Abstract
Objective
To examine the prevalence of antenatal maternal hypoglycemia after initiation of pharmacotherapy for gestational diabetes mellitus (GDMA2) and its association with pregnancy outcomes.
Study Design
Retrospective cohort of GDMA2 women receiving either insulin or oral hypoglycemic agents. Composite neonatal outcome included macrosomia, jaundice, respiratory distress syndrome, large for gestational age, shoulder dystocia, birth trauma, 5-minute Apgar < 7, and neonatal hypoglycemia, and was compared between women with and without hypoglycemia using bivariate and multivariate analyses.
Results
Of 489 women included in the study, 95 (19.4%) had at least one episode of hypoglycemia, most often in the setting of glyburide. Newborns exposed to maternal hypoglycemia had higher rates of the composite neonatal outcome (54.7% vs. 38.3%, p = 0.004). After controlling for confounding factors, maternal hypoglycemia remained independently associated with the composite neonatal outcome (aOR = 1.69, 95% CI 1.04–2.72).
Conclusion
Maternal hypoglycemia in GDMA2 was associated with higher rates of adverse neonatal outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Gestational diabetes mellitus (GDM) affects up to 10% of pregnant women in the United States every year [1]. For many women, pregnancy is the first exposure to insulin or oral hypoglycemic agents and the subsequent side effects of these medications. One of the most concerning side effects of glucose-lowering medications as reported in large randomized trials is hypoglycemia [2, 3]. Maternal hypoglycemia is seen in up to 66% of pregnant women receiving treatment for GDM [2,3,4,5,6]. The risk of hypoglycemia was noted to be higher with insulin compared to oral hypoglycemic agents in some studies [2,3,4,5], but not in others [6], and the definition of hypoglycemia has varied over time [2,3,4,5,6]. Further information on perinatal outcomes accompanying maternal hypoglycemia is lacking.
The effects of hypoglycemia on maternal health can be profound as seen in women with type 1 diabetes mellitus (T1DM) in pregnancy, with approximately one quarter to three quarters of pregnant women experiencing severe symptoms or requiring treatment [7,8,9]. The effects of hypoglycemia on the fetuses of women with T1DM are more challenging to elucidate as some studies conclude there is no difference, while others suggest there may be both short and long-term impacts on fetal neurologic development [10]. Moreover, the initial studies on hypoglycemia were specific to T1DM and did not include women with GDM [7,8,9,10]. To date, the data on the impact of hypoglycemia on maternal and neonatal outcomes in women with GDM is limited. A few studies investigated the association between maternal hypoglycemia at 1-hour glucose screening test or at 3-hour confirmatory test [11,12,13,14,15]. They found higher rates of small-for-gestational age (SGA), higher rates of preeclampsia and eclampsia, and higher rates of neonatal intensive care unit (NICU) admissions [11, 12, 14]. However, these studies evaluated hypoglycemia only at the time of 1-hour or 3-hour testing, and not the iatrogenic hypoglycemia secondary to pharmacotherapy for GDMA2.
A 1989 study by Langer et al. found that lower average glycemic levels were associated with SGA infants [16]. This study did not specifically evaluate hypoglycemia, and the mean maternal glucose level in the group with SGA was 88 mg/dL (4.9 mmol/L). From animal studies, concerning effects of severe, prolonged hypoglycemia have been demonstrated in rats with resulting cranial, eye, and skeletal abnormalities in addition to SGA [17,18,19]. To date, these findings have not been reproduced in human studies [10].
Therefore, given the limited data describing the impact of maternal hypoglycemia on perinatal outcomes in women with GDMA2, the aim of this study was to evaluate risk factors for hypoglycemia and to assess the association between maternal episodes of hypoglycemia and neonatal outcomes in women with GDMA2. We hypothesized that women who experienced hypoglycemia would have worse neonatal outcomes than those who did not.
