Abstract
Obinutuzumab (GA101) and ibrutinib show excellent efficacy for treatment of chronic lymphocytic leukemia (CLL). Preclinical investigations and a complementary safety profile were in support of testing their combined use. The exploratory CLL2-BIG-trial evaluated a sequential combination therapy following a recently proposed strategy. Two courses of bendamustine were used for debulking in patients with a high tumor load, followed by six courses of induction therapy with ibrutinib and GA101, followed by an MRD-triggered maintenance phase. The results of a pre-planned analysis at the end of the induction phase are presented. 61 patients were included, 30 previously untreated and 31 with relapsed/refractory CLL. 44 patients received bendamustine. During induction, neutropenia (14.8%) and thrombocytopenia (13.1%) were the most common CTC grade 3 and 4 events. One fatality (duodenitis) occurred. The overall response rate was 100%. 54.1% of patients achieved a partial remission, 41% a clinical complete remission (cCR) without confirmation by CT scan or bone marrow (BM) biopsy according to protocol and 4.9% a cCR with incomplete recovery of the BM. 29 patients (47.5%) had no detectable (<10−4) minimal residual disease assessed by flow cytometry in peripheral blood. In conclusion, the BIG regimen is a safe and highly effective therapy for CLL.
Similar content being viewed by others
Introduction
The current standard first-line treatment for the majority of patients with chronic lymphocytic leukemia (CLL) consists of chemotherapy combined with a monoclonal antibody targeting CD20 (chemoimmunotherapy) [3,4,5]. However, chemoimmunotherapies, and in particular the FCR regimen composed of fludarabine, cyclophosphamide and rituximab, are often associated with side effects, notably myelosuppression and infections and fail to obtain durable remissions in high risk CLL patients [6, 7].
Therefore, we wished to design novel combination therapies using effective agents [8] such as the second-generation CD20-antibody obinutuzumab (GA101) or the Bruton kinase inhibitor ibrutinib to create less toxic, but highly effective therapies for CLL [4, 9]. Combining ibrutinib with rituximab yielded promising results for the therapy of relapsed CLL patients [10]. Therefore we hypothesized that the combination of the potent anti-CD20 antibody obinutuzumab with ibrutinib might further improve their efficacy. This rationale was also supported by preclinical data showing that CLL cells from ibrutinib-induced lymphocytosis are responsive to obinutuzumab [11].
Materials and methods
This prospective, open-label, multicenter phase II trial investigated the efficacy and safety of the novel BIG-regimen based on the so-called triple-T concept of a tailored and targeted treatment aiming at total eradication of minimal residual disease (MRD) [12].
Inclusion and exclusion criteria are listed in the appendix.
The trial was designed for treatment-naïve and relapsed/refractory CLL patients irrespective of comorbidities and high versus low risk genetic features.
62 patients were to be recruited according to a predefined allocation for the two strata of first-line (FL) and relapsed/refractory (RR) patients. Each stratum was foreseen to include one third of the patients at minimum, the last third was to be filled flexibly with patients from both strata.
Study oversight and conduct
The study was approved by the institutional review board and independent health authorities at each participating institution and was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All patients provided informed consent.
The sponsor of the trial, designed as an investigator-initiated trial by the German CLL Study Group (GCLLSG), was the University of Cologne. The trial was supported financially by F. Hoffmann–La Roche Ltd., Basel, Switzerland, and Janssen-Cilag, Neuss, Germany, which also provided the study medication.
Investigators from the GCLLSG analyzed the data. All authors affirmed the completeness and accuracy of the data and the adherence of the study to the protocol. The CLL2-BIG study is registered at http://www.clinicaltrials.gov as # NCT02345863.
Treatment
Prior start of induction therapy, physicians had the option to recommend the use of two courses of bendamustine as a mild debulking therapy to patients with a higher tumor load, defined as an absolute lymphocyte count ≥ 25 × 109/l and/or lymph nodes ≥ 5 cm, unless an intolerance or refractoriness to bendamustine existed. Bendamustine monotherapy was administered on day 1 and 2 at a daily dose of 70 mg/m² for all patients, FL or RR, for a maximum of two cycles, each cycle with a duration of 28 days.
