Abstract
Objective
To evaluate the short-term outcomes of implementing a care bundle emphasizing frequent hemodynamic assessments by echocardiography in neonates with congenital diaphragmatic hernia (CDH).
Study design
This was a retrospective cohort study of infants with CDH admitted to a quaternary perinatal unit from January 2013 to March 2021. The primary composite outcome was defined as mortality or use of extracorporeal membrane oxygenation or need for respiratory support at discharge.
Results
We identified 37 and 20 CDH infants in Epoch I and II, respectively. More patch repairs (50% vs. 21.9%, p = 0.035) and echocardiograms (6[4–8] vs. 1[0–5], p = 0.003) were performed in Epoch II. While there were no differences in the primary outcome, there was a reduction in mortality in Epoch II (0% vs. 27%, p = 0.01).
Conclusion
With the implementation of a CDH care bundle with an emphasis on hemodynamic assessment, we demonstrated a significant reduction in mortality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Congenital diaphragmatic hernia (CDH) is one of the most common major congenital anomalies, occurring in 1 in 4000–5000 live births [1]. It is associated with significant morbidity and mortality, primarily due to physiological derangement caused by pulmonary hypoplasia and pulmonary hypertension [2, 3]. Furthermore, the severity of pulmonary hypertension and cardiac dysfunction in infants with CDH are major determinants of patient survival and outcomes [4, 5]. In Canada, the mortality rate for infants with CDH is around 21% and the survivors can have significant long-term health concerns [6, 7].
Targeted neonatal echocardiography (TnEcho) is a non-invasive imaging technique used to assess the cardiac structure and function of the newborn infants in the neonatal intensive care unit (NICU) and is performed primarily by specially trained neonatologists [8, 9]. The hemodynamic information derived can be used to estimate pulmonary pressures and predict pulmonary hypertension severity with reasonable accuracy to guide clinical decision making [10]. Patel et al. demonstrated that 58% of infants with CDH had impaired right ventricular function within the first 48 h of life [4]. It has been demonstrated that right ventricular diastolic dysfunction is associated with CDH severity and predicts early outcomes in CDH [11]. Lusk et al. reported that weekly echocardiographic assessments of pulmonary hypertension for up to 6 weeks in infants with CDH were helpful in predicting short-term outcomes [12].
Substantial variations in practice have been reported and multicenter standardization of CDH management in Europe has led to improvement in outcomes [13]. In 2018, a new protocol on CDH management was jointly developed by Canadian centers [14]. Though TnEcho has been shown to improve neonatal outcomes by guiding cardiovascular care such as management of patent ductus arteriosus [15,16,17,18], it is unclear if including regular and frequent bedside hemodynamic assessments with TnEcho peri- and post-operatively can improve the outcomes of infants with CDH, who are at high risk of pulmonary hypertension and ventricular dysfunction.
In this study, we aimed to evaluate the impact of a CDH care bundle with emphasis on dedicated hemodynamic assessments by TnEcho on the short-term outcomes of neonates with CDH. We hypothesized that bundle implementation would improve short term patient outcomes.
Methods
Study design and patients
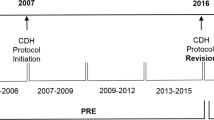
This was a retrospective cohort study of infants born at gestational age (GA) ≥ 34 weeks with diagnosis of CDH based on postnatal radiographic evidence and/or antenatal scans and admitted to the British Columbia Women’s Hospital NICU (Vancouver BC, Canada) from January 2013 to March 2021. Infants were excluded if they had a major congenital heart defect (except for a patent ductus arteriosus, patent foramen ovale, atrial septal defect, and small ventricular septal defect) or hypoxic ischemic encephalopathy. We obtained patient demographics and cardiorespiratory data from a local neonatal database that derived information from clinical charts. The cohort was divided into two epochs (epoch I = January 2013–December 2017; epoch II = January 2018–March 2021). During epoch II, we introduced a quality improvement bundle consisting of four modifications in clinical care:
-
1.)