Material and methods
This was a retrospective cohort study of women with a singleton gestation who delivered in a single tertiary care center between 2011 and 2019. Institutional review board approval was obtained prior to study initiation. Women were excluded from the study if they had multiple gestations, fetal anomalies, or if weekly glucose information was unavailable. Women were diagnosed with GDM using Carpenter–Coustan two-step approach and were included if they were 18 years of age or older, met criteria for GDMA2, and were prescribed either insulin or an oral hypoglycemic agent (metformin or glyburide) in pregnancy for glycemic control. Women were considered to have experienced hypoglycemia if at least one value less than 70 mg/dL (or 3.9 mmol/L) was identified in their weekly blood glucose logs after pharmacotherapy initiation. Baseline maternal characteristics, pregnancy information, and neonatal outcomes were collected via chart abstraction.
The primary outcome was a composite neonatal outcome that included macrosomia, defined as birth weight greater than 4000 grams [20], jaundice requiring phototherapy, respiratory distress syndrome (RDS), large for gestational age (LGA) defined as fetal weight greater than the 90th percentile [21], shoulder dystocia defined as failure to deliver the anterior shoulder requiring additional maneuvers to facilitate delivery [22], birth trauma, as documented in the neonatal medical record, 5-minute Apgar score < 7, and neonatal hypoglycemia, defined as two or more episodes of glucose levels less than 40 mg/dL (2.2 mmol/L). Secondary outcomes included each individual neonatal outcome from the composite outcome, as well as small for gestational age (SGA), defined as birth weight less than the 10th percentile [21], neonatal intensive care unit (NICU) admission, preterm birth, defined as birth prior to 37 weeks, stillbirth, and neonatal death.
All statistical analyses were performed with Stata version 14.2 (StataCorp College Station, TX). Bivariate comparisons of maternal characteristics and pregnancy outcomes were performed in women with GDMA2 stratified by presence or absence of maternal hypoglycemia. The composite outcome and each secondary outcome were compared using Student’s T-test, Chi-square, Fisher exact test, and Mann-U Whitney as appropriate. Multivariate analysis was performed using logistic regression while controlling for confounding factors including maternal age, race and ethnicity, and parity, as well as those variables that differed between groups in the bivariate analysis with a p-value of <0.10. A separate backward regression was done to analyze the relationship between hypoglycemia and maternal characteristics.
Results
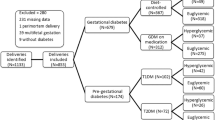
Out of 531 women with GDMA2, 20 were excluded due to multiple gestations, 4 were excluded due to fetal anomalies, and 18 were excluded due to unavailable weekly glucose logs information after initiation of pharmacotherapy. Among the 489 women who were included in the study, 95 (19.4%) were identified as experiencing at least one episode of hypoglycemia after initiation of pharmacotherapy for GDM. There were no differences between groups regarding maternal characteristics including maternal age, body mass index (BMI), nulliparity, insurance status, race and ethnicity, marital status, tobacco use, presence of chronic hypertension, managing provider (Endocrinologist, General OBGYN, or Maternal-Fetal Medicine physicians), or gestational age at GDM diagnosis or pharmacotherapy initiation (Table 1). However, women who had episodes of hypoglycemia were more likely to have started glyburide as a first-line pharmacotherapy (62.8% of women with hypoglycemia versus 34.5% without hypoglycemia, p < 0.001). Among women who experienced hypoglycemia, the average number of episodes was 1.9 ± 1.7 over a two-week period and three patients required admission for hypoglycemia. A total of 5 out of 57 (8.8%) of women on metformin experienced hypoglycemia, whereas 31 out of 237 (13.1%) and 59 out of 195 (30.3%) of women experienced hypoglycemia on insulin and glyburide, respectively.
Table 2 describes pregnancy outcomes stratified by presence of maternal hypoglycemia. In bivariate analyses, the rate of the primary outcome, the composite neonatal outcome, was more frequent among women with hypoglycemic episodes compared with controls (54.7% vs. 38.3%, p = 0.004). When looking at individual outcomes, infants of women who experienced hypoglycemic episodes were more likely to have the following adverse outcomes: 5-minute Apgar score < 7, birth trauma, and neonatal hypoglycemia (Table 2). When examining secondary outcomes, there was one case of stillbirth in the hypoglycemia group and no cases of stillbirth or neonatal death in the control group. The rest of the secondary outcomes did not differ between the groups. After controlling for confounding variables, including maternal age, nulliparity, medication chosen as first line agent, maternal race and ethnicity, and type of managing provider, increased rates of the composite neonatal outcome and neonatal hypoglycemia persisted in women who experienced hypoglycemic episodes (Table 3).
