Abstract
Following the achievement of deep molecular response on tyrosine kinase inhibitors (TKIs), approximately half of patients with chronic myeloid leukemia (CML) can discontinue TKI and remain in treatment-free remission (TFR). The ALLG CML8 study enrolled 40 imatinib-treated patients with undetectable BCR-ABL1 mRNA (approximately MR4.5). Molecular relapse was defined as detectable BCR-ABL1 on two consecutive tests or any single value >0.1%. With a median follow-up of 8.6 years (range 5.7–11.2 years), 18 patients remain in continuous TFR (45.0%; 95% confidence interval 31.9−63.4%). The latest relapse detected was 27 months after stopping imatinib. No patient progressed to advanced phase. Twenty-two patients met criteria for imatinib re-treatment and all regained undetectable molecular response. Nine patients in long-term TFR were monitored by highly sensitive individualized BCR-ABL1 DNA PCR in a sufficient number of samples to enable more precise quantification of residual leukemia. BCR-ABL1 DNA decreased from a median of MR5.0 in the first year of TFR to MR6.1 in the sixth year of TFR. Our results support the long-term safety and remarkable stability of response after imatinib discontinuation in appropriately selected CML patients. Serial high sensitivity testing provides a new and unexpected finding of gradually reducing CML cells in patients in long-term TFR.
Similar content being viewed by others
Introduction
Multiple prospective clinical trials cumulatively involving more than 2000 patients with chronic myeloid leukemia (CML) have examined the feasibility and safety of treatment-free remission (TFR) after withdrawal of tyrosine kinase inhibitor (TKI) treatment following achievement of a sustained deep molecular response (DMR), variably defined as a molecular response (MR) of BCR-ABL1 ≤ 0.01% (MR4.0) or better on the International Scale (IS) [1,2,3,4,5]. Despite slightly differing inclusion criteria and definitions of molecular relapse being used these trials have shown remarkably consistent results, with TFR rates of around 40–60% at 2 years, and the majority of instances of molecular relapse occurring in the first 6 months after TKI withdrawal. There is considerable interest among patients and clinicians in the potential to stop TKI treatment [6]. The recent CML treatment recommendations of the US National Comprehensive Cancer Network (available at www.nccn.org) and the European Society of Medical Oncology [7] for the first time included criteria for a TFR attempt outside the clinical trial setting. With increasing numbers of patients attempting TFR it is important to have longer-term data on the safety and durability of this approach.
The Australasian Leukaemia & Lymphoma Group (ALLG) CML8 study (TWISTER; ACTRN 12606000118505) commenced accrual in July 2006 and enrolled 40 imatinib-treated patients with undetectable measurable residual disease (UMRD; approximately equivalent to MR4.5, or BCR-ABL1 ≤ 0.0032%) by real-time quantitative reverse transcriptase-polymerase chain reaction (RQ-PCR). We previously reported a TFR rate of 42.7% with a median follow-up of 3.6 years [1]. The French STIM study commenced at around the same time and had very similar inclusion criteria [2]. The long-term outcome of the 100 patients in the STIM study was recently updated with a median follow-up of 6.4 years and reported 38% of patients alive and in TFR [8]. These two studies offer us the longest available follow-up of patients in TFR.
In the TWISTER study we previously demonstrated the presence of persistent BCR-ABL1-positive cells, detectable by highly sensitive individualized genomic DNA PCR (sensitivity MR6.2), even in patients with sustained TFR for up to 5 years [1, 9]. The intronic BCR-ABL1 fusion sequence is essentially unique to each individual patient [10], effectively eliminating the risk of false positive results, and this enables us to achieve a level of sensitivity and specificity which is significantly greater than that of conventional RQ-PCR [9]. In the present study we extend the period of follow-up to demonstrate the long-term durability of TFR and the safety of TKI discontinuation in selected patients. In addition we show evidence for a gradual reduction in the level of circulating BCR-ABL1-positive cells during TFR.
