same patient. In accordance with primary patient samples, echinomycin treatment of iPS cell-derived progenitors from JAK2V617F-positive PV patients resulted in a differential effect on JAK2V617F-positive vs. -negative cells. The selective targeting of malignant cells is a crucial factor in the reduction of side effects during therapy and potential eradication of the malignant clone. Treatment of JAK2V617F 32D cells, primary patient BMMCs/PBMCs and iPS-cell derived progenitors demonstrated that pharmacological inhibition of HIF-1 exerted specific effects for cells bearing the JAK2V617F mutation.
To examine the underlying mechanisms upon HIF-1 inhibition or KD, we investigated the metabolic reprogramming to aerobic glycolysis. Elevated protein levels of p-PDK1 were observed in several leukemic cell lines, including JAK2V617F positive HEL cells and inhibition of JAK2 with AG490 reduced phosphorylation of PDK1 [57]. MPN patients are characterized by increased LDH serum levels, which are predictive for reduced survival [58]. Another group reported the upregulation of PFKFB3 in JAK2V617F positive cells [59]. Further, they observed a strong growth reduction of these cells upon treatment with a PFKFB3 inhibitor and KD blocked in vivo tumor formation in mice. We identified elevated mRNA levels of Pdk1, Ldha, Pfkfb3, Glut1, and Glut3 in Jak2V617F expressing cells, which were decreased by echinomycin treatment. In addition to that, Pdk1, Ldha, Glut1, and Glut3 were significantly downregulated by HIF-1α KD after H2A2 treatment. We performed microarray analysis of HIF-1 target genes in MPN patient derived CD34+ cells and observed the upregulation of several genes involved in metabolic reprogramming, corroborating our cell line results. Although mechanistic proof is missing our correlations are strengthened by just recently published data from Nageswara Rao et al. who have shown the importance of metabolic changes in Jak2V617F-positive mice [60]. They observed elevated expression levels of Hif-1α and several HIF-1 target genes including Pdk1, Glut1, Glut3, Max, Aldoa, and Pfkfb3 in Jak2V617F positive mice corroborating our transcriptomic analysis of human CD34+ cells from JAK2V617F-positive MPN patients. Inhibition of glycolysis with a PFKFB3 inhibitor induced apoptosis and attenuated proliferation in several human JAK2V617F-positive cell lines suggesting a potential mechanism [60]. We hypothesize that enhanced proliferation, accompanied by the addiction to certain fuel sources, might render JAK2V617F-positive cells vulnerable to HIF-1 inhibition or depletion. Further experiments unraveling the impact of metabolic changes in respect of the differential regulation of HIF-1 in MPN are of high interest and planned for the future.
Collectively, our data provide evidence that HIF-1 is required for JAK2V617F-positive MPN cell survival and indicate that targeting HIF-1 represents a novel therapeutic approach in classical Philadelphia-chromosome-negative MPN. Our work provides a foundation for further studies, such as in vivo experiments, the investigation of other HIF-1 inhibitors and target genes and the impact of HIF-1 in CALR and MPL mutated MPN.
References
Spivak JL. The chronic myeloproliferative disorders: clonality and clinical heterogeneity. Semin Hematol. 2004;41 2 Suppl 3:1–5.
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90.
Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270.
Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–90.
Tefferi A, Lasho TL, Gilliland G. JAK2 mutations in myeloproliferative disorders. N Engl J Med. 2005;353:1416–7. author reply 1416–7.
Bose P, Verstovsek S. JAK2 inhibitors for myeloproliferative neoplasms: what is next? Blood. 2017;130:115–25.
Mesa RA, Vannucchi AM, Mead A, Egyed M, Szoke A, Suvorov A, et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial. Lancet Haematol. 2017;4:e225–36.
Vainchenker W, Leroy E, Gilles L, Marty C, Plo I, Constantinescu SN. JAK inhibitors for the treatment of myeloproliferative neoplasms and other disorders. F1000Res. 2018;7:82.
Graham AM, Presnell JS. Hypoxia Inducible Factor (HIF) transcription factor family expansion, diversification, divergence and selection in eukaryotes. PLoS ONE. 2017;12:e0179545.
Kaelin WG Jr., Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402.
Gezer D, Vukovic M, Soga T, Pollard PJ, Kranc KR. Concise review: genetic dissection of hypoxia signaling pathways in normal and leukemic stem cells. Stem Cells. 2014;32:1390–7.