All patients were to receive six cycles of induction treatment with the combination of obinutuzumab and ibrutinib, each cycle with a duration of 28 days.
Obinutuzumab was administered intravenously at 100 mg on day 1 and 900 mg on day 2 (or 1000 mg on day 1 if treatment was well tolerated) as well as at 1000 mg on day 8 and 15 during the first cycle. Subsequently, obinutuzumab was given intravenously at 1000 mg on day 1 of each cycle. Prophylaxis for infusion-related reactions (IRR) and tumor lysis syndrome (TLS) prior to obinutuzumab included hydration and premedication with allopurinol, paracetamol (acetaminophen), antihistamines and glucocorticoids.
Oral, daily intake of 420 mg ibrutinib was added to the obinutuzumab therapy starting on the first day of the second cycle of the induction therapy to avoid an enhanced risk of IRR or TLS caused by the typical, ibrutinib-induced lymphocytosis. Henceforward, ibrutinib intake was continued on a daily basis.
Patients responding to induction therapy by judgment of the investigator continued with maintenance therapy, consisting of 420 mg ibrutinib per day and intravenous application of 1000 mg obinutuzumab every 3 months. In absence of unacceptable toxicity, maintenance therapy was applied until achieving an MRD-negative remission, confirmed by two consecutive, negative results of MRD testing of the peripheral blood within 3 months, progression, start of new therapy or for up to 24 months, whichever occurred first. Investigators were allowed to continue treatment in patients with MRD negativity in the peripheral blood, if MRD remained detectable in the bone marrow.
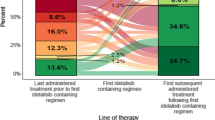
An overview of the trial procedures is shown in Fig. 1.
Assessments and endpoints
A central screening process prevented inclusion of patients with violations of eligibility criteria. Confirmation of CLL diagnosis was performed centrally by immunophenotyping of circulating lymphocytes.
Before initiation of therapy, prognostic parameters including cytogenetic aberrations by means of fluorescence in-situ hybridization, mutational analysis of the immunoglobulin heavy-chain variable-region gene and tumor protein 53 (TP53), as well as the serum parameters beta-2-microglobulin and thymidine kinase were assessed in the central laboratories of the GCLLSG. A CT scan was recommended to evaluate the tumor load.
The primary endpoint of the trial was the overall response rate (ORR) at final restaging defined as the proportion of patients having achieved a complete remission (CR), a CR with incomplete recovery of the bone marrow (CRi), partial remission (PR) or PR with lymphocytosis. Final restaging was defined as the staging performed 3 months after the start of the last induction cycle administered. At this time point, patients still received treatment as part of the maintenance therapy.
Response to therapy and disease progression were assessed using iwCLL criteria [13]. In general, full confirmation of CR using these iwCLL criteria was planned after the first cycle of maintenance therapy (3 months after final restaging) and will be reported later, as the data are currently incomplete. A clinical CR (cCR) was defined in the protocol as absence of disease by clinical examination and blood counts, without assessment by CT or bone marrow biopsy.
Secondary endpoints included the analysis of safety parameters, assessment of MRD evaluation at final restaging, ORR after debulking, the ORR in predefined subgroups as well as progression-free survival (PFS).
Other secondary endpoints were best response achieved until 9 months after the start of the last induction cycle administered, ORR after the end of maintenance therapy, CR rate, event-free, overall and treatment-free survival as well as the duration of response. These parameters were not part of the final primary endpoint analysis and will be analyzed later based on extended follow-up data.
Adverse events (AE) were coded with MedDRA and reported according to the National Cancer Institute Common Terminology Criteria (NCI-CTC) version 4.0.
MRD assessment of the peripheral blood and the bone marrow was performed in a central laboratory at baseline and final restaging by four-color flow cytometry [14].