A dedicated hemodynamic assessment program with TnEcho after structural heart disease had been ruled out by our pediatric cardiology team – a team of two TnEcho-trained neonatologists provided frequent TnEcho assessment of pulmonary hypertension, cardiac output, and ventricular dysfunction every 24–48 h on average, before and after CDH repair, till the resolution of systemic pulmonary hypertension. TnEcho studies were performed using the Vivid E9 cardiovascular ultrasound system (GE Medical Systems, Milwaukee Wisconsin, USA) with a 6- or 12-MHz high-frequency phased-array transducer probe. For detailed TnEcho protocol, please refer to the supplementary file.
-
2.)
Daily bedside multidisciplinary care rounds during the acute phase of illness – consisting of a team of neonatologists, cardiologists, anesthetists, surgeons and/or extracorporeal membrane oxygenation (ECMO) specialists to review the clinical care of patient at the bedside daily (morning and evening rounds) to develop a management plan per consensus of expertize to enhance timely communication amongst stakeholders.
-
3.)
Implementation of the Canadian CDH Collaborative clinical practice guideline into bedside care [14] – including use of a guidelines “app” on communication devices at the bedside with systematic documentation of compliance or variance with individual recommendations.
-
4.)
Shift to use of high frequency jet ventilation for stabilization and repair of high risk CDH – previously we used high frequency ventilation (oscillator or jet) as a “rescue” mode for infants whose ventilatory or oxygenation targets could not be met with conventional mechanical ventilation and were either at risk of significant barotrauma or as an attempt to avoid ECMO.
Demographics and clinical parameters
Demographic and clinical data were collected during the infant’s NICU stay. Data on neonatal demographics (GA at birth, birth weight, small for GA, sex, inborn vs. outborn, mode of delivery, Apgar score at 5 min <7, score for neonatal acute physiology II (SNAP II) ≥ 28 [19, 20], and age of surgery) were collected from medical charts, as per the Canadian Neonatal Network Abstractors’ manual [21]. Prenatal diagnosis, use of fetal MRI, presence of associated malformations, and operative data (age at surgery, CDH Study Group (CDHSG) defect size [22], surgical approach and closure type) were collected from the Canadian Pediatric Surgery Network data registry [23] and hospital electronic database. Details of medical treatment including ventilation and use/duration of medications and echocardiogram findings were collected from medical charts, echocardiography database and the pharmacy department database. Short-term neonatal outcomes (mortality, length of invasive and non-invasive respiratory support, type of invasive ventilation, ECMO requirement, and length of hospitalization), and discharge parameters (survival to discharge, respiratory support and feeding support on discharge) were collected from medical charts.
Outcomes measurements
The primary composite outcome of any one of mortality during the birth hospitalization, use of ECMO, and need for respiratory support (including continuous positive airway pressure/non-invasive ventilation/tracheostomy) at discharge was compared between epoch I and epoch II. Secondary outcomes include individual short-term outcomes and morbidities, duration of all cardiotherapeutic agents, and use of inhaled nitric oxide, benzodiazepines, opioids, and neuromuscular blockers.
Statistical analyses
Descriptive statistics (frequencies [%] or median [interquartile range, [IQR]]) were used to characterize the demographics and clinical parameters. Between-epoch differences were assessed using Mann–Whitney U test for continuous variables and Chi-square or Fisher’s exact tests for categorical variables. Significance was set at p < 0.05. All analyses were performed in R (version 4.2.1) using R-studio (version 1.4.1717).
Results
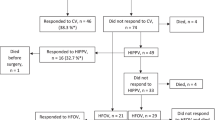
A total of 57 infants with CDH met the inclusion criteria, of which 37 were in epoch I and 20 in epoch II. Baseline characteristics, in terms of GA, birth weight, and delivery mode, were similar among the infants during both epochs (Table 1). There was increasing use of fetal MRI during epoch II (epoch I = 24.3% vs epoch II = 55%; p = 0.042). There were no differences between side of defect, liver herniation, CDHSG staging, presence of significant pre-operative pulmonary hypertension, or methods of surgery (Table 1). There was a significant increase in patch closure rate among the infants with CDH during epoch II (epoch I = 21.9% vs epoch II = 50%; p = 0.035) (Table 1).
