Abstract
Objective
To estimate the association of transpyloric feeding (TPF) with the composite outcome of tracheostomy or death for patients with severe bronchopulmonary dysplasia (sBPD).
Study design
Retrospective multi-center cohort study of preterm infants <32 weeks with sBPD receiving enteral feedings. We compared infants who received TPF at 36, 44, or 50 weeks post-menstrual age to those who did not receive TPF at any of those timepoints. Odds ratios were adjusted for gestational age, small for gestational age, male sex, and invasive ventilation and FiO2 at 36 weeks.
Results
Among 1039 patients, 129 (12%) received TPF. TPF was associated with an increased odds of tracheostomy or death (aOR 3.5, 95% CI 2.0–6.1) and prolonged length of stay or death (aOR 3.1, 95% CI 1.9–5.2).
Conclusions
Use of TPF in sBPD after 36 weeks was infrequent and associated with worse in-hospital outcomes, even after adjusting for respiratory severity at 36 weeks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Bronchopulmonary dysplasia (BPD) affects 10,000 preterm infants annually in the United States, and is the most common complication of preterm birth [1]. Despite improving outcomes of survival in preterm infants, incidence of BPD has not improved over time [2]. Most research in BPD focuses on its pathophysiology and strategies to prevent BPD [3, 4]. There is less evidence, and widely variable practices in the management of established BPD, in particular, severe BPD (sBPD), which is generally defined by the need for positive pressure respiratory support at 36 weeks post-menstrual age [5,6,7]. In particular, there is a lack of clear evidence to guide the nutritional management of these infants, though it is known that growth in infants with sBPD correlates with in-hospital outcomes including survival without tracheostomy [8,9,10,11].
One strategy employed in these infants is to administer feeds transpylorically, directly into the small intestine [12,13,14,15]. This is based on evidence that microaspiration of gastric contents might contribute to ongoing lung injury in infants with sBPD [16, 17]. Transpyloric feeds (TPF) are employed as a lung protective strategy in other critically ill children [18, 19]. However, the efficacy of TPF in sBPD is unclear, with one meta-analysis suggesting no benefit [12, 13]. In fact, at least one recent study suggested that some infants might experience an increase in hypoxemic events when receiving TPF compared to gastric feeds [20]. This suggests that TPF may, in fact, result in harm to certain infants with established sBPD. Consequently, it remains unclear if use of TPF to decrease microaspiration into the lungs from gastroesophageal reflux may lessen pulmonary inflammation and improve disease outcomes over time. Additionally, the safety and effect of TPF on growth is unclear [21].
This study aims to leverage a national registry of infants with sBPD from the BPD Collaborative to examine if survival without tracheostomy is associated with the use of TPF in established sBPD compared to those who receive exclusively gastric feeds at 36, 44, and 50 weeks post-menstrual age. In addition, we evaluated secondary outcomes of prolonged length of stay or death, gastrostomy placement, and late weight and linear growth velocities. We hypothesized that sBPD infants who received TPF would have no difference in rate of survival without tracheostomy compared to infants who received exclusively gastric feeds.
Subjects and methods
Data source
We selected study participants enrolled in the BPD Collaborative Registry. The BPD Collaborative is a consortium of interdisciplinary BPD programs with expertise in caring for sBPD that aims to promote research to improve the care of this population [5, 22]. The BPD Collaborative Registry includes patients with BPD cared for at member institutions after 36 weeks PMA during their initial NICU hospitalization. The registry includes demographic data, discharge data, and interim clinical data at three key timepoints: 36 weeks PMA, 44 weeks PMA, and 50 weeks PMA. This includes information about the subjects’ ventilatory support and nutrition at the three key timepoints. In this study, we queried the registry for patients with sBPD born between January 1, 2015 and September 20, 2022. At the time of the analysis, twelve BPD Collaborative Centers had contributed data meeting study inclusion criteria: Children’s Hospital of Philadelphia, Boston Children’s Hospital, Nationwide Children’s Hospital, Children’s Mercy Hospital, Joe DiMaggio Children’s Hospital, Vanderbilt University Medical Center, University of Massachusetts Memorial Medical Center, Women and Infants Hospital of Rhode Island, Arkansas Children’s Hospital, Children’s Hospital of Los Angeles, Phoenix Children’s Hospital, and University of Michigan C.S. Mott Children’s Hospital. Each contributing hospital obtained institutional review board approval to participate in the BPD Collaborative Registry. Informed consent was obtained at sites where the local review board determined doing so was necessary.