Statistical analysis
The ORR at the end of induction therapy as the primary endpoint of the trial was used to determine the sample size for the study using a two-sided one-sample binomial- test with an overall significance level of 5% and an assumed power of 80%. The ORR of an uninteresting regimen was assumed to be at least 75%, and it was expected to improve this rate to 90% with the BIG regimen. Thus, the efficacy of the BIG regimen regarding a mixed population was confirmed if the ORR was at least 90% (response rate of an active regime) respectively and it would be assessed to be not effective if the ORR was 75% or less.
This lower boundary of efficacy of 75% ORR corresponded to an expected ORR of a mixed CLL population and was composed of the expected ORR of relapsed/refractory as well as previously untreated (first-line) patients. For relapsed/refractory patients an ORR of 64% [15] was expected and for first-line patients it was expected to achieve an ORR of 90% [16] approximately. Concerning different allocations of RR/FL-patients a fix lower limit of 1/3 and an upper limit of 2/3 was considered resulting in a flexible recruitment of 1/3 to 2/3 per stratum.
The ORR at the end of induction therapy was defined as the proportion of patients having achieved a CR, a CRi, clinical CR/CRi, PR or PR with lymphocytosis. Patients without any documented response assessment were kept and labeled as ‘non-responder’ in the analysis.
The primary dataset for the primary endpoint analysis was derived from the full analysis set (FAS). This dataset included all patients enrolled into the trial who received at least two complete cycles of induction therapy.
Analyses of time-to-event endpoints were performed on the FAS applying Kaplan–Meier methods including Kaplan–Meier estimates. PFS was measured from the date of enrollment to the date of first disease progression (as defined by the iwCLL response criteria [13]) or death by any cause. Analyses of other response rates including MRD were reported in a similar way as the analysis of the primary endpoint.
Safety analyses included all patients who received at least one dose of any compound of the study treatment.
Results
Patients
Between January and August 2015, 66 patients with CLL from a total of 17 trial sites in Germany were enrolled.
Until the end of the second cycle of induction therapy, two patients withdrew their consent, one patient died due to sepsis and two patients stopped treatment due to AE, in particular systemic inflammatory response syndrome in combination with renal failure and general physical health deterioration, in the other case parainfluenza pneumonia in combination with deep vein thrombosis. Accordingly, 61 patients constituted the FAS for patients receiving induction therapy. 30 of these patients were previously untreated and 31 patients relapsed/refractory with a median of one prior treatment line (range 1–5).
The FAS for patients receiving debulking constituted of 44 patients, whereas 49 patients received at least one cycle of bendamustine as debulking therapy, 30 of them were treatment-naïve and 19 relapsed/refractory.
An overview of the trial is shown in Fig. 1. The patient demographics are shown in Table 1.
Efficacy
Bendamustine debulking
35 patients received two cycles of bendamustine as planned by the study protocol. Besides the above described patients excluded from FAS, nine patients (20.5%) stopped debulking prematurely; disease progression occurred in one patient (2.3%) whereas seven patients (15.9%) discontinued due to AE. These events included rash in two treatment naïve patients as well as hypersensitivity, lung infection, diarrhea and carotid artery stenosis in one patient, respectively. Furthermore, four AE, namely thrombophlebitis, fatigue, herpes virus infection and nausea were reported in one patient of the relapsed/refractory cohort. In one patient (2.3%) treatment was discontinued due to investigator´s decision.
29 patients (18 FL, 11 RR) responded to bendamustine monotherapy as debulking resulting in an ORR of 65.9%. According to the iwCLL criteria [13] all responses were rated as PR as responses were not confirmed by bone marrow biopsy and/or CT scans. In this trial, four patients (9.1%; 1 FL (3.7%), 3 RR (17.3%)) with PR were rated as clinical CR as they showed a normalized blood counts and without any detectable lymphadenopathy. Three patients (6.8%; 2 FL (7.4%), 1 RR (5.9%)) achieved a PR without any detectable lymphadenopathy but with incomplete recovery of the bone marrow and were therefore counted as clinical CRi. 11 patients (25.0%; 6 FL (22.2%), 5 RR (29.4%)) achieved stable disease (SD) whereas two patients (4.5%; 1 FL (3.7%), 1 RR (5.9%)) progressed during the debulking period of the study. For two patients the response was not evaluable.