The median number of total echocardiograms performed pre-operatively was the same in the two epochs, however, there was an increase in the number of post-operative echocardiogram assessments during epoch II (epoch I = 1 [0–5] vs epoch II = 6 [4–8]; p = 0.003) (Table 1).
There was no significant difference in the primary outcome (any one of mortality, use of ECMO, and discharge on respiratory support) between epoch I (35%) and II (25%) (Table 1). There was a significant decline in overall mortality among the infants with CDH during epoch II (epoch I = 27% [n = 10] vs epoch II = 0% [n = 0]; p = 0.010) (Table 2). During epoch II, use of dobutamine (epoch I = 3.7% vs epoch II = 80%; p < 0.01), milrinone (epoch I = 18.5% vs epoch II = 50%; p = 0.049), hydrocortisone (epoch I = 22.2% vs epoch II = 55%; p = 0.045), and vasopressin (epoch I = 7.4% vs epoch II = 35%; p = 0.045) was higher (Table 2). The duration of epinephrine use was shorter (epoch I = 3 [3–4] days vs epoch II = 1 [1–1.3] days; p = 0.008) (Table 2). No differences were seen in the duration of use for other cardiotherapeutic medications. No differences were observed in the use of benzodiazepines, opioids, and neuromuscular blockers, or short-term neonatal outcomes such as duration of ventilation and length of hospital stay (Table 2).
Discussion
There is little question that pulmonary hypertension and cardiac dysfunction are the primary drivers of morbidity and mortality in infants with CDH [24, 25]. While there was no difference in the composite primary outcome (any one of mortality, use of ECMO, and respiratory on discharge), we demonstrated a significant reduction in overall mortality from 27.0% in epoch I to 0% in epoch II without any increase in short-term neonatal morbidity. Although the two groups seemed generally comparable, Epoch II had a higher rate of patch repair, a known predictor of adverse outcome [26], which makes the finding of a lower mortality rate in Epoch II notable.
CDH affects a plethora of organ systems, thus an expert multidisciplinary approach is necessary to optimize patient outcomes [27]. Standardization offered by evidence-based clinical practice guidelines reduces unnecessary practice variation and has been recognized as a contributor to improved outcomes. Most Canadian centers have implemented the CDH app into bedside care to support clinical decision-making in real time [28]. The other relevant practice change have been our group’s preferential use of high frequency jet ventilation as a primary strategy in high risk CDH [29].
Incorporating TnEcho into a standardized hemodynamic assessment program has had a significant impact on the clinical management of infants in the NICU [17, 30]. In the CDH population, objective measure of echocardiogram-estimated pulmonary pressure has been used to guide the timing of CDH repair, with fewer acute decompensations compared to patients whose treatment decisions were made without strict echocardiographic criteria [31].
The implementation of a TnEcho program in the NICU requires collaboration with pediatric cardiologists for initial training and maintenance of competence. Neonatologist-performed TnEcho assessment is quick and can provide real-time hemodynamic information to guide diagnosis and therapeutic intervention [9]. Complete echocardiogram (i.e., structural and functional) are usually performed by cardiologists and are strongly recommended as the initial assessment tool in CDH care [25, 32]. A recent study demonstrated that echocardiography findings at 3 standardized times points (within 72 h of birth, 24–48 h pre-operative, and 24–48 h post-operative) were useful in guiding the initiation and choice of cardiotherapeutic agents as well as readiness for surgery [33]. Rather than performing TnEcho assessment at prescribed times, we used causal uncertainty of a hemodynamic state as the trigger for frequent echocardiographic assessment, particularly in the most vulnerable postoperative period where we observed a 6-fold increase in utilization in Epoch II compared to Epoch I.