Study population
This retrospective cohort study was performed in accordance with STROBE reporting guidelines [23]. The registry includes subjects admitted to BPD centers born <32 weeks gestation who develop sBPD, as defined by the 2001 National Institutes of Health Consensus criteria (premature infants requiring invasive mechanical ventilation, non-invasive positive airway pressure, or nasal cannula with effective FiO2 0.30 at 36 weeks PMA), and do not have a significant congenital or genetic abnormality contributing to their lung disease [24]. All registry subjects who were admitted at one or more of the key timepoints (36, 44, and 50 weeks PMA) were included. Subjects were excluded who were not on enteral feeds at any of the key timepoints (i.e. NPO at 36 weeks, 44 weeks, and 50 weeks), had no information on enteral feeding route at the key timepoints, or did not have outcome data recorded.
Definition of exposure variable
The exposure variable of TPF was defined in the registry as receiving jejunal feeding at one or more of the three key timepoints assessed – 36 weeks, 44 weeks, or 50 weeks PMA. Non-exposed subjects were those who received gastric or oral feeding at all of the key timepoints. Data from timepoints that occurred after the primary study outcome (tracheostomy and/or death) were excluded.
Study outcomes
The primary outcome of our study was the composite outcome of death or tracheostomy, which has been reported in other studies examining outcomes in more severe forms of BPD [25,26,27,28]. A tracheostomy is meant to represent an outcome related to the severity of an infant’s lung disease rather than an adverse outcome in itself [29, 30]. As death and tracheostomy can be competing in-hospital adverse outcomes, a composite outcome of death or tracheostomy was used. The following secondary outcomes were examined in statistical analyses: the composite outcome of death or prolonged length of stay (defined as PMA at discharge ≥75th percentile for the cohort, 56.4 weeks), death alone, tracheostomy among survivors, gastrostomy or jejunostomy in survivors, and weight velocity from 36 weeks PMA until discharge. Weight velocity was calculated in g/kg/d using an exponential model [31].
Covariates
We identified covariates to be included in the regression models a priori known to be associated with disease severity in established BPD, which could therefore be associated with both the exposure (jejunal feeding) and outcome. [7, 32, 33]. These a priori covariates included: gestational age in weeks, small for gestational age (SGA) status (defined as <10th percentile for weigh at birth), and sex. In addition, we included invasive mechanical ventilation (type 2 severe BPD) and FiO2 at 36 weeks PMA as covariates to adjust for baseline severity of lung disease when sBPD is diagnosed. Each covariate had complete data for all participants and was included in final multivariate regression models.
Statistical analysis
Descriptive statistics for the cohort are presented as median with interquartile range (IQR) for continuous data and number (n) with percentage (%) for categorical data. Univariable analyses between the primary and secondary outcomes and exposure to TPF are tested using the chi-square test. Logistic regression models were fit to the composite outcome of death or tracheostomy to determine the adjusted association with TPF. Logistic regression models were also constructed for the outcomes of prolonged length of stay or death, and death alone. Linear models were constructed for the outcome of weight velocity after 36 weeks.
Sub-group analyses with similar outcomes and models were conducted using exposure to TPF at 36 weeks, 44 weeks, and 50 weeks PMA individually, to test for different effects of TPF at each of the key timepoints in the registry. Additionally, a sensitivity analysis was performed restricting the population to invasively ventilated infants at 36 weeks PMA.
R Version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analysis. All P-values are two-sided with a significance threshold of <0.05.