A separate backward regression analysis was performed to analyze the relationship between maternal characteristics and hypoglycemia after pharmacotherapy initiation. We identified the following risk factors for maternal hypoglycemia: maternal non-Hispanic Black race and ethnicity (aOR 6.53, 95% CI 2.10–20.29) and glyburide use as a first-line therapy for GDM (aOR 3.51, 95% CI 1.43–8.62) (Table 4). GDMA2 management by General OBGYN or Endocrinology was noted to be associated with lower rates of hypoglycemia compared to management by Maternal-Fetal Medicine specialists (MFM) (aOR 0.36, 95% CI 0.14–0.94 and aOR 0.23, 95% CI 0.07–0.82, respectively) (Table 4).
Discussion
Our study identified hypoglycemia in 19.4% of women at the onset of medication initiation for GDMA2, with persistently higher rates of the composite neonatal outcome after controlling for confounding factors. Use of glyburide and maternal non-Hispanic Black race and ethnicity were independently associated with an increased risk for experiencing hypoglycemia and having received initial diabetic care from a General OB/GYN or Endocrinologist was associated with a decreased risk compared with MFM.
Maternal hypoglycemia in women with GDMA2 has been reported as a side effect observed in randomized trials [2,3,4,5,6]. The 19.4% rate of hypoglycemia we identified in our study falls within the wide range seen in previous randomized studies—between 2% to 66% of subjects [2,3,4,5,6]. The substantial variance in the overall rate of hypoglycemia among these studies is largely due to both the wide-ranging frequency of glucose assessments performed and the definition of hypoglycemia utilized [2,3,4,5,6]. For example, study by Yogev et al. utilized a cutoff of 50 mg/dL (2.8 mmol/L) for women undergoing continuous glucose monitoring and found that 26 of 55 (47.3%) of women with GDM experienced hypoglycemia [5]. Langer et al. used a cutoff of 40 mg/dL (2.2 mmol/L) to define maternal hypoglycemia and when evaluating blood glucose values 4 times per day, found rates between 2–20% [4].
In regard to the effect of medication utilized on rates of hypoglycemia, Yogev et al. found hypoglycemia was more prominent with insulin use (63%) compared to glyburide (28%) [5]. Langer et al. also showed higher rates of hypoglycemia with insulin (20%) compared to glyburide (4%) [4]. Conversely, Jacobson et al. evaluated over 500 women and found that the rates of maternal hypoglycemia were higher with glyburide (20%) than with insulin (8%) [6] The reason behind these differences is difficult to ascertain; however, it is likely attributable to the varied definition of hypoglycemia and the frequency with which blood glucose values were assessed.
In addition to the use of glyburide, we found that non-Hispanic Black maternal race and ethnicity were associated with higher rates of hypoglycemia among women with GDMA2. Prior studies have found that Black women demonstrate a lower prevalence of GDM when compared to Hispanic and Asian counterparts [23,24,25]. These published studies did not investigate the occurrence of maternal hypoglycemia. A 1993 study of Black women in South Africa found 14.4% prevalence of hypoglycemia in insulin-dependent diabetic mothers, but close to 60% of patients in that study had pregestational diabetes [26]. While there is data demonstrating Black women are at greater risk of developing preeclampsia and hypertension after GDM diagnosis [27,28,29], our study is the first to suggest non-Hispanic Black women with GDMA2 may be more likely to experience hypoglycemia. For a genetic explanation, we look at a 2015 study by Schmella et al. which found that Black women were more likely to harbor a polymorphism in the gene for lipoprotein lipase, resulting in lower triglyceride concentrations in the third trimester [30]. While this may initially seem protective, the study concludes that the effects of this polymorphism are not well understood in overall pregnancy outcomes, and may result in maternal hypoglycemia [30].