Out of five patients with del(17p), one PR and one SD were reported. Two patients were progressive after debulking; in one patient no response was documented.
According to the study protocol, patients with PD after debulking were allowed to continue study treatment with obinutuzumab and ibrutinib in the induction phase.
Induction therapy
At the end of induction, the combination of obinutuzumab and ibrutinib showed an ORR of 100% (95% confidence interval, 94.1–100.0%; p < 0.001) by investigator assessment at final restaging. Statistically, the primary endpoint was met and the null hypothesis could be rejected.
Response rates after induction therapy by investigator assessment are presented in Table 2; Fig. 2 shows the improvement of laboratory values during study therapy and Fig. 3 the improvement of response over the time.
Improvement of laboratory values during study therapy. The courses of median absolute lymphocyte count (ALC; %), hemoglobin (g/dl) and platelets (109/l) with the appropriate interquartile ranges are shown for all patients as well as for patients with and without prior debulking. DB = Prior first dose of bendamustine. C1 = prior first dose of obinutuzumab. C2 = prior first dose of combination therapy with ibrutinib and obinutuzumab. IST interim staging (after three cycles of induction therapy), IR initial response (after 6 cycles of induction therapy), FR final restaging
MRD samples were available in 57 patients. 29 (47.5%) patients showed MRD negativity of the peripheral blood at final restaging as defined by MRD values below 10−4 by four-color flow cytometry. In contrast, 15 patients (24.6%; 8 FL (26.7%), 7 RR (22.6%)) had intermediate MRD-levels, defined as a level ≥ 10−4 and <10−2, whereas MRD positivity (≥10−2) was found in 13 patients (21.3%; 4 FL (13.3%), 9 RR (29.0%)) at final restaging.
16 FL (53.3%) and 13 RR (41.9%) patients became MRD negative at final restaging. Among all patients achieving a cCR, 19 patients (76.0%) were MRD negative, among those with cCRi one patient (33.3%) and 9 patients of those with PR (27.3 %) were MRD negative. Regarding the effects of bendamustine debulking on MRD negativity, 17 patients received bendamustine (58.6%), and 7 patients did not (46.7%).
An MRD negative status was achieved in one patient with del(17p) (12.5%), in seven patients with del(11q) (50.0%), in seven patients with trisomy 12 (70.0%), in eight patients with del(13q) (44.4%) and in six patients with normal karyotype according to fluorescent in situ hybridization (FISH) (54.5%).
Time to first documentation of MRD negativity and treatment discontinuation is shown in Fig. 4.
Time to first documentation of MRD negativity and treatment discontinuation. Plot shows duration of treatment (full length of bar), time point of first documentation of MRD negativity in peripheral blood (blue boxes) and iwCLL response (color of the bars, see figure legend). One patient discontinued treatment due to fatal dueodenitis. iwCLL International Workshop for Chronic Lymphocytic Leukemia, PD progressive disease, SD stable disease, PR partial remission, clinical CR/CRi complete response or complete response with incomplete marrow recovery, MRD minimal residual disease
After a median observation time of 15.4 months, there were three events for PFS including two events of PD and one treatment related death resulting in an estimated PFS of 95.5% at 15 months. All events occurred in patients of the RR stratum (Fig. 5 “Estimated progression free survival (PFS)”).