Results
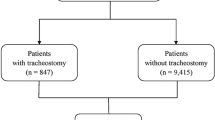
Of 1254 eligible patients enrolled in the BPD Collaborative registry for the study period, 1039 were included in the final cohort (Fig. 1). These represented patients across twelve centers. Among the 1039 included patients, 129 (12%) were exposed to TPF at one or more of the three key timepoints; 59/931 (6%) at 36 weeks PMA, 74/588 (13%) at 44 weeks PMA, and 45/337 (13%) at 50 weeks PMA. They were compared to the 910 (88%) who were not receiving TPF at any of the key timepoints after 36 weeks. TPF use and outcomes varied by center (Supplemental Fig. 1).
The demographics and clinical characteristics of the cohort, stratified by exposure to TPF, are shown in Table 1. Subjects exposed to TPF were born to mothers who were older, more likely to have mothers of Black race, had lower birthweight, and were more likely to be SGA.
Infants exposed to TPF at any of the key timepoints were more likely to be invasively ventilated, require higher ventilator pressures at each of the key timepoints, and require higher FiO2 at 36 and 44 weeks PMA, than those who did not receive TPF at any key timepoint (Table 2). Total fluids (ml/kg/day) were lower only at 50 weeks PMA in the TPF exposed group, and weight was lower at 36 and 44 weeks PMA in the TPF exposed group. Infants receiving TPF at any point were less likely to be receiving any oral feeds at 36, 44, and 50 weeks PMA, and were less likely to be receiving oral feeds at discharge (19% vs 69%, p < 0.001).
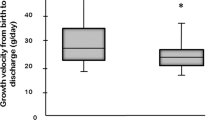
Weight velocity from 36 weeks PMA to discharge was not different between groups (9.2 g/kg/day in TPF exposed infants, 9.6 g/kg/d in non-exposed infants, p = 0.7 by t-test). Infants who received TPF at any point were more likely to receive acid blockade (36% vs 19%, p < 0.001), including histamine-2 blockers or proton pump inhibitors. Only three infants in each group received prokinetic agents. Thirty-one infants (24%) in the TPF group underwent Nissen fundoplication, while only 15 infants (1.6%) in the no-TPF group underwent the procedure (p < 0.001).
Among surviving subjects with discharge feeding route data, only 3 of 100 (3%) who received TPF at any point of the study remained on TPF at hospital discharge. Among infants receiving TPF at 36 or 44 weeks’ PMA, many were not maintained on TPF at later key timepoints (see Supplemental Table 1).
In unadjusted analyses, the primary outcome of tracheostomy or death occurred in 49 (42%) of patients exposed to TPF versus 111 (12%) of patients not-exposed to TPF (p < 0.001, Fig. 2). Prolonged length of stay or death occurred in 65 (56%) of patients exposed to TPF versus 183 (21%) of patients not-exposed to TPF (p < 0.001). Death alone occurred in 11 (9.5%) and 31 (3.5%) of exposed and non-exposed patients, respectively (p = 0.006). Among survivors, 38 (36%) of TPF exposed infants and 80 (9.3%) of non-exposed infants required tracheostomy, and 87 (75%) of TPF exposed infants and 296 (33%) of non-exposed infants required gastrostomy or jejunostomy (both p < 0.001).
Univariate analyses between TPF exposure and the outcomes of (A) Tracheostomy or Death, (B) Prolonged Length of Stay (LOS) or Death, (C) Death Prior to Discharge, (D) Tracheostomy among Survivors, and (E) Gastrostomy among Survivors. **p < 0.01, ***p < 0.001, ****p < 0.0001, by chi-squared test. Exposure defined as receiving transpyloric feeds at 36 weeks, 44 weeks, or 50 weeks PMA. Error bars represent standard error of the proportion. Prolonged LOS = length of stay > PMA 56.4 weeks (75%ile of cohort).