Materials and methods
Treatment and monitoring by RQ-PCR
The inclusion criteria, monitoring, and re-treatment schedules for the TWISTER study were described previously [1]. Eligible patients had sustained UMRD for at least 2 years on imatinib treatment. In subsequent years there has been a move away from UMRD to define absolute levels of BCR-ABL1, such as MR4.5 (BCR-ABL1 ≤ 0.0032%). For comparison, the equivalent median sensitivity of RQ-PCR on the day of stopping imatinib was MR4.8 (range 4.0–5.3). RQ-PCR monitoring was performed in a central laboratory (SA Pathology, Adelaide) [11, 12] monthly for the first 12 months after discontinuation, every 2 months in the second year, then every 3 months thereafter. The protocol-defined trigger to restart imatinib was two consecutive positive RQ-PCR results at any level, without requiring either an increase in BCR-ABL1 or loss of a major molecular response (MMR; BCR-ABL1IS ≤ 0.1%). In patients who restarted imatinib there was 1 year of central monitoring during re-treatment [1]. The protocol was subsequently amended to add a further 4 years of follow-up following completion of the monitoring or re-treatment phase during which the patients were monitored in their respective local laboratories, all of which report BCR-ABL1IS [13]. At last follow-up the only patients being monitored in the central laboratory were those patients treated in Adelaide. We circulated a data collection form to investigators to update survival and disease status (including molecular response and CML treatment). The study was approved by the ethics committees of the participating hospitals. All samples were collected with informed consent in accordance with the Institutional Ethics-approved protocols and with reference to the Declaration of Helsinki.
DNA Q-PCR
The BCR-ABL1 genomic breakpoint sequence was identified in 26 patients (13 who had relapsed and 13 in stable TFR) for whom we had diagnostic material available. Genomic DNA was extracted from peripheral blood leukocytes and BCR-ABL1 DNA PCR was performed as described previously [9, 14]. Serial testing was performed during the protocol-defined follow-up period, and at later time points in 20 Adelaide patients (9 patients in long-term TFR and 11 patients who restarted imatinib treatment). Briefly, the amount of amplifiable DNA in a sample was determined by interpolation from a standard curve for the GUSB control gene. Nested PCR was performed with 500 ng amplifiable genomic DNA in each first round PCR and real-time Q-PCR in the second round to give a semiquantitative value. Typically, 20 replicates were performed to give a limit of detection of MR6.2 (one in ~2 million cells). At very low levels of measurable residual disease (MRD) the assay is digital: each PCR replicate contains either one target molecule or none. When all replicates were positive, a standard curve (comprising serial dilution of the patient’s diagnostic DNA) was used to estimate copy number [9, 14], with the modification that Poisson statistics were used to exclude replicates that gave outlying values [15]. This modification was necessary due to the potential for minor differences in PCR efficiency in a nested assay to produce widely varying threshold cycle numbers in the second round real-time Q-PCR.
Statistical analysis
Statistical tests were performed using GraphPad Prism 7 statistical software (GraphPad Prism Inc., La Jolla, CA, USA). R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria) was used to estimate the cumulative incidence of molecular response by the Kaplan−Meier method and to prepare loess curves (locally weighted scatterplot smoothing, an adaptation of the least squares method for averaging serial data) to fit the BCR-ABL1 DNA data over time. A P value of <0.05 was considered statistically significant.
Results
Patient characteristics
Forty patients were enrolled and their characteristics at study entry were previously described [1]. Key features are summarized in Table 1. At the time of last follow-up the median age of the patients was 69 years (range 38–91 years).
Overall and molecular relapse-free survival
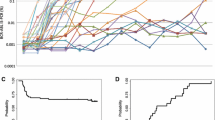
Of the 40 patients enrolled, 37 remained alive at last contact, 18 of whom were in continuous TFR with a median follow-up of 8.6 years (range 5.7–11.2 years). No patient progressed to accelerated phase or blast crisis, and all three deaths were considered unrelated to CML (one each from plasma cell myeloma, cardiac failure, unknown cause with UMRD on the last available test). The three deaths were all among patients who had recommenced imatinib treatment and therefore had no effect on molecular relapse-free survival, which was 45% at 8 years (95% confidence interval 31.9−63.4%) (Fig. 1). No patient recommenced TKI treatment without prior molecular relapse. The latest molecular relapse was at 27 months, as previously reported [1].
BCR-ABL1 DNA PCR during stable TFR
DNA Q-PCR was performed in nine Adelaide patients who remained in stable TFR for a median of 8.6 years (range 5.7–10.8 years). In two of the patients (#4, 5) BCR-ABL1 DNA became undetectable in the last 4 years whereas, remarkably, two of the patients (#8, 9) had detectable BCR-ABL1 DNA at every measurement over 6.5 and 10.5 years, respectively (Fig. 2 and Supplementary Figure 1). In order to test whether there was a reduction in BCR-ABL1 DNA over time we plotted a loess curve using the quantitative values from all nine patients. This showed a continuous rate of decline during the first few years of TFR with an inflection at 3 years, beyond which the data were too sparse to determine whether there was any further decline (Fig. 3a). In the six patients who had quantifiable BCR-ABL1 DNA in more than 50% of samples during the first year of TFR, the median level of BCR-ABL1 reduced significantly from MR5.0 during the first year after imatinib discontinuation to MR6.1 in the sixth year of follow-up (P = 0.03; Fig. 3b).