Nombela-Arrieta C, Pivarnik G, Winkel B, Canty KJ, Mahoney JE, Park S-Y, et al. Quantitative imaging of hematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol. 2013;15:533–43.
Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402.
Vukovic M, Sepulveda C, Subramani C, Guitart AV, Mohr J, Allen L, et al. Adult hematopoietic stem cells lacking Hif-1alpha self-renew normally. Blood. 2016;127:2841–6.
Guitart AV, Subramani C, Armesilla-Diaz A, Smith G, Sepulveda C, Gezer D, et al. Hif-2α is not essential for cell-autonomous hematopoietic stem cell maintenance. Blood. 2013;122:1741–5.
Zhou H-S, Carter BZ, Andreeff M. Bone marrow niche-mediated survival of leukemia stem cells in acute myeloid leukemia: Yin and Yang. Cancer Biol Med. 2016;13:248–59.
Wellmann S, Guschmann M, Griethe W, Eckert C, Stackelberg A, Lottaz C, et al. Activation of the HIF pathway in childhood ALL, prognostic implications of VEGF. Leukemia. 2004;18:926–33.
Wang Y, Liu Y, Malek SN, Zheng P, Liu Y. Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem Cell. 2011;8:399–411.
Zhang H, Li H, Xi HS, Li S. HIF1α is required for survival maintenance of chronic myeloid leukemia stem cells. Blood. 2012;119:2595–607.
Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, et al. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32:614–21.
Percy MJ, Furlow PW, Lucas GS, Li X, Lappin TR, McMullin MF, et al. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med. 2008;358:162–8.
Gardie B, Percy MJ, Hoogewijs D, Chowdhury R, Bento C, Arsenault PR, et al. The role of PHD2 mutations in the pathogenesis of erythrocytosis. Hypoxia. 2014;2:71–90.
Čokić VP, Mossuz P, Han J, Socoro N, Beleslin-Čokić BB, Mitrović O, et al. Microarray and proteomic analyses of myeloproliferative neoplasms with a highlight on the mTOR signaling pathway. PLoS ONE. 2015;10:1–23.
Harada H, Itasaka S, Kizaka-Kondoh S, Shibuya K, Morinibu A, Shinomiya K, et al. The Akt/mTOR pathway assures the synthesis of HIF-1alpha protein in a glucose- and reoxygenation-dependent manner in irradiated tumors. J Biol Chem. 2009;284:5332–42.
Marty C, Lacout C, Droin N, Le Couédic JP, Ribrag V, Solary E, et al. A role for reactive oxygen species in JAK2 V617F myeloproliferative neoplasm progression. Leukemia. 2013;27:2187–95.
Vener C, Novembrino C, Bamonti Catena F, Fracchiolla NS, Gianelli U, Savi F, et al. Oxidative stress is increased in primary and post-polycythemia vera myelofibrosis. Exp Hematol. 2010;38:1058–65.
Jung SN, Yang WK, Kim J, Kim HS, Kim EJ, Yun H, et al. Reactive oxygen species stabilize hypoxia-inducible factor-1 alpha protein and stimulate transcriptional activity via AMP-activated protein kinase in DU145 human prostate cancer cells. Carcinogenesis. 2008;29:713–21.
Xu Q, Liu GM, Wang FY, Zhang LJ, Liang WT, Cheng ZY. The effect of ruxolitinib on the expression of VEGF and HIF-1 alpha in leukemia HEL cells. Sichuan Da Xue Xue Bao Yi Xue Ban. 2016;47:669–73.
Mitsumori T, Nozaki Y, Kawashima I, Yamamoto T, Shobu Y, Nakajima K, et al. Hypoxia inhibits JAK2V617F activation via suppression of SHP-2 function in myeloproliferative neoplasm cells. Exp Hematol. 2014;42:783–92 e781.
Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, et al. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–94.
Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–107.
Zhang J, Su L, Ye Q, Zhang S, Kung H, Jiang F, et al. Discovery of a novel Nrf2 inhibitor that induces apoptosis of human acute myeloid leukemia cells. Oncotarget. 2017;8:7625–36.
Yamaguchi Y, Kanzaki H, Katsumata Y, Itohiya K, Fukaya S, Miyamoto Y, et al. Dimethyl fumarate inhibits osteoclasts via attenuation of reactive oxygen species signalling by augmented antioxidation. J Cell Mol Med. 2018;22:1138–47.
Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, et al. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005;65:9047–55.
Machado-Neto JA, Traina F. Reactive oxygen species overload promotes apoptosis in JAK2V617F-positive cell lines. Rev Bras Hematol Hemoter. 2016;38:179–81.
Ahn JS, Li J, Chen E, Kent DG, Park HJ, Green aR. JAK2V617F mediates resistance to DNA damage-induced apoptosis by modulating FOXO3A localization and Bcl-xL deamidation. Oncogene. 2016;35:2235–46.
Chen E, Beer PA, Godfrey AL, Ortmann CA, Li J, Costa-Pereira AP, et al. Distinct clinical phenotypes associated with JAK2V617F reflect differential STAT1 signaling. Cancer Cell. 2010;18:524–35.
Oku S, Takenaka K, Kuriyama T, Shide K, Kumano T, Kikushige Y, et al. JAK2 V617F uses distinct signalling pathways to induce cell proliferation and neutrophil activation. Br J Haematol. 2010;150:334–44.
Menrad H, Werno C, Schmid T, Copanaki E, Deller T, Dehne N, et al. Roles of hypoxia-inducible factor-1α (HIF-1α) versus HIF-2α in the survival of hepatocellular tumor spheroids. Hepatology. 2010;51:2183–92.
Schulz K, Milke L, Rübsamen D, Menrad H, Schmid T, Brüne B. HIF-1α protein is upregulated in HIF-2α depleted cells via enhanced translation. FEBS Lett. 2012;586:1652–7.
Scheuermann TH, Li Q, Ma H-W, Key J, Zhang L, Chen R, et al. Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat Chem Biol. 2013;9:271–6.
Nagel R, Semenova EA, Berns A. Drugging the addict: non-oncogene addiction as a target for cancer therapy. EMBO Rep. 2016;17:1516–31.
Peng G, Liu Y. Hypoxia-inducible factors in cancer stem cells and inflammation. Trends Pharmacol Sci. 2015;36:374–83.
Lu L, Chen Y, Zhu Y. The molecular basis of targeting PFKFB3 as a therapeutic strategy against cancer. Oncotarget. 2017;8:62793–802.
Amati B, Land H. Myc-Max-Mad: a transcription factor network controlling cell cycle progression, differentiation and death. Curr Opin Genet Dev. 1994;4:102–8.
Ruiz A, Dror E, Handschin C, Furrer R, Perez-Schindler J, Bachmann C, et al. Over-expression of a retinol dehydrogenase (SRP35/DHRS7C) in skeletal muscle activates mTORC2, enhances glucose metabolism and muscle performance. Sci Rep. 2018;8:636.
Kucharzewska P, Christianson HC, Belting M. Global profiling of metabolic adaptation to hypoxic stress in human glioblastoma cells. PLoS ONE. 2015;10:e0116740.
Iommarini L, Porcelli AM, Gasparre G, Kurelac I. Non-canonical mechanisms regulating hypoxia-inducible factor 1 alpha in cancer. Front Oncol. 2017;7:286.
Masoud GN, Li W. HIF-1?? pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378–89.
Marty C, Lacout C, Martin A, Hasan S, Jacquot S, Birling MC, et al. Myeloproliferative neoplasm induced by constitutive expression of JAK2V617F in knock-in mice. Blood. 2010;116:783–7.
Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408.
Abd-Aziz N, Stanbridge EJ, Shafee N. Bortezomib attenuates HIF-1- but not HIF-2-mediated transcriptional activation. Oncol Lett. 2015;10:2192–6.
Dokucu AI, Ozturk H, Ozturk H, Tuncer MC, Yilmaz F. The effects of molsidomine on hypoxia inducible factor alpha and Sonic hedgehog in testicular ischemia/reperfusion injury in rats. Int Urol Nephrol. 2009;41:101–8.
Wang Y, Liu Y, Tang F, Bernot KM, Schore R, Marcucci G, et al. Echinomycin protects mice against relapsed acute myeloid leukemia without adverse effect on hematopoietic stem cells. Blood. 2014;124:1127–35.
Romanov VS, Abramova MV, Svetlikova SB, Bykova TV, Zubova SG, Aksenov ND, et al. p21(Waf1) is required for cellular senescence but not for cell cycle arrest induced by the HDAC inhibitor sodium butyrate. Cell Cycle. 2010;9:3945–55.