Safety
Bendamustine debulking
For a debulking safety population consisting of 49 patients, 131 AE grade 1–5 were reported in 37 patients, 65 (49.6%) during the first and 66 (50.4%) during the second cycle. One patient of the RR cohort died due to pulmonary sepsis (CTC grade 5; 0.8%). 63 (48.1%) AE were CTC grade 1, 46 (35.1%) CTC grade 2, 17 (13.0%) CTC grade 3 and four (3.1%) CTC grade 4. 73 AE (55.7%) were rated as related to bendamustine whereas 21 AE (16.0%) lead to an adjustment of the study medication.
The most common toxicities (according to system organ class [SOC] category) observed were gastrointestinal and general disorders (19.1% each), infections and infestations (13.0%). Blood and lymphatic system disorders occurred in 7.6% of all reported AE.
All grade 3–5 toxicities observed during debulking therapy are shown in Table 3.
Induction treatment
The induction safety population included 62 patients who received at least one compound of the induction treatment. Sixty of them completed six cycles of induction therapy.
In total, 412 AE were reported in 60 out of 62 patients of the induction safety population including 240 AE (58.3%) related to study therapy. AE occurred mostly during the first cycle (25.2%) and were graded as CTC grade 1 (55.8%). 64 AE (15.5%) resulted in an adjustment of the study drugs.
One grade 5 AE occurred in a 74-year old female patient with four prior therapies for CLL who died after the fifth course of induction therapy due to duodenitis related to study medication. Her medical history included no relevant concomitant diseases. She was hospitalized due to reduced general condition and cachexia as a result of diarrhea, nausea and loss of appetite. As reason for diarrhea no pathogens were detected. For clarification duodenoscopy was performed and duodenitis was found. Furthermore, atrial fibrillation as well as changing vigilance and renal failure appeared. She died in an onward decreasing general condition.
During induction therapy, 25 events of infusion related reactions (IRR) related to obinutuzumab occurred in 23 patients (37.1%). Only three events were CTC grade 3 (12%), whereas eleven events were CTC grade 1 and 2, respectively. IRR occurred more often in patients without prior debulking (58.8% without versus 28.9% with bendamustine debulking). CTC grade 3 IRR occurred twice in patients without prior debulking, once in a patient with prior debulking.
In total, 412 AE were reported in 60 patients out of 62 patients of the induction safety population including 240 AE (58.3%) related to study therapy. AEs occurred mostly during the first cycle (25.2%) and were graded as CTC grade 1 (55.8%). 64 AE (15.5%) resulted in an adjustment of ibrutinib or obinutuzumab.
For obinutuzumab, one dose modified treatment cycle was performed in three patients (4.8%), respectively. Two dose modifications were performed due to AE and one due to administrative reasons.
366 cycles of ibrutinib were applied in 62 patients. 72 cycles (19.7%) were dose modified, 30 cycles (41.7%) due to AE in 16 patients (25.8%). 30 patients (48.4%) temporarily stopped ibrutinib with a median of 8 days on zero-dose (range 1–54).
Of note, no event of atrial fibrillation occurred during ibrutinib therapy. Furthermore, seven mild bleeding events (< CTC Grade 3) were reported.
Grade 3–5 toxicities observed during induction therapy are shown in Table 3.
Discussion
The study reports the results of a sequential combination therapy consisting of bendamustine debulking followed by ibrutinib and obinutuzumab in CLL patients (BIG regimen) based on the so-called triple-T concept of a targeted and tailored treatment aiming at total eradication of MRD [12].
The population tested in this exploratory study was heterogeneous regarding the number of prior treatment lines, the age at study entry, the creatinine clearance, the CLL-IPI risk group and the CIRS score as an indicator for comorbidities.
The trial population appeared rather favorable as demonstrated by the relatively high frequency of patients with del(13q) and a relatively low number of patients with del(17p), particularly amongst RR patients.
Efficacy results after debulking therapy with bendamustine were comparable to previously reported findings for bendamustine monotherapy in CLL [17, 18].
An interesting observation of the trial was that the occurrence of IRR following first infusions of obinutuzumab was reduced from 58.8 to 28.9% in patients with bendamustine debulking compared to patients without prior debulking. Results for IRR following the first infusion of obinutuzumab were consistent with previous reports [4].
