Abstract
Objective
Considerable variation in the care of extremely low gestational age infants (ELGAN) contributes to the variation in incidence of bronchopulmonary dysplasia (BPD). We compared management and outcomes of two neonatal centres with different respiratory support strategies.
Study design
Retrospective cohort study of infants <28 weeks gestational age treated at two units in Australia and the UK between 2015 and 2017.
Result
Of 492 infants, the overall incidence of BPD for extremely preterm infants was 62.20% and was similar across both sites (64.84% at Monash vs. 60.65% at Oxford). Independent predictors for the development of BPD or mortality included the days on mechanical ventilation (MV, adjusted OR 1.13, 95% Cl 1.07–1.19) and use of inhaled nitric oxide (adjusted OR 13.42, 95% Cl 1.75–103.28).
Conclusion
Primary choice of non-invasive respiratory support had no significant impact on BPD development. Duration of MV and using nitric oxide were independent predictors for death or BPD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Bronchopulmonary dysplasia (BPD) is a chronic lung condition that affects preterm infants and, in spite of advances in modern neonatal intensive care, this condition remains a major cause of neonatal mortality and morbidity. Data from high-income countries, like for instance the Canadian Neonatal Network, show that BPD affects almost 70% of all neonates born less than 28 weeks’ gestation [1]. Whilst the survival rates of preterm neonates are increasing due to modern advances in neonatal intensive care, unfortunately these developments have not been successful in reducing the incidence of BPD [2].
Despite attempts to standardise perinatal management of preterm infants [3], neonatal practices [4, 5] and outcomes often vary markedly between countries: [2, 6] The investigators of the International Network for Evaluating Outcomes of Neonates (iNeo) and the Vermont Oxford Network found that there exists marked variation in both practices and outcomes between neonatal centres [7, 8]. Thus, there is scope for further collaboration between centres, even across different countries, to identify if variations in outcomes exist, and if these discrepancies can be explained by differences in practices. We chose Monash Newborn (Australia) and the Oxford Newborn Care Unit (UK) as both centres are comparable in terms of level of care provided, staff expertise and adjunct paediatric services, however they differ in their choice of primary respiratory support. The aim of this study was to test the hypothesis that choice of primary respiratory support impacts the BPD rate, by comparing the respiratory management and outcomes of two tertiary neonatal centres with differing respiratory support strategies.
Methods
Setting
A comparative study was undertaken between two neonatal intensive care units, specifically investigating the respiratory and mortality outcomes of extremely low gestational age infants (ELGAN). Both Monash Newborn at Monash Children’s Hospital (Melbourne, Australia) and the Oxford Newborn Care Unit at the John Radcliffe Hospital (Oxford, United Kingdom) are tertiary intensive care units (NICUs) that provide specialist medical and surgical care for neonates. Both units advocate a non-invasive approach at birth to respiratory care for extremely preterm infants, however, have fundamental differences in the provision of non-invasive respiratory support.
Study design
We conducted an observational retrospective study of all extremely preterm neonates admitted to Monash Newborn and the Oxford Newborn Care Unit who met the inclusion criteria, over a period of 3 years, from 2015 to 2017. Infants were included in the study if they were delivered with a gestation age up to 27 weeks and 6 days and admitted to either Monash Newborn or the Oxford Newborn Care Unit from 1st of January 2015 until the 31st of December 2017. The lowest gestational age admitted at Oxford was 22.9 weeks, compared to 23.2 weeks at Monash. Infants were excluded from the study if they were diagnosed with a major congenital anomaly.
Study outcomes
The primary outcome of this study was a composite outcome of BPD or death. Infants were defined as meeting the outcome of BPD if they received any respiratory support (such as synchronised intermittent positive pressure ventilation, nasal CPAP, nasal high flow therapy (nHFT, with or without supplemental oxygen) for a chronic pulmonary disorder at 36 weeks’ postmenstrual age.