BCR-ABL1 DNA values over time. a, b Values of patients in long-term TFR and c, d for patients re-treated with imatinib. a, c The graphs represent the loess analysis. The BCR-ABL1 DNA values for every patient are indicated by dot-dashed lines. The blue solid line represents a fitted loess line (locally weighted scatterplot smoothing). The gray zone represents the 95% confidence interval of the loess line. For illustrative purposes the undetectable values are assigned a value of MR6.2 corresponding to the limit of detection of the method. The total number of patients (red) and samples (black) are shown for each year. b, d Decline of BCR-ABL1 DNA. The blue dots represent the median of DNA BCR-ABL1 values per year per patient. Median with 95% CI are represented. Wilcoxon matched-paired signed rank test performed. IM imatinib
Response to imatinib re-treatment
Twenty-two patients met the study criteria for re-treatment with imatinib, and all 22 regained UMRD after a median of 3.3 months of re-treatment (range 0.0–17.4 months). One patient lost UMRD, but remained in MR4.5 (BCR-ABL1 0.002%); this patient had spontaneously returned to UMRD at the time of restarting imatinib. No patient has developed imatinib resistance. Two patients switched to second-generation TKIs after regaining UMRD on imatinib: one to dasatinib because of chronic imatinib toxicities (periorbital edema requiring blepharoplasty, and nausea), and one to nilotinib with the aim of achieving a deeper molecular response to enable a subsequent TFR attempt.
BCR-ABL1 DNA Q-PCR during imatinib re-treatment
DNA Q-PCR was performed in 11 Adelaide patients who relapsed and recommenced imatinib treatment. We previously performed DNA PCR in these patients after regaining UMRD (median 3.2 months; range 1.1–9.9 months) and showed that the level of BCR-ABL1 DNA returns to a level similar to that prior to imatinib discontinuation [1]. In this follow-up analysis we analysed the BCR-ABL1 DNA level over time with a median of 6.2 years (range 1.3–9 years) of follow-up during re-treatment (Fig. 3c) Again we performed a loess analysis, which appeared to show three distinct phases: a rapid decline in BCR-ABL1 DNA during the first 2 years of re-treatment, followed by the same biphasic pattern that we described above for the patients in continuous TFR (Fig. 3c). In nine patients there were sufficient numbers of samples after the first year to compare the values of BCR-ABL1 DNA. The median level of BCR-ABL1 DNA reduced significantly from MR4.7 during the first year of re-treatment to MR5.9 in the fifth year of re-treatment (P = 0.02; Fig. 3d).
Second and subsequent TFR attempts
Twelve of the 22 patients who regained UMRD later attempted TKI discontinuation for a second time (TFR2). In contrast to the primary study definition of molecular relapse [1], the trigger for re-treatment after TFR2 was at the discretion of the treating physician. For these 12 patients the median time to regain UMRD after restarting imatinib after the first TFR attempt was similar to the overall cohort (median 3.2 months; range 0.9–17.4 months), and the median duration of TKI re-treatment prior to TFR2 was 5.9 years (range 1.7–9.7 years). Three of these patients attempted TFR2 in a clinical trial (ACTRN12615001169538, a Phase Ib study of lenalidomide in combination with imatinib for adult patients with CML in second molecular remission). The clinical outcome of the 12 patients is summarized in Table 2. Six patients restarted TKI treatment: four after loss of MMR (restarted imatinib at 1, 3, 8, and 72 months, respectively); and two after loss of MR4.5, remaining in MMR (restarted TKI at 2 months (imatinib) and 7 months (nilotinib)). The latter patient (#30) subsequently stopped nilotinib 32 months later, and remained in TFR3 with UMRD at last follow-up after 16 months off treatment. One patient (#25) died from an unknown cause after 13 months in TFR2 with no detectable BCR-ABL1 transcripts. The remaining five patients remained alive and in MMR (n = 1) or stable MR4.5 (n = 4) after a median of 17 months (range 12–44 months) following TKI discontinuation.