In adjusted analyses (Fig. 3), controlling for GA, SGA, male sex, FiO2 at 36 weeks, and invasive ventilation at 36 weeks PMA, TPF was associated with an increased adjusted odds of tracheostomy or death (aOR 3.5, 95% CI 2.0–6.1, p < 0.001). TPF was also associated with an increased odds of prolonged length of stay (discharge PMA > 56.4 weeks) or death (aOR 3.1, 95% CI 1.9–5.2, p < 0.001) and placement of tracheostomy (aOR 4.0, 95% CI 2.2–7.3, p < 0.001) and gastrostomy or jejunostomy (aOR 4.9, 95% CI 2.6–8.6, p < 0.001) among survivors, but was not associated with an increased odds of death alone (aOR 1.5, 95% CI 0.5–3.5).
Logistic regression models with the outcomes listed and the exposure of transpyloric feeding, controlling for GA, SGA, male sex, FiO2 at 36 weeks, and invasive ventilation at 36 weeks PMA. aOR are plotted along with 95% confidence intervals. Prolonged LOS = length of stay > PMA 56.4 weeks (75%ile of cohort).
Subanalyses were conducted using the exposure variable of TPF at 36, 44, and 50 weeks PMA individually in order to determine if there were different effects of TPF at different ages (Supplementary Fig. 2). TPF exposure at 44 weeks and 50 weeks PMA was associated with tracheostomy and/or death, and exposure at 36 weeks PMA nearly reached significance for this association as well. In addition, a sensitivity analysis restricted to infants who were invasively ventilated at 36 weeks PMA (n = 256) yielded similar results; the aOR for tracheostomy or death with TPF was slightly lower but still significant (aOR 2.5, 95% CI 1.2–5.4) (Supplementary Fig. 3).
Discussion
In this study, we show that among infants being treated for sBPD after 36 weeks PMA at BPD collaborative centers, use of TPF at 36, 44, or 50 weeks PMA was associated with worse in-hospital outcomes, including higher rates of tracheostomy or death as well as prolonged length of stay or death and tracheostomy and gastrostomy or jejunostomy among survivors. This remained true after controlling for respiratory illness severity at 36 weeks PMA (use of invasive ventilation and FiO2 at 36 weeks). The association we observed between TPF and adverse in-hospital outcomes raises uncertainty regarding the purported benefits of routine TPF use in infants with established sBPD.
This is the first study, to our knowledge, to estimate the association of TPF and in-hospital outcomes in babies with established sBPD. TPF has been employed as a nutritional strategy to treat sBPD based on the suspicion that chronic microaspiraton of gastric contents from esophageal reflux contributes to respiratory pathology in these patients. This was based on the finding of a correlation between biomarkers in tracheal aspirates suggesting chronic aspiration and pulmonary outcomes in preterm infants [16]. However, TPF has not been shown to consistently improve pulmonary outcomes in preterm infants, though only a small amount of evidence exists on this subject [13]. The results of this analysis are congruent with the results of one prior n-of-1 crossover trial, which suggested worse episodes of hypoxemia of babies receiving TPF compared to continuous gastric feeds [20]. This may be due to the reflux of gastric contents containing higher concentrations of acid and pepsin without a dilutional or neutralizing effect from feeds, as well as increased risk of bile acid reflux due to tube-related pyloric stenting [34]. TPF when used without benefit may add to the medical burden of these infants, as it requires near-continuous feeding into the small intestine which cannot accommodate bolus feeds, and requires skilled expertise to replace if the feeding tube becomes dislodged [35].