Our results also suggest that management of GDMA2 by an MFM specialist was associated with increased rates of maternal hypoglycemia. This may be due to discrepancies in what constitutes a diagnosis of diabetes in pregnancy or intensity of treatment approaches [31]. Further, standards set by the American College of Obstetrician-Gynecologists, American Diabetes Association, and International Association of Diabetes and Pregnancy Study Groups all differ in medication initiation criteria after medical nutritional therapy failure [32]. Additionally, MFM physicians are more likely to care for complex patients with GDM, potentially increasing their risk of complications, including hypoglycemia.
Finally, our study found that GDMA2 women who experience hypoglycemia are more likely to have neonates with hypoglycemia. While there is ample evidence to demonstrate a direct association between GDM and neonatal hypoglycemia [33, 34], there is limited data examining the relationship between maternal hypoglycemia and neonatal hypoglycemia. Given oral hypoglycemic agents’ ability to cross the placenta, studies have shown glyburide specifically increases the likelihood of neonatal hypoglycemia—but its presence in umbilical cord blood is highly variable [35]. Genetic variances in drug metabolism in the context of pregnancy may be considered as an explanation, though it remains to be studied. Prior studies have found that appropriate antenatal glucose control is less likely to lead to neonatal hypoglycemia, but a very tight maternal glucose control may contribute further to this outcome [36].
Our study has several limitations. First, exclusion of subjects without glucose information available may have led to an incidental bias. Second, we performed dichotomized analysis by presence or absence of hypoglycemia episodes. We did not control the analysis for the number of hypoglycemic episodes, due to overall small number of women that had multiple episodes of hypoglycemia. In addition, we did not collect data on the dose of oral antidiabetic medication or insulin regimen that each patient in our study has received and thus unable to comment on the association between hypoglycemia and medication dose. We also did not control for temporal relationship between timing of hypoglycemia and delivery. Strengths of our study include a granular review of medical charts of each study subject including review of the weekly blood glucose log and an innovative research question.
In conclusion, we have described neonatal outcomes of women who experience hypoglycemia while undergoing pharmacologic treatment of GDMA2. Our study identified that women who were hypoglycemic were more likely to be on glyburide, to be Non-Hispanic Black, and to have an MFM as the managing provider. Infants exposed to maternal hypoglycemia were more likely to experience a composite adverse neonatal outcome. Further prospective studies are needed to elucidate the full impact of maternal hypoglycemia on pregnancy outcomes after pharmacotherapy initiation for GDM.
References
Gestational Diabetes. https://www.cdc.gov/diabetes/basics/gestational.html. Accessed December 27, 2020.
Nicholson W, Bolen S, Witkop CT, Neale D, Wilson L, Bass E. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: a systematic review. Obstet Gynecol. 2009;113:193–205.
Dhulkotia JS, Ola B, Fraser R, Farrell T. Oral hypoglycemic agents vs insulin in management of gestational diabetes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203:457.e1–457.e9. https://doi.org/10.1016/j.ajog.2010.06.044
Langer O, Conway D, Berkus M, Xenakis EMJ, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N. Engl J Med. 2000;343:1134–8.
Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Undiagnosed asymptomatic hypoglycemia: diet, insulin, and glyburide for gestational diabetic pregnancy. Obstet Gynecol. 2004;104:88–93.
Jacobson GF, Ramos GA, Ching JY, Kirby RS, Ferrara A, Field DR. Comparison of glyburide and insulin for the management of gestational diabetes in a large managed care organization. Am J Obstet Gynecol. 2005;193:118–24. https://doi.org/10.1016/j.ajog.2005.03.018
Mathiesen ER, Kinsley B, Amiel SA, Heller S, McCance D, Duran S, et al. Maternal glycemic control and hypoglycemia in type 1 diabetic pregnancy: a randomized trial of insulin aspart versus human insulin in 322 pregnant women. Diabetes Care. 2007;30:771–6. https://doi.org/10.2337/dc06-1887
Rosenn BM, Miodovnik M. Glycemic control in the diabetic pregnancy: is tighter always better?. J Matern Neonatal Med. 2000;9:29–34. https://doi.org/10.3109/14767050009020509.