Discussion
In this updated report of the TWISTER study we confirm the stability of TFR with a median follow-up in excess of 8 years. No molecular relapse has been observed beyond 27 months after imatinib discontinuation, consistent with the STIM study in which the latest relapse was reported at 22 months [8]. Notably, one patient stopping imatinib for a second time lost MMR after 6 years. Extremely late relapses have been reported even after allogeneic stem cell transplantation [16, 17]. At present, there are very few patients with follow-up in TFR for more than a decade. Therefore, until we have data from much larger numbers of patients followed in very long-term TFR we continue to recommend RQ-PCR monitoring indefinitely at least twice per year. We also provide long-term follow-up on the stability of re-treatment with imatinib: no patient developed resistance and the commonest cause for discontinuation was a second TFR attempt.
We used highly sensitive BCR-ABL1 DNA PCR to monitor the depth of response in serial samples taken during stable TFR. The number of patients in whom this analysis could be performed was limited by sample availability. The precision of any MRD assay operating near the limit of detection will be determined primarily by sampling error, which can be modeled using the Poisson distribution [18, 19]. Nevertheless, by pooling the data from the evaluable patients we were able to demonstrate a clear reduction in the proportion of BCR-ABL1-positive cells circulating in the peripheral blood. The reduction of MRD during the time in TFR could be explained by two hypotheses: gradual extinction of long-lived lineage-committed cells that lack self-renewal capacity or depletion of slowly proliferating CML precursor cells. Multiple studies have shown an association between immunological cell subsets and successful TFR [20,21,22,23,24], and immunological depletion of residual leukemic cells could occur even in the absence of TKI treatment. However, stochastic effects at play in the switch between self-renewal and proliferation may also lead to the random extinction of leukemic stem cells [25], so that no active process need be postulated to explain this observation.
Around half of TFR attempts result in re-treatment with a TKI and almost all of these patients rapidly regain a DMR [4, 5]. After UMRD is restored conventional RNA-based RQ-PCR cannot be used to determine whether there is further response in the CML clone. Using highly sensitive patient-specific BCR-ABL1 DNA PCR we were able to show that there is a gradual reduction in the CML clone with prolonged re-treatment, even though UMRD is regained typically within a few months. The triphasic response that we observed in patients receiving re-treatment is consistent with mathematical models describing a steep first phase of response reflecting the depletion of differentiated leukemic cells [26,27,28]. This burst of proliferation at molecular relapse must arise from a residual population of CML progenitor cells, and the slower second phase of reduction in BCR-ABL1 could correspond to depletion of this progenitor population by continuing TKI treatment [26,27,28].
The number of patients in a second DMR after an unsuccessful attempt at TFR is increasing over time, and the question of how to manage patients who may wish to have a second TFR attempt is gaining importance. The French CML collaborative group recently reported on 70 patients who recommenced imatinib after an unsuccessful TFR attempt, and who later stopped TKI treatment for a second time after regaining a DMR (RE-STIM) [29]. The estimated proportion of patients remaining in stable MMR without treatment was 42% at 2 years. In our own cohort of 12 patients attempting TFR2 the proportion of patients remaining in TFR2 was 60% (if two patients restarting TKI without loss of MMR are excluded). Considering the small number of patients (three of whom were in a Phase 1 study that might have influenced the outcome of the TFR attempt) these proportions are broadly similar. Several factors might contribute to the change in status of these patients from relapse at TFR1 to remission at TFR2. Firstly, the A-STIM study demonstrated that when the trigger for re-treatment is MMR the proportion of patients remaining in TFR rises from around 40% to around 60% [3]. This suggests that perhaps 20% of patients who were re-treated in TWISTER (and STIM) might have sustained MMR at the first TFR attempt, had the re-treatment trigger been less strict (noting that one of our patients originally “relapsed” without loss of MR4.5). Differing criteria for TKI re-treatment will influence the reported rate of TFR. Secondly, the total duration of TKI treatment in patients who achieved TFR at the second cessation attempt was very long at a median of 12.7 years (range 11.3−14.8 years). An interim analysis of the EuroSKI study reported that each additional year of DMR on imatinib prior to discontinuation was associated with approximately 2–3% absolute increase in the probability of TFR at 6 months after stopping [30]. Our results showing reducing levels of BCR-ABL1 DNA during prolonged re-treatment could potentially help explain why a longer duration of TKI treatment results in a higher proportion of patients in stable TFR. The optimal duration of TKI treatment for an individual patient cannot be determined at present, and there is a need for reliable biomarkers to predict TFR outcome. Prospective clinical trials with consistent inclusion criteria for patients attempting TFR2 are needed to help identify factors that may predict the outcome of a second discontinuation attempt.