Clinical management of neonates with CDH should prioritize pharmacologic stabilization of hemodynamics status [34, 35]. Rather than simply focusing on blood pressure numbers, the use of real-time TnEcho to provide information on cardiac output and ventricular function led to change in management in 40% of infants, particularly the choice of most appropriate inotropic support agents in rapidly changing hemodynamic situations [9]. We speculate that the increased use of TnEcho was responsible for the increased use of dobutamine (80% vs. 3.7%), milrinone (50% vs. 18.5%), hydrocortisone (55% vs. 22.2%), and vasopressin (35% vs. 7.4%) in epoch II patients, because of the recognition and targeted therapy on pulmonary hypertension. While dopamine is one of the most widely used cardiotherapeutic agents in the NICU, experts recommend shifting away from the use of dopamine due to concerns of exacerbating pulmonary pressures [36]. Dobutamine and/or milrinone can be used as an adjunct to iNO to augment pulmonary vasodilation [37, 38]. They have been used for the treatment and management of refractory pulmonary hypertension in infants with hypoxic respiratory failure due to other causes [37, 39, 40]. Adrenal insufficiency has been reported to be prevalent among infants with CDH and infants with low cortisol levels were associated with increased severity of illness [41]. While there have been no evidence-based guidelines for cardiotherapeutic therapy for infants with CDH [42], experts recommend that the treatment of infants with CDH should focus on the stabilization of cardiopulmonary status over surgical repair, leading to the increased use of echocardiography to manage hemodynamic function and guide clinical decisions [43]. Recognizing the underlying pathophysiology of each patient at specific time points by dedicated TnEcho assessment to provide tailor-made hemodynamic support is of utmost importance and was a driving factor in the overall increased use of pharmacologic hemodynamic support in Epoch II. Although it is impossible to attribute the improvement in outcome to an individual aspect of this integrated approach, we feel that targeted cardiotherapeutics based on hemodynamic assessment with TnEcho likely played a substantial role in the reduction of mortality observed in the post-implementation cohort.
This study has limitations inherent in retrospective review. Due to the relatively small number of patients in each cohort, the study is likely underpowered to address all the short-term outcomes and control for confounding variables. TnEcho assessment was one of the components of a care bundle. Ascertaining the precise contribution of each component to the observed improvement in patient outcomes presents a complex challenge. In our previous study, we showed that HFJV was found to be safe in this group of patients, though it did not showed a statistically significant improvement in terms of mortality/ECMO use/need for respiratory support at discharge [29]. Future research with longitudinal follow-up data from multiple centers could provide valuable information on the impact of management and treatment strategies over time as well as to develop a standardized follow-up protocol in this population [44].
In conclusion, with the introduction of a CDH care bundle with emphasis on frequent and standardized hemodynamic assessment by TnEcho, we demonstrated a significant reduction in overall mortality without an increase in short-term neonatal morbidity in this cohort of infants with CDH.
Data availability
The database for this research article is not available because of identifiable patient particulars in a relatively small number of patients.
References
Paoletti M, Raffler G, Gaffi MS, Antounians L, Lauriti G, Zani A. Prevalence and risk factors for congenital diaphragmatic hernia: a global view. J Pediatr Surg. 2020;55:2297–307.
Mohseni-Bod H, Bohn D. Pulmonary hypertension in congenital diaphragmatic hernia. Semin Pediatr Surg. 2007;16:126–33.
Thibeault DW, Haney B. Lung volume, pulmonary vasculature, and factors affecting survival in congenital diaphragmatic hernia. Pediatrics. 1998;101:289–95.
Patel N, Kipfmueller F. Cardiac dysfunction in congenital diaphragmatic hernia: pathophysiology, clinical assessment, and management. Semin Pediatr Surg. 2017;26:154–8.
Dillon PW, Cilley RE, Mauger D, Zachary C, Meier A. The relationship of pulmonary artery pressure and survival in congenital diaphragmatic hernia. J Pediatr Surg. 2004;39:307–12.
Baird R, MacNab YC, Skarsgard ED. Mortality prediction in congenital diaphragmatic hernia. J Pediatr Surg. 2008;43:783–7.
Safavi A, Synnes AR, O’Brien K, Chiang M, Skarsgard ED, Chiu PP. Multi-institutional follow-up of patients with congenital diaphragmatic hernia reveals severe disability and variations in practice. J Pediatr Surg. 2012;47:836–41.
Tissot C, Singh Y. Neonatal functional echocardiography. Curr Opin Pediatr. 2020;32:235–44.
El-Khuffash AF, McNamara PJ. Neonatologist-performed functional echocardiography in the neonatal intensive care unit. Semin Fetal Neonatal Med. 2011;16:50–60.