Rosenn BM, Miodovnik M, Holcberg G, Khoury J, Siddiqi T. Hypoglycemia: the price of intensive insulin therapy for pregnant women with insulin-dependent diabetes mellitus. Obstet Gynecol. 1995;85:417–22. https://doi.org/10.1016/0029-7844(94)00415-A
Ter Braak EWMT, Evers IM, Erkelens DW, Visser GHA. Maternal hypoglycemia during pregnancy in type 1 diabetes: maternal and fetal consequences. Diabetes Metab Res Rev. 2002;18:96–105. https://doi.org/10.1002/dmrr.271
Vadakekut ES, McCoy SJB, Payto ME. Association of maternal hypoglycemia with low birth weight and low placental weight: a retrospective investigation. J Am Osteopath Assoc. 2011;111:148–52. https://doi.org/10.7556/jaoa.2011.111.3.148
Pugh SK, Doherty DA, Magann EF, Chauhan SP, Hill JB, Morrison JC. Does hypoglycemia following a glucose challenge test identify a high risk pregnancy? Reprod Health. 2009;6:1–7. https://doi.org/10.1186/1742-4755-6-10
Feinberg JH, Magann EF, Morrison JC, Holman JR, Polizzotto MJ. Does maternal hypoglycemia during screening glucose assessment identify a pregnancy at-risk for adverse perinatal outcome? J Perinatol. 2005;25:509–13. https://doi.org/10.1038/sj.jp.7211336
Abell DA, Beischer NA. Evaluation of the three hour oral glucose tolerance test in detection of significant hyperglycemia and hypoglycemia in pregnancy. Diabetes. 1975;24:874–80. https://doi.org/10.2337/diab.24.10.874
Raviv S, Wilkof-Segev R, Maor-Sagie E, Naeh A, Yoeli Y, Hallak M, et al. Hypoglycemia during the oral glucose tolerance test in pregnancy-maternal characteristics and neonatal outcomes. Int J Gynaecol Obstet. 2021; 18. https://doi.org/10.1002/ijgo.14037.
Langer O, Levy J, Brustman L, Anyaegbunam A, Merkatz R, Divon M. Glycemic control in gestational diabetes mellitus-How tight is tight enough: small for gestational age versus large for gestational age? Am J Obstet Gynecol. 1989;161:646–53. https://doi.org/10.1016/0002-9378(89)90371-2
Kuwata C, Saeki N, Honda K, Matsuoka T, Tsuchiya Y, Shimomura K. Effects of maternal hypoglycemia on fetal eye and skeleton development in rats. Reprod Toxicol. 2017;71:135–41. https://doi.org/10.1016/j.reprotox.2017.05.009
Tanigawa K, Kawaguchi M, Tanaka O, Kato Y. Skeletal malformations in rat offspring: long-term effect of maternal insulin-induced hypoglycemia during organogenesis. Diabetes. 1991;40:1115–21. https://doi.org/10.2337/diab.40.9.1115
Jensen VFH, Mølck AM, Lykkesfeldt J, Bøgh IB. Effect of maternal hypoglycaemia during gestation on materno-foetal nutrient transfer and embryo-foetal development: evidence from experimental studies focused primarily on the rat. Reprod Toxicol. 2018;77:1–24. https://doi.org/10.1016/j.reprotox.2018.01.007
ACOG. PB216 Macrosomia (2020). Obstet Gynecol. 2019;133:168–86.
Duryea EL, Hawkins JS, McIntire DD, Casey BM, Leveno KJ. A revised birth weight reference for the United States. Obstet Gynecol. 2014;124:16–22. https://doi.org/10.1097/AOG.0000000000000345
ACOG. ACOG Practice Bulletin Shoulder Dystocia. ACOG. 2017;2017:1118–32.