Including the three patients who entered a phase 1 study of TFR2, a total of 24 out of 40 patients in the TWISTER study (60%) have eventually achieved TFR. No patient developed hematological relapse, TKI resistance, or progression of CML. Unnecessarily prolonged TKI treatment may expose the patient to rare late-emerging TKI toxicities, impairment of quality of life, and increased financial burden. Our results confirm the long-term safety and durability of TFR after imatinib and show, for the first time, evidence that MRD continues to fall in these patients despite the absence of ongoing therapy.
References
Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122:515–22.
Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–35.
Rousselot P, Charbonnier A, Cony-Makhoul P, Agape P, Nicolini FE, Varet B, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32:424–30.
Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17–23.
Saussele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. 2016;30:1638–47.
Villemagne Sanchez LA, O’Callaghan C, Gough K, Hall K, Kashima Y, Seymour JF, et al. Patient perceptions of treatment-free remission in chronic myeloid leukemia. Leuk Lymphoma. 2018;59:406–15.
Hochhaus A, Saussele S, Rosti G, Mahon FX, Janssen J, Hjorth-Hansen H, et al. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv41–iv51.
Etienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Charbonnier A, et al. Long-term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35:298–305.
Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Bartley PA, et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia. 2010;24:1719–24.
Ross DM, O’Hely M, Bartley PA, Dang P, Score J, Goyne JM, et al. Distribution of genomic breakpoints in chronic myeloid leukemia: analysis of 308 patients. Leukemia. 2013;27:2105–7.
Branford S, Fletcher L, Cross NC, Muller MC, Hochhaus A, Kim DW, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008;112:3330–8.
Branford S, Hughes TP, Rudzki Z. Monitoring chronic myeloid leukaemia therapy by real-time quantitative PCR in blood is a reliable alternative to bone marrow cytogenetics. Br J Haematol. 1999;107:587–99.
Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia—a Europe Against Cancer program. Leukemia. 2003;17:2318–57.
Pagani IS, Dang P, Kommers IO, Goyne JM, Nicola M, Saunders VA, et al. BCR-ABL1 genomic DNA PCR response kinetics during first-line imatinib treatment of chronic myeloid leukemia. Haematologica. 2018. 189787. https://doi.org/10.3324/haematol.2018.189787. [Epub ahead of print].
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–22.
Sekhri A, Liu D, Rasul M, Ahmed N, Ahmed T, Seiter K. Very late relapse of chronic myelogenous leukemia after allogeneic bone marrow transplantation. Leuk Res. 2009;33:1291–3.
Goldman JM, Majhail NS, Klein JP, Wang Z, Sobocinski KA, Arora M, et al. Relapse and late mortality in 5-year survivors of myeloablative allogeneic hematopoietic cell transplantation for chronic myeloid leukemia in first chronic phase. J Clin Oncol. 2010;28:1888–95.
Cross NC, White HE, Colomer D, Ehrencrona H, Foroni L, Gottardi E, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015;29:999–1003.
Rawer D, Borkhardt A, Wilda M, Kropf S, Kreuder J. Influence of stochastics on quantitative PCR in the detection of minimal residual disease. Leukemia. 2003;17:2527–8. author reply 2528−31
Hughes A, Clarson J, Tang C, Vidovic L, White DL, Hughes TP, et al. CML patients with deep molecular responses to TKI have restored immune effectors and decreased PD-1 and immune suppressors. Blood. 2017;129:1166–76.
Ohyashiki K, Katagiri S, Tauchi T, Ohyashiki JH, Maeda Y, Matsumura I, et al. Increased natural killer cells and decreased CD3(+)CD8(+)CD62L(+) T cells in CML patients who sustained complete molecular remission after discontinuation of imatinib. Br J Haematol. 2012;157:254–6.
Ilander M, Olsson-Stromberg U, Schlums H, Guilhot J, Bruck O, Lahteenmaki H, et al. Increased proportion of mature NK cells is associated with successful imatinib discontinuation in chronic myeloid leukemia. Leukemia. 2017;31:1108–16.