McCrary AW, Barker PCA, Torok RD, Spears TG, Li JS, Hornik CP, et al. Agreement of an echocardiogram-based diagnosis of pulmonary hypertension in infants at risk for bronchopulmonary dysplasia among masked reviewers. J Perinatol. 2019;39:248–55.
Moenkemeyer F, Patel N. Right ventricular diastolic function measured by tissue doppler imaging predicts early outcome in congenital diaphragmatic hernia. Pediatr Crit Care Med. 2014;15:49–55.
Lusk LA, Wai KC, Moon-Grady AJ, Steurer MA, Keller RL. Persistence of pulmonary hypertension by echocardiography predicts short-term outcomes in congenital diaphragmatic hernia. J Pediatr. 2015;166:251–6.e1.
Reiss I, Schaible T, van den Hout L, Capolupo I, Allegaert K, van Heijst A, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium consensus. Neonatology. 2010;98:354–64.
Puligandla PS, Skarsgard ED, Offringa M, Adatia I, Baird R, Bailey M, et al. Diagnosis and management of congenital diaphragmatic hernia: a clinical practice guideline. Cmaj. 2018;190:E103–e12.
Yadav K, Hebert A, Lavie-Nevo K, Kuan MTY, Castaldo M, Osiovich H, et al. Targeted neonatal echocardiography for patent ductus arteriosus in neonates reduces the surgical ligation rate without affecting healthcare outcomes. Transl Pediatr. 2023;12:137–45.
Ting JY, Resende M, More K, Nicholls D, Weisz DE, El-Khuffash A, et al. Predictors of respiratory instability in neonates undergoing patient ductus arteriosus ligation after the introduction of targeted milrinone treatment. J Thorac Cardiovasc Surg. 2016;152:498–504.
El-Khuffash A, Herbozo C, Jain A, Lapointe A, McNamara PJ. Targeted neonatal echocardiography (TnECHO) service in a Canadian neonatal intensive care unit: a 4-year experience. J Perinatol. 2013;33:687–90.
Alammary D, Narvey M, Soni R, Elsayed Y, Louis D. Targeted neonatal echocardiography service in neonatal intensive care in Manitoba, Canada. J Perinatol. 2022;42:655–9.
Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138:92–100.
Kipfmueller F, Schroeder L, Melaku T, Geipel A, Berg C, Gembruch U, et al. Prediction of ECMO and mortality in neonates with congenital diaphragmatic hernia using the SNAP-II Score. Klin Padiatr. 2019;231:297–303.
The Canadian Neonatal Network. The Canadian Neonatal Network Abstractor’s Mannual 2023 [updated March 8, 2023. Available from: https://www.canadianneonatalnetwork.org/portal/Portals/0/CNN%20Manuals/CNN%20Manual_20230308.pdf.
Harting MT, Lally KP. The congenital diaphragmatic hernia study group registry update. Semin Fetal Neonatal Med. 2014;19:370–5.
Puligandla PS, Skarsgard ED. The canadian pediatric surgery network congenital diaphragmatic hernia evidence review project: developing national guidelines for care. Paediatr Child Health. 2016;21:183–6.
Ferguson DM, Gupta VS, Lally PA, Luco M, Tsao K, Lally KP, et al. Early, postnatal pulmonary hypertension severity predicts inpatient outcomes in congenital diaphragmatic hernia. Neonatology. 2021;118:147–54.
Patel N, Massolo AC, Kraemer US, Kipfmueller F. The heart in congenital diaphragmatic hernia: Knowns, unknowns, and future priorities. Front Pediatr. 2022;10:890422.
Brindle ME, Brar M, Skarsgard ED. Patch repair is an independent predictor of morbidity and mortality in congenital diaphragmatic hernia. Pediatr Surg Int. 2011;27:969–74.
van den Hout L, Sluiter I, Gischler S, De Klein A, Rottier R, Ijsselstijn H, et al. Can we improve outcome of congenital diaphragmatic hernia? Pediatr Surg Int. 2009;25:733–43.