Yuen L. Gestational diabetes mellitus: challenges for different ethnic groups. World J Diabetes. 2015;6:1024 https://doi.org/10.4239/wjd.v6.i8.1024
Hedderson M, Ehrlich S, Sridhar S, Darbinian J, Moore S, Ferrara A. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care. 2012;35:1492–8. https://doi.org/10.2337/dc11-2267
Ferrara A, Hedderson MM, Quesenberry CP, Selby JV. Prevalence of gestational diabetes mellitus detected by the National Diabetes Data Group or the Carpenter and Coustan plasma glucose thresholds. Diabetes Care. 2002;25:1625–30. https://doi.org/10.2337/diacare.25.9.1625
Huddle K, England M, Nagar A. Outcome of pregnancy in diabetic women in Soweto, South Africa 1983-1992. Diabet Med. 1993;10:290–4.
Bentley-Lewis R, Powe C, Ankers E, Wenger J, Ecker J, Thadhani R. Effect of race/ethnicity on hypertension risk subsequent to gestational diabetes mellitus. Am J Cardiol. 2014;113:1364–70. https://doi.org/10.1016/j.amjcard.2014.01.411
Nguyen BT, Cheng YW, Snowden JM, Esakoff TF, Frias AE, Caughey AB. The effect of race/ethnicity on adverse perinatal outcomes among patients with gestational diabetes mellitus. Am J Obstet Gynecol. 2012;207:322.e1–322.e6. https://doi.org/10.1016/j.ajog.2012.06.049
Mocarski M, Savitz DA. Ethnic differences in the association between gestational diabetes and pregnancy outcome. Matern Child Health J. 2012;16:364–73. https://doi.org/10.1007/s10995-011-0760-6
Schmella MJ, Ferrell RE, Gallaher MJ, Lykins DL, Althouse AD, Roberts JM, et al. The −93T/G LPL promoter polymorphism is associated with lower third-trimester triglycerides in pregnant African American Women. Biol Res Nurs. 2015;17:429–37. https://doi.org/10.1177/1099800414561475
Agarwal M. Consensus in gestational diabetes MELLITUS: looking for the holy grail. J Clin Med. 2018;7:123 https://doi.org/10.3390/jcm7060123
Caissutti C, Berghella V, Scientific evidence for different options for GDM screening and management: controversies and review of the literature. Biomed Res Int. 2017;2017. https://doi.org/10.1155/2017/2746471
Harris DL, Weston PJ, Harding JE. Incidence of neonatal hypoglycemia in babies identified as at risk. J Pediatr. 2012;161:787–91. https://doi.org/10.1016/j.jpeds.2012.05.022
Cho HY, Jung I, Kim SJ, The association between maternal hyperglycemia and perinatal outcomes in gestational diabetes mellitus patients: a retrospective cohort study. Medicine. 2016;95. https://doi.org/10.1097/MD.0000000000004712
Hamel MS, Kanno LM, Has P, Beninati MJ, Rouse DJ, Werner EF. Intrapartum glucose management in women with gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol. 2019;133:1171–7. https://doi.org/10.1097/AOG.0000000000003257
Schwartz RA, Rosenn B, Aleksa K, Koren G. Glyburide transport across the human placenta. Obstet Gynecol. 2015;125:583–8. https://doi.org/10.1097/AOG.0000000000000672.
Author information
Authors and Affiliations
Contributions
RH generated the research question, did chart abstraction, collected patient data, cleaned the data set, designed the analysis plan, perform the statistical analysis, and wrote the first draft of the manuscript. V.S. did chart abstraction, literature review, and wrote the first draft of the introduction and results section. CD did chart abstraction, reviewed and edited the final draft. MC did chart abstraction, reviewed and edited the final draft. AP help generate the research question, design the analysis plan, and edited the final manuscript several times.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work was done in full alignment with ethical and consent guidelines and was performed in accordance with the Declaration of Helsinki. The study was approved by the Medical College of Wisconsin’s Institutional Review Board.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This research was previously presented on February 5th, 2020 at the Society for Maternal Fetal Medicine 40th Annual Pregnancy Meeting in Grapevine, Texas.
Rights and permissions
About this article
Cite this article
Harrison, R.K., Saravanan, V., Davitt, C. et al. Antenatal maternal hypoglycemia in women with gestational diabetes mellitus and neonatal outcomes. J Perinatol 42, 1091–1096 (2022). https://doi.org/10.1038/s41372-022-01350-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01350-4
- Springer Nature America, Inc.