Imagawa J, Tanaka H, Okada M, Nakamae H, Hino M, Murai K, et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol. 2015;2:e528–535.
Rea D, Henry G, Khaznadar Z, Etienne G, Guilhot F, Nicolini F, et al. Natural killer-cell counts are associated with molecular relapse-free survival after imatinib discontinuation in chronic myeloid leukemia: the IMMUNOSTIM study. Haematologica. 2017;102:1368–77.
Traulsen A, Lenaerts T, Pacheco JM, Dingli D. On the dynamics of neutral mutations in a mathematical model for a homogeneous stem cell population. J R Soc Interface. 2013;10:20120810.
Roeder I, Horn M, Glauche I, Hochhaus A, Mueller MC, Loeffler M. Dynamic modeling of imatinib-treated chronic myeloid leukemia: functional insights and clinical implications. Nat Med. 2006;12:1181–4.
Michor F, Hughes TP, Iwasa Y, Branford S, Shah NP, Sawyers CL, et al. Dynamics of chronic myeloid leukaemia. Nature. 2005;435:1267–70.
Tang M, Gonen M, Quintas-Cardama A, Cortes J, Kantarjian H, Field C, et al. Dynamics of chronic myeloid leukemia response to long-term targeted therapy reveal treatment effects on leukemic stem cells. Blood. 2011;118:1622–31.
Legros L, Nicolini FE, Etienne G, Rousselot P, Rea D, Giraudier S, et al. Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer. 2017;123:4403–10.
Saussele S, Richter J, Guilhot J, Gruber FX, Hjorth-Hansen H, Almeida A, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19:747–57.
Acknowledgements
The authors acknowledge the important contribution of the TWISTER study patients and coordinators, and the ALLG as the study sponsor. This research was funded by the Australian National Health & Medical Research Council (Project grant #1051965), the Royal Adelaide Hospital Health Services Charitable Gifts Board, and Novartis Pharmaceuticals.
Author information
Authors and Affiliations
Consortia
Contributions
DMR designed and supervised the study, performed experiments, analysed data, and prepared the manuscript; ISP performed the experiments, analysed data, and prepared the manuscript; NS, JFS, AKM, RJF CKA, ASY, APG, and APS contributed essential clinical data and reviewed the manuscript; PD and VAS performed experiments and reviewed the manuscript; JB contributed RQ-PCR data and reviewed the manuscript; CHK and DTY analysed data, and reviewed the manuscript; DLW supervised research and reviewed the manuscript; SB contributed RQ-PCR data, supervised research, and reviewed the manuscript; TPH designed the study, supervised research, and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
DMR has received research funding from Novartis and Celgene, and honoraria from Novartis and BMS. AKM received honoraria from Novartis, BMS, and Specialised Therapeutics. ASY has received research funding from Novartis, BMS and Celgene, and honoraria from Novartis and BMS. DTY and TPH have received research funding and honoraria from Novartis, BMS, and Ariad. APG is an advisory board member for Novartis, BMS, Roche, Takeda, and MSD. APS has received honoraria from Novartis, BMS, Celgene, Roche, Amgen, and Specialised Therapeutics. SB has received research funding from Novartis, Ariad and Otsuka, honoraria from Novartis, BMS, Otsuka, Qiagen and Ariad, and is an advisory board member for Novartis and Qiagen. The remaining authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ross, D.M., Pagani, I.S., Shanmuganathan, N. et al. Long-term treatment-free remission of chronic myeloid leukemia with falling levels of residual leukemic cells. Leukemia 32, 2572–2579 (2018). https://doi.org/10.1038/s41375-018-0264-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-018-0264-0
- Springer Nature Limited
This article is cited by
-
Single-cell analysis of immune recognition in chronic myeloid leukemia patients following tyrosine kinase inhibitor discontinuation
Leukemia (2024)
-
Computational modeling reveals key factors driving treatment-free remission in chronic myeloid leukemia patients
npj Systems Biology and Applications (2024)
-
Tyrosine Kinase Inhibitor Discontinuation in Chronic Myeloid Leukemia: Strategies to Optimize Success and New Directions
Current Hematologic Malignancy Reports (2024)
-
Cardiovascular Adverse Events of Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia: Clinical Relevance, Impact on Outcome, Preventive Measures and Treatment Strategies
Current Treatment Options in Oncology (2023)
-
Guidelines for the treatment of chronic myeloid leukemia from the NCCN and ELN: differences and similarities
International Journal of Hematology (2023)