Data collection
Anonymised data from Monash and Oxford was extracted from patients’ electronic medical records (BadgerNet Neonatal, Clevermed 2018: Edinburgh, UK) onto a spreadsheet using Microsoft Excel for Mac, Version 16.10 (Microsoft 2017: Redmond, Washington, USA). Data was collected on maternal characteristics, infant characteristics, birth and resuscitation, respiratory management practices, other practices and discharge management, co-morbidities and the study outcomes. At Monash Newborn, a number of data fields are routinely collected for the Australian and New Zealand Neonatal Network. Similarly, clinical data at all National Health Service neonatal units across the United Kingdom are extracted and sent to the NNRD periodically. The data dictionaries used for both databases were cross-checked to ensure equivalence of the data fields. The investigators manually extracted data from patient records at Oxford if data fields required for the study were not available in routinely collected data.
Infants were resuscitated according to the 2015 AHA and ERC resuscitation guidance update. nHFT was administered at Oxford by the Vapotherm Precision Flow Plus device (Vapotherm Inc. 2018: Exeter, New Hampshire, USA). For administration of MV, both conventional ventilation and HFO (high frequency oscillation) and nCPAP, the Acutronic Fabian HFO 4-in-1 High Frequency Oscillation Ventilator was used (Acutronic Medical Systems AG 2018: Zurich, Switzerland). At Monash Newborn, nCPAP was delivered by the Bubble CPAP system (Fisher & Paykel Healthcare Limited, Auckland, New Zealand). Mechanical ventilation, conventional and HFO was delivered by the Dräger Babylog® VN500 neonatal ventilator (Drägerwerk AG & Co. KGaA, 2018) and the SLE5000 Neonatal Ventilator with High Frequency Oscillation (SLE Specialised Laboratory Equipment, Croydon, UK). Nasal HFT at Monash was administered by OptiflowTM Junior (Fisher & Paykel Healthcare Limited, Auckland, New Zealand).
Data analysis
Statistical analysis was performed using Stata/IC Version 14.0 for Mac (StataCorp LLC 2015: Texas, USA). Continuous variables with a non-normal distribution were reported as medians with interquartile ranges (IQR). To compare two independent population medians, the two-sample Wilcoxon rank-sum test was used. For categorical data, the chi-squared (χ2) test was performed, except when the expected cell frequency was less than 5, in which case Fisher’s exact test was used. Variables with over 10% missing observations were excluded.
To adjust for confounders and analyse the contribution of various determinants on the development of the composite outcome, a multiple logistic regression model was built using a forward selection approach. We used a p-value of <0.25 for candidate variables. Collinear variables (such as birthweight and gestational age) was not included simultaneously. Model diagnostics were performed, including specification link test for single-equation models and the Hosmer and Lemeshow’s test. Standardised survival curve analysis was performed adjusting for gestational age, birthweight and gender [9]. Start dates were measured from date of birth, end dates corresponded with the date infants came off respiratory support and date of death was used for date of censoring.
Ethical approval was granted by the Monash Health Human Research Ethics Committee. The project was classified as a retrospective service evaluation according to the UK Health Research Authority and was approved by the clinical audit lead at the John Radcliffe Hospital as per local guidelines. All individual patient data was de-identified.
Results
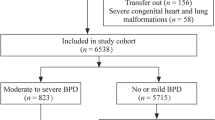
A total of 7760 infants were admitted to either the Oxford Newborn Care Unit or Monash Newborn from the period during 1st of January 2015 until the 31st of December 2017. Five hundred and twenty-one infants met the inclusion criteria according to gestational age and ranged from 22.9 weeks to 27.9 weeks gestational age. Of these infants, 29 were excluded due to the presence of a major congenital anomaly. Overall, a total of 492 infants were included in the study—310 from the Oxford Newborn Care Unit and 182 from Monash Newborn. Three hundred and sixty-six infants (74% of the study population) met the primary combined outcome of BPD or death (Fig. 1).