Similarly, it was reassuring to see a relatively low rate of TLS in this trial. Only one laboratory TLS event occurred during induction therapy, while three cases of TLS were documented after bendamustine infusions. Therefore, a debulking for patients with a high tumor burden using bendamustine may have beneficial effects with regard to major side effects, TLS and IRR caused by obinutuzumab. However, bendamustine therapy may come at the price of its own side effects, most notably infections, as shown by this study. The role of bendamustine debulking for treatment efficacy remains uncertain as response rates were comparable in patients with and without debulking, although MRD negativity seems to occur more often in patients with prior debulking.
Overall, the BIG regimen showed a very promising therapeutic efficacy with an ORR of 100% and a MRD negativity rate of 47.5% in the peripheral blood based on the FAS. Although one patient died after the fifth cycle of induction treatment, the estimated PFS is 95.5% after a median observation time of 15.4 months. The response rates seen here in this trial compare favorably to what was reported for standard chemoimmunotherapy. In first line treatment, the BR regimen achieved an ORR of 96% and a median PFS of 41.7, the FCR regimen was reported to achieve an ORR of 95% and a median PFS of 55.2 months [6]. In relapsed/refractory CLL, the BR regimen yielded an ORR of 59% and a median PFS of 15.2 months [15].
When compared to ibrutinib monotherapy with response rates of 86% in first-line treatment of CLL [19] and to an ORR of 71% in the treatment of relapsed/refractory CLL [9] the BIG regimen with the addition of obinutuzumab to ibrutinib seems to improve the efficacy of CLL therapy both in the frontline and the relapsed/refractory setting. In a combination study using rituximab and ibrutinib in a high-risk study population, a similar ORR of 95% was reached, but only one out of 40 patients achieved MRD negativity [10]. Therefore we hypothesize that the addition of obinutuzumab improves treatment outcome over ibrutinib monotherapy and ibrutinib plus rituximab, because the rates of MRD negative remissions are increased [20]. Longer follow-up will be needed to determine whether the BIG regimen improves long-term outcome.
In addition, the planned continuation of the combination of ibrutinib and obinutuzumab as maintenance therapy may even improve the quality of responses over the time as shown for ibrutinib therapy in CLL [19, 21].
Finally, the study also demonstrates that ibrutinib can be safely combined with obinutuzumab in CLL patients. No cumulative toxicity occurred when compared to ibrutinib monotherapy [19] or combination therapy of ibrutinib plus rituximab [10]. No increases of bleeding or cardiac events were observed. Moreover, the rate of AE compared favorably with chemoimmunotherapy in particular with regard to hematological toxicity [4, 6, 15].
In conclusion, the BIG regimen showed very promising efficacy and a good safety profile for treatment of treatment-naïve and relapsed/refractory CLL patients.
References
von Tresckow J, Cramer P, Bahlo J, Engelke A, Langerbeins P, Fink A-M, et al. CLL2-BIG—a novel treatment regimen of bendamustine followed by GA101 and ibrutinib followed by ibrutinib and GA101 maintenance in patients with chronic lymphocytic leukemia (CLL): interim results of a phase II-trial. Blood. 2015;126:4151.
von Tresckow J, Cramer P, Bahlo J, Robrecht S, Engelke A, Langerbeins P, et al. CLL2-BIG—a novel treatment regimen of bendamustine followed by GA101 and ibrutinib followed by ibrutinib and GA101 maintenance in patients with chronic lymphocytic leukemia (CLL): results of a phase II-trial. Blood. 2016;128:640.
Hillmen P, Gribben JG, Follows GA, Milligan D, Sayala HA, Moreton P, et al. Rituximab plus chlorambucil as first-line treatment for chronic lymphocytic leukemia: Final analysis of an open-label phase II study. J Clin Oncol. 2014;32:1236–41.
Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–10.