LaRusso K, Puligandla PS, Anna GS. The canadian congenital diaphragmatic hernia collaborative mobile app: a national guideline implementation strategy. Am J Perinatol. 2020;37:S66–S70.
Al Kharusi A, Al-Maawali A, Traynor M, Adreak N, Ting J, Skarsgard ED. High frequency jet ventilation for congenital diaphragmatic hernia. J Pediatr Surg. 2023;58:799–802.
Papadhima I, Louis D, Purna J, Deshpande P, Diambomba Y, Lee S, et al. Targeted neonatal echocardiography (TNE) consult service in a large tertiary perinatal center in Canada. J Perinatol. 2018;38:1039–45.
Deeney S, Howley LW, Hodges M, Liechty KW, Marwan AI, Gien J, et al. Impact of objective echocardiographic criteria for timing of congenital diaphragmatic hernia repair. J Pediatr. 2018;192:99–104.e4.
Snoek KG, Reiss IKM, Greenough A, Capolupo I, Urlesberger B, Wessel L, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium Consensus - 2015 Update. Neonatology. 2016;110:66–74.
Soni R, Soni N, Chakkarapani A, Gupta S, Yajamanyam PK, Ali SKM, et al. The utility of serial echocardiography parameters in management of newborns with Congenital Diaphragmatic Hernia (CDH) and predictors of mortality. Pediatr Cardiol. 2023;44:354–66.
Aggarwal S, Shanti C, Agarwal P, Lelli J, Natarajan G. Echocardiographic measures of ventricular-vascular interactions in congenital diaphragmatic hernia. Early Hum Dev. 2022;165:105534.
Dingeldein M. Congenital diaphragmatic hernia: management & outcomes. Adv Pediatr. 2018;65:241–7.
McNamara PJ, Giesinger RE, Lakshminrusimha S. Dopamine and neonatal pulmonary hypertension - pressing need for a better pressor? J Pediatr. 2022;246:242–50.
Jain A, McNamara PJ. Persistent pulmonary hypertension of the newborn: Advances in diagnosis and treatment. Semin Fetal Neonatal Med. 2015;20:262–71.
Giesinger RE, McNamara PJ. Hemodynamic instability in the critically ill neonate: an approach to cardiovascular support based on disease pathophysiology. Semin Perinatol. 2016;40:174–88.
Mohamed A, Nasef N, Shah V, McNamara PJ. Vasopressin as a rescue therapy for refractory pulmonary hypertension in neonates: case series. Pediatr Crit Care Med. 2014;15:148–54.
Qasim A, Jain SK. Milrinone use in persistent pulmonary hypertension of the newborn. NeoReviews. 2020;21:e165–e78.
Kamath BD, Fashaw L, Kinsella JP. Adrenal insufficiency in newborns with congenital diaphragmatic hernia. J Pediatr. 2010;156:495–7.e1.
Lakshminrusimha S, Keszler M, Kirpalani H, Van Meurs K, Chess P, Ambalavanan N, et al. Milrinone in congenital diaphragmatic hernia – a randomized pilot trial: study protocol, review of literature and survey of current practices. Matern Health, Neonatol Perinatol. 2017;3:27.
Skarsgard ED. Preoperative management of congenital diaphragmatic hernia. Curr Treat Options Pediatr. 2022;8:232–45.
Hollinger LE, Harting MT, Lally KP. Long-term follow-up of congenital diaphragmatic hernia. Semin Pediatr Surg. 2017;26:178–84.
Acknowledgements
We thank Dr. Alfonso Solimano for his contribution to the implementation of the TnEcho protocol.
Author information
Authors and Affiliations
Contributions
MTYK, KY, ES and JYT conceptualized and designed the study. MTYK, KY and JT collected the data. All authors analyzed and interpreted the data. MK and JYT drafted the initial manuscript. All authors critically reviewed and revised the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuan, M.T.Y., Yadav, K., Castaldo, M. et al. The impact of a care bundle with an emphasis on hemodynamic assessment on the short-term outcomes in neonates with congenital diaphragmatic hernia. J Perinatol 44, 348–353 (2024). https://doi.org/10.1038/s41372-023-01807-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01807-0
- Springer Nature America, Inc.