The results of this study do not necessarily suggest that TPF results in worse outcomes; as a retrospective study, we are limited by confounders on the indications for TPF in infants with sBPD. Those infants with greater respiratory support needs at 36 weeks PMA were most likely to receive TPF; however, a sensitivity analysis restricted to infants receiving invasive ventilation at 36 weeks yielded similar results. Even after controlling for respiratory illness severity at 36 weeks PMA, those who received TPF were most likely to need long-term respiratory support via a tracheostomy or die prior to discharge. We were also limited by the availability of data at specific time points – 36, 44, and 50 weeks PMA – without detail to how long these patients were receiving TPF; for example, an infant who received 3 days of TPF at 44 weeks PMA would be considered in the exposed cohort, much like an infant who received 8 weeks of TPF from 36 weeks PMA to 44 weeks PMA. Additionally, as we did not look at TPF prior to 36 weeks, it remains possible that TPF has a protective role in preterm babies with evolving lung disease prior to the formal diagnosis of BPD [12]. It is also notable as only a small percentage of babies in this study who received TPF were discharged with TPF or with Nissen fundoplication.
Prospective studies are needed to better determine if and at what age specific nutritional interventions, including TPF, affect in-hospital outcomes in sBPD patients or patients with evolving lung disease. Nonetheless, this retrospective analysis does not suggest an advantage to employing TPF as a strategy to reduce the likelihood of tracheostomy or death in infants with established sBPD. This may be due to a number of reasons. First of all, it is possible that reflux aspiration is just as frequent, and perhaps worse, with TPF as with gastric feeds. In fact, it has been shown that the number of reflux events per hour during feeding periods is higher than non-feeding periods in infants receiving TPF [36]. Additionally, gastric feeding may serve as an important buffer for the low stomach pH caused by gastric secretions, and thus may reduce injury to the esophageal and respiratory tract caused by the enzymes contained in gastric secretions. Lastly, TPF is not physiologic, and may have unintended results in feed tolerance, which could have impacts on respiratory stability and support required.
Strengths of this study include its large size, specifically the largest to our knowledge cohort of sBPD infants receiving TPF. It also benefits from being a multicenter study, as indications for TPF may vary by institution. At sBPD centers, these indications may include severe respiratory failure, inability to wean from invasive or non-invasive ventilation approaching term age, and gastric feeding intolerance or reflux thought to worsen respiratory disease. The detailed respiratory and nutritional support at multiple time points after 36 weeks PMA is also unique among neonatal registries, and particularly advantageous the study of infants with established sBPD.
There are important limitations of this study. First, as above, the retrospective nature of the study does not imply causality between TPF and the outcomes of interest, particularly tracheostomy and death; TPF may simply be a marker of illness severity, and there may be residual confounding beyond ventilation support and FiO2 at 36 weeks’ PMA. However, our results suggest that there does not seem to be a significant benefit to the use of TPF in this cohort. We also did not have information on the indication for use of TPF; specifically, if they were employed due to a diagnosis of gastroesophageal reflux, severity of respiratory illness, a specific phenotype of sBPD (such a pulmonary hypertension or airway malacia), or as a general treatment strategy for sBPD in itself. Likewise, we did not have available data if gastric feeds were run continuously or via bolus. As previously mentioned, we were also unable to account for any dose-dependent effect based on true duration of TPF or effect of TPF at other time points than the three data points analyzed; however, subanalyses at individual data points (36, 44 and 50 weeks PMA) suggest similar trends at each data point. There also may be center by center variability in the indications for TPF that is unaccounted for that do in fact correlate with the outcomes of interest, specifically tracheostomy, which may also vary center by center. This practice variability was not accounted for in the analysis, and could have resulted in confidence intervals that are artificially more narrow. Regarding safety, we were also unable to account for history of necrotizing enterocolitis or gastrointestinal complications of postpyloric feeding, which were not available in the registry. Finally, the cohort studied includes sBPD patients who are being cared for at BPD collaborative centers; these patients are most likely to have severe disease and adverse in-hospital outcomes. The results of this study are not generalizable to premature infants prior to 36 weeks PMA who are at risk for development of BPD; further study is needed in this population.
To conclude, among infants with sBPD, the use of TPF after 36 weeks PMA was associated with adverse in-hospital outcomes, including: (1) tracheostomy or death prior to discharge, (2) prolonged length of stay or death prior to discharge, and (3) tracheostomy and gastrostomy or jejunostomy among survivors. Weight trends were similar in each population. Future prospective trials are needed to determine how specific nutritional interventions, including TPF, affect respiratory and growth outcomes for patients with established sBPD, and if there are subpopulations or phenotypes of sBPD that may respond favorably to this strategy.