Foa R, Del Giudice I, Cuneo A, Del Poeta G, Ciolli S, Di Raimondo F, et al. Chlorambucil plus rituximab with or without maintenance rituximab as first-line treatment for elderly chronic lymphocytic leukemia patients. Am J Hematol. 2014;89:480–6.
Eichhorst B, Fink A-M, Bahlo J, Busch R, Kovacs G, Maurer C, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17:928–42.
Fischer K, Bahlo J, Fink AM, Goede V, Herling CD, Cramer P, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127:208–15.
Cramer P, von Tresckow J, Bahlo J, Engelke A, Langerbeins P, Fink AM, et al. CLL2-BXX Phase II trials: sequential, targeted treatment for eradication of minimal residual disease in chronic lymphocytic leukemia. Future Oncol. 2018;14:499–513.
Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42.
Burger JA, Keating MJ, Wierda WG, Hartmann E, Hoellenriegel J, Rosin NY, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15:1090–9.
Ysebaert L, Klein C, Quillet-Mary A. CLL cells from ibrutinib-induced lymphocytosis of relapsed/refractory chronic lymphocytic leukemia patients are responsive to obinutuzumab, but not rituximab, ex vivo. Blood. 2015;126:4157.
Hallek M. Signaling the end of chronic lymphocytic leukemia?: new frontline treatment strategies. Blood. 2013;122:3723–34.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56.
Böttcher S, Ritgen M, Fischer K, Stilgenbauer S, Busch RM, Fingerle-Rowson G, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol. 2012;30:980–8.
Fischer K, Cramer P, Busch R, Stilgenbauer S, Bahlo J, Schweighofer CD, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011;29:3559–66.
Fischer K, Cramer P, Busch R, Bottcher S, Bahlo J, Schubert J, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012;30:3209–16.
Bergmann M, Goebeler M, Herold M, Emmerich B, Wilhelm M, Ruelfs C, et al. Efficacy of bendamustine in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase I/II study of the German CLL Study Group. Haematologica. 2005;90:1357–64.
Knauf WU, Lissitchkov T, Aldaoud A, Liberati AM, Loscertales J, Herbrecht R, et al. Bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukaemia: updated results of a randomized phase III trial. Br J Haematol. 2012;159:67–77.
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. New Engl J Med. 2015;373:2425–37.
Kovacs G, Robrecht S, Fink AM, Bahlo J, Cramer P, Tresckow Jv, et al. Minimal residual disease assessment improves prediction of outcome in patients with chronic lymphocytic leukemia (CLL) who achieve partial response: comprehensive analysis of two phase III studies of the German CLL Study Group. J Clin Oncol. 2016;34:3758–65.
Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497–506.
Acknowledgements
This study was initiated and organized by the German CLL Study Group as an academic trial with the University of Cologne being the sponsor, and financial support by F. Hoffmann La Roche and Janssen-Cilag. The authors wish to express their gratitude towards all patients participating in the trial and their families, as well as the physicians and trial staff at the sites. Furthermore, we thank all study team members involved; in particular, we acknowledge Johanna Wesselmann and Irene Stodden for their excellent contribution. Finally, we thank Dr. Birgit Fath and the monitors from the competence network malignant lymphoma (Kompetenznetz Maligne Lymphome) for facilitating the conduct of this trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JvT: Research funding (F. Hoffmann-LaRoche, Janssen-Cilag), honoraria and consultant or advisory board member (AbbVie, Janssen-Cilag and F. Hoffmann-LaRoche), travel grants (AbbVie, Celgene, F. Hoffmann-LaRoche and Janssen-Cilag). PC: Research funding (AbbVie, Gilead, GlaxoSmithKline/Novartis, F. Hoffmann-LaRoche, and Janssen-Cilag), honoraria (F. Hoffmann-LaRoche and Janssen-Cilag), consultant or advisory board member (AbbVie, AstraZeneca, Janssen-Cilag and Novartis) and travel grants (Astellas, F. Hoffmann LaRoche, Gilead, Janssen-Cilag and Mundipharma). JB: Honoraria and travel grants (F. Hoffmann-LaRoche). PL: Research funding (Janssen-Cilag), Honoraria (AbbVie, Janssen-Cilag and F. Hoffmann-LaRoche), travel grants (AbbVie, F. Hoffmann-LaRoche, Janssen-Cilag and Mundipharma) AF: Research funding (Celgene), honoraria (F. Hoffman-LaRoche) and travel grants (F. Hoffmann-LaRoche, Mundipharma, Abbvie and Celgene). OA: Honoraria and consultant or advisory board member (AbbVie, Gilead and F. Hoffmann-LaRoche), travel grants (AbbVie, Gilead, F. Hoffmann-LaRoche and Janssen-Cilag). TI: Travel grants (F. Hoffmann-LaRoche). MR: Research funding (AbbVie; F. Hoffmann-LaRoche), consultant or advisory board member (AbbVie, BMS, F. Hoffmann-LaRoche) and travel grants (F. Hoffmann-LaRoche). KF: Travels grants (F. Hoffmann-LaRoche). CMW: Honoraria, consultant or advisory board member, research funding and travel grants (AbbVie, Gilead, F. Hoffmann-LaRoche, Janssen-Cilag and Mundipharma). KK: Research funding, honoraria, consultant or advisory board member and speaker´s bureau (F. Hoffmann-LaRoche, Janssen-Cilag and Mundipharma). SS: Research support, honoraria, consultant or advisory board member, speaker´s bureau and travel support (AbbVie, Amgen, Astra-Zeneca, Celgene, F. Hoffmann-LaRoche, Gilead, GlaxoSmithKline, Janssen-Cilag and Novartis). SB: Reasearch funding (AbbVie, Celgene and F. Hoffmann-LaRoche), honoraria (AbbVie, Becton Dickinson, F. Hoffmann-LaRoche), consultant or advisory board member (F. Hoffmann-LaRoche), travel grants (Celgene, F. Hoffmann-LaRoche). BE: Research support, consultant or advisory board member and travel support (AbbVie, Celgene, F. Hoffmann-LaRoche, Gilead, GlaxoSmithKline, Janssen-Cilag, Mundipharma). MH: Research funding (AbbVie, Celgene, F. Hoffmann-LaRoche, Gilead, Janssen-Cilag, Mundipharma and Pharmacyclics), honoraria and speaker´s bureau (AbbVie, Boehringer-Ingelheim, Celgene, F. Hoffmann-LaRoche, Gilead, Janssen-Cilag, Mundipharma and Pharmacyclics) and consultant or advisory board member (AbbVie, F. Hoffmann-LaRoche, Gilead, Janssen-Cilag). The remaining authors declare that they have no conflict of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
von Tresckow, J., Cramer, P., Bahlo, J. et al. CLL2-BIG: sequential treatment with bendamustine, ibrutinib and obinutuzumab (GA101) in chronic lymphocytic leukemia. Leukemia 33, 1161–1172 (2019). https://doi.org/10.1038/s41375-018-0313-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-018-0313-8
- Springer Nature Limited
This article is cited by
-
Chronic Lymphocytic Leukemia: Time-Limited Therapy in the First-Line Setting and Role of Minimal Residual Disease
Current Oncology Reports (2024)
-
Sequential treatment with bendamustine, obinutuzumab (GA101) and Ibrutinib in chronic lymphocytic leukemia (CLL): final results of the CLL2-BIG trial
Leukemia (2022)
-
Richter transformation in chronic lymphocytic leukemia (CLL)—a pooled analysis of German CLL Study Group (GCLLSG) front line treatment trials
Leukemia (2021)
-
Prognostic value of high-sensitivity measurable residual disease assessment after front-line chemoimmunotherapy in chronic lymphocytic leukemia
Leukemia (2021)
-
Is There a Role for Chemotherapy in the Era of Targeted Therapies?
Current Hematologic Malignancy Reports (2020)