Lv B, Li F, Fang J, Xu L, Sun C, Han J, et al. Hypoxia inducible factor 1alpha promotes survival of mesenchymal stem cells under hypoxia. Am J Transl Res. 2017;9:1521–9.
Hitosugi T, Fan J, Chung TW, Lythgoe K, Wang X, Xie J, et al. Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism. Mol Cell. 2011;44:864–77.
Shah S, Mudireddy M, Hanson CA, Ketterling RP, Gangat N, Pardanani A, et al. Marked elevation of serum lactate dehydrogenase in primary myelofibrosis: clinical and prognostic correlates. Blood Cancer J. 2017;7:657.
Reddy MM, Fernandes MS, Deshpande A, Weisberg E, Inguilizian HV, Abdel-Wahab O, et al. The JAK2V617F oncogene requires expression of inducible phosphofructokinase/fructose-bisphosphatase 3 for cell growth and increased metabolic activity. Leukemia. 2012;26:481–9.
Nageswara Rao T, Hansen N, Hilfiker J, Rai S, Majewska JM, Lekovic D, et al. JAK2 mutant hematopoietic cells display metabolic alterations that can be targeted to treat myeloproliferative neoplasms. Blood. 2019. https://doi.org/10.1182/blood.2019000162.
Acknowledgements
This work was supported by the Genomics Facility, a core facility of the Interdisciplinary Center for Clinical Research (IZKF) Aachen within the Faculty of Medicine at RWTH Aachen University. This work was supported by the Core Facility Flow Cytometry, a Core Facility of the Interdisciplinary Center for Clinical Research (IZKF) Aachen within the Faculty of Medicine at RWTH Aachen University. This study was supported by a research grant from the German Research Foundation (DFG KO2155/6–1) to SK, from the Interdisciplinary Centre for Clinical Research within the faculty of Medicine at the RWTH Aachen University (O3–3) and by START grant by the Faculty of Medicine in Aachen to DG. Biomaterial samples were provided by the RWTH centralized Biomaterial Bank Aachen (RWTH cBMB, Aachen, Germany) in accordance with the regulations of the biomaterial bank and the approval of the ethics committee of the medical faculty, RWTH Aachen. The Kranc laboratory is funded by Cancer Research UK (Senior Fellowship and Programme Grant), Medical Research Council, The Barts Charity, Bloodwise, and the Kay Kendall Leukaemia Fund. The pMSCV-IRES-GFP plasmids containing Jak2WT or Jak2V617F were a kind gift from the lab of Dr. Gary Gilliland. Parts of this work were part of the PhD thesis of JB and the bachelor’s thesis of AH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SK reports advisory board activity for Pfizer, Incyte/Ariad, Novartis, AOP Pharma, BMS, CTI, Roche, Baxalta, Sanofi, honoraria from Novartis, BMS, Pfizer, Incyte/Ariad, Shire, Roche, AOP Pharma, Janssen, research funding from Novartis Foundation, BMS, Novartis, and other financial disclosures (i.e., travel support) from Alexion, Novartis, BMS, Incyte/Ariad, AOP Pharma, Baxalta, CTI, Pfizer, Sanofi, Celgene, Shire, and Janssen. THB reports consultancy from Pfizer, Novartis, Janssen, Merck, Incyte/Ariad and research funding from Pfizer and Novartis. DG reports advisory board activity for AMGEN. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Baumeister, J., Chatain, N., Hubrich, A. et al. Hypoxia-inducible factor 1 (HIF-1) is a new therapeutic target in JAK2V617F-positive myeloproliferative neoplasms. Leukemia 34, 1062–1074 (2020). https://doi.org/10.1038/s41375-019-0629-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0629-z
- Springer Nature Limited
This article is cited by
-
Novel cancer treatment paradigm targeting hypoxia-induced factor in conjunction with current therapies to overcome resistance
Journal of Experimental & Clinical Cancer Research (2023)
-
MAPK14 over-expression is a transcriptomic feature of polycythemia vera and correlates with adverse clinical outcomes
Journal of Translational Medicine (2021)
-
Dnmt3a is downregulated by Stat5a and mediates G0/G1 arrest by suppressing the miR-17-5p/Cdkn1a axis in Jak2V617F cells
BMC Cancer (2021)
-
The crosstalk between HIFs and mitochondrial dysfunctions in cancer development
Cell Death & Disease (2021)
-
Early and late stage MPN patients show distinct gene expression profiles in CD34+ cells
Annals of Hematology (2021)