Data availability
A de-identified dataset is available upon request.
References
Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314:1039–51.
Martin JA, Osterman MJK. Describing the Increase in Preterm Births in the United States, 2014-6. NCHS Data Brief. 2018;312:1–8.
Jobe AH. Mechanisms of lung injury and bronchopulmonary dysplasia. Am J Perinatol. 2016;33:1076–8.
Kennedy KA, Cotten CM, Watterberg KL, Carlo WA. Prevention and management of bronchopulmonary dysplasia: lessons learned from the neonatal research network. Semin Perinatol. 2016;40:348–55.
Abman SH, Collaco JM, Shepherd EG, Keszler M, Cuevas-Guaman M, Welty SE, et al. Interdisciplinary care of children with severe bronchopulmonary dysplasia. J Pediatr. 2017;181:12–28.e1.
Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am J Respir Crit Care Med. 2019;200:751–9.
Jensen EA, Edwards EM, Greenberg LT, Soll RF, Ehret DEY, Horbar JD. Severity of bronchopulmonary dysplasia among very preterm infants in the United States. Pediatrics. 2021;148:e2020030007.
Poindexter BB, Martin CR. Impact of nutrition on bronchopulmonary dysplasia. Clin Perinatol. 2015;42:797–806.
Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–61.
Natarajan G, Johnson YR, Brozanski B, Farrow KN, Zaniletti I, Padula MA, et al. Postnatal weight gain in preterm infants with severe bronchopulmonary dysplasia. Am J Perinatol. 2014;31:223–30.
Kielt MJ, Logan JW, Backes CH, Reber KM, Nelin LD, Shepherd EG. In-hospital outcomes of late referrals for established bronchopulmonary dysplasia. J Perinatol. 2021;41:1972–82.
Wallenstein MB, Brooks C, Kline TA, Beck RQ, Yang W, Shaw GM, et al. Early transpyloric vs gastric feeding in preterm infants: a retrospective cohort study. J Perinatol. 2019;39:837–41.
Watson J, McGuire W. Transpyloric versus gastric tube feeding for preterm infants. Cochrane Database Syst Rev. 2013;2:CD003487.
Malcolm WF, Smith PB, Mears S, Goldberg RN, Cotten CM. Transpyloric tube feeding in very low birthweight infants with suspected gastroesophageal reflux: impact on apnea and bradycardia. J Perinatol. 2009;29:372–5.
Shimokaze T, Yamamoto K, Miyamoto Y, Toyoshima K, Katsumata K, Saito T. Acute respiratory effect of transpyloric feeding for respiratory exacerbation in preterm infants. J Perinat Med. 2021;49:383–7.
Farhath S, He Z, Nakhla T, Saslow J, Soundar S, Camacho J, et al. Pepsin, a marker of gastric contents, is increased in tracheal aspirates from preterm infants who develop bronchopulmonary dysplasia. Pediatrics. 2008;121:e253–259.
Radford PJ, Stillwell PC, Blue B, Hertel G. Aspiration complicating bronchopulmonary dysplasia. CHEST. 1995;107:185–8.
Jiyong J, Tiancha H, Huiqin W, Jingfen J. Effect of gastric versus post-pyloric feeding on the incidence of pneumonia in critically ill patients: observations from traditional and Bayesian random-effects meta-analysis. Clin Nutr. 2013;32:8–15.
Lyons KA, Brilli RJ, Wieman RA, Jacobs BR. Continuation of transpyloric feeding during weaning of mechanical ventilation and tracheal extubation in children: a randomized controlled trial. JPEN J Parenter Enteral Nutr. 2002;26:209–13.
Jensen EA, Zhang H, Feng R, Dysart K, Nilan K, Munson DA, et al. Individualising care in severe bronchopulmonary dysplasia: a series of N-of-1 trials comparing transpyloric and gastric feeding. Arch Dis Child Fetal Neonatal Ed. 2020;105:399–404.
Wallenstein MB, Stevenson DK. Need for reassessment of early transpyloric feeding in preterm infants. JAMA Pediatr. 2018;172:1004–5.
Guaman MC, Gien J, Baker CD, Zhang H, Austin ED, Collaco JM. Point prevalence, clinical characteristics, and treatment variation for infants with severe bronchopulmonary dysplasia. Am J Perinatol. 2015;32:960–7.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296.
Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9.
Murthy K, Savani RC, Lagatta JM, Zaniletti I, Wadhawan R, Truog W, et al. Predicting death or tracheostomy placement in infants with severe bronchopulmonary dysplasia. J Perinatol. 2014;34:543–8.
Murthy K, Porta NFM, Lagatta JM, Zaniletti I, Truog WE, Grover TR, et al. Inter-center variation in death or tracheostomy placement in infants with severe bronchopulmonary dysplasia. J Perinatol. 2017;37:723–7.
Wu KY, Jensen EA, White AM, Wang Y, Biko DM, Nilan K, et al. Characterization of disease phenotype in very preterm infants with severe bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2020;201:1398–406.
Kielt MJ, Logan JW, Backes CH, Conroy S, Reber KM, Shepherd EG, et al. Noninvasive respiratory severity indices predict adverse outcomes in bronchopulmonary dysplasia. J Pediatr. 2021;242:129–36.
DeMauro SB, D’Agostino JA, Bann C, Bernbaum J, Gerdes M, Bell EF, et al. Developmental outcomes of very preterm infants with tracheostomies. J Pediatr. 2014;164:1303–1310.e2.
Gien J, Abman SH, Baker CD. Interdisciplinary care for ventilator-dependent infants with chronic lung disease. J Pediatr. 2014;165:1274–5.
Patel AL, Engstrom JL, Meier PP, Kimura RE. Accuracy of methods for calculating postnatal growth velocity for extremely low birth weight infants. Pediatrics. 2005;116:1466–73.
Bose C, Van Marter LJ, Laughon M, O’Shea TM, Allred EN, Karna P, et al. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics. 2009;124:e450–458.
Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183:1715–22.
Rosen R, Lurie M, Kane M, DiFilippo C, Cohen A, Freiberger D, et al. Risk factors for bile aspiration and its impact on clinical outcomes. Clin Transl Gastroenterol. 2021;12:e00434.
Hawk H, Valdivia H. Bedside methods for transpyloric feeding tube insertion in hospitalized children: a systematic review of randomized and non-randomized trials. J Pediatr Nurs. 2021;60:238–46.
Rosen R, Hart K, Warlaumont M. Incidence of gastroesophageal reflux during transpyloric feeds. J Pediatr Gastroenterol Nutr. 2011;52:532–5.
Funding
This work was supported by the Gerber Foundation (JC Levin) and NIH/NHBLI K23 HL136851 (LP Hayden).
Author information
Authors and Affiliations
Contributions
JCL, MJK, KTL conceptualized the manuscript. JCL and MJK conducted the analyses. JCL, MJK, LPH, SEC, and KTL interpreted the results of the analysis. JCL, MJK, LPH, LDN contributed data. WET, MCG, SHA, LDN, RLR, and KTL provided supervision, resources, and oversight. JCL wrote the initial draft of the manuscript; all authors reviewed the manuscript and approved its final version for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Levin, J.C., Kielt, M.J., Hayden, L.P. et al. Transpyloric feeding is associated with adverse in-hospital outcomes in infants with severe bronchopulmonary dysplasia. J Perinatol 44, 307–313 (2024). https://doi.org/10.1038/s41372-024-01867-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-024-01867-w
- Springer Nature America, Inc.
This article is cited by
-
Transpyloric feeding in severe BPD: a call for prospective trials
Journal of Perinatology (2024)