The overall incidence of BPD in infants born under 28 weeks’ gestation was 62.20% (n = 306) and was similar across Monash and Oxford (64.84% at Monash vs. 60.65% at Oxford, p = 0.355). The overall mortality rate was 12.80% (n = 63), though Oxford had a significantly higher mortality rate compared to Monash (6.59% at Monash vs. 16.45% at Oxford, p = 0.002). Three hundred and sixty-six infants (74.39%) met the combined outcome of mortality or BPD, however there was no difference between the two sites (70.88% at Monash vs. 76.45% at Oxford, p = 0.172).
Oxford had a higher percentage of outborn infants than Monash (15.38% at Monash vs. 30.03% at Oxford, p < 0.001) (Table 1), however being an outborn infant was not associated with increased odds of developing the combine outcome of BPD or mortality (OR 1.00, 95% Cl 0.62–1.59, p = 0.984) or mortality alone (OR 0.78, 95% Cl 0.41–1.48, p = 0.441).
Respiratory management practices
Overall, 82.01% (n = 892) of infants received surfactant, though Oxford had significantly higher rates of surfactant administration than Monash (74.18% at Monash vs. 86.82% at Oxford, p < 0.001). Seventy-one infants (14.43%) received inhaled nitric oxide (INO) and as with surfactant, Oxford had higher rates of INO administration (6.04% at Monash vs. 19.35% at Oxford, p < 0.001).
The primary mode of non-invasive ventilation was nasal CPAP at Monash and high flow nasal cannula therapy (nHFT) at Oxford. The use of nCPAP at Monash was 95.05% amongst ELGANs and infants spent a median of 884 h [IQR 606–1167] on nCPAP. Three hundred and ninety-nine (81.10%) infants received nHFT and there was no difference in terms of number of infants receiving nHFT at Monash and Oxford (83.52% at Monash vs. 79.68% at Oxford, p = 0.294).
After adjusting for potential confounders (gestational age, sepsis confirmed on culture, days of MV, INO and the presence of preterm labour), factors that were associated with significantly lower odds of developing the combined outcome of BPD or death were higher gestational age, higher birthweight, preterm labour and breast milk upon discharge. Factors that were associated with significantly higher odds of developing the combined outcome (BPD or mortality) included confirmed sepsis on culture, INO, days of MV and requiring oxygen upon discharge (Table 2).
Standardised survival curve analysis demonstrated that infants at Monash spent longer periods of time on respiratory support than infants at Oxford, even when adjusting for gestational age, birthweight and gender. Similarly, infants with BPD were exposed to longer periods of time on respiratory support than infants who did not develop BPD (Figs. 2 and 3).
Discussion
Despite significant differences in the choice of primary non-invasive respiratory support, there was no difference in the rates of composite outcome (BPD or mortality) between the two units. The overall incidence of BPD alone for extremely preterm infants in this study was 62.20% and was similar across Monash and Oxford (64.84% at Monash vs. 60.65% at Oxford, p = 0.355). Monash had a significantly lower crude mortality rate compared to Oxford (6.59% vs. 16.45%, p = 0.002), though this number does not account for the differences in illness severity and infant characteristics, such as birthweight, between the two study sites. Data from the Vermont Oxford Network shows that the BPD rate at Oxford for infants less than 29 weeks gestational age is 46.6%, compared to our higher BPD rate of 60.65% at Oxford. This discrepancy is likely due to our study population including all infants less that 28 weeks gestational age with no lower gestational age margin and irrespective of birth weight.
Infants at both study sites were comparable in terms of gender, gestational age, maternal age, ethnicity, singleton births, caesarean deliveries, Apgar scores at 1 and 5 min and temperature upon admission. However, the birthweight of infants born at Oxford was significantly lower than for those born at Monash (median 835 grams [IQR 697–969] at Monash vs. 800 g [IQR 660–935] at Oxford; p = 0.028), which is likely to account in part for the higher crude mortality rate found at Oxford.
The main practice difference was the choice of primary non-invasive respiratory support for treatment of respiratory distress syndrome—nHFT was used in Oxford and nCPAP was used in Monash. Oxford had significantly higher rates of intubation at birth, surfactant administration and use of INO. Although there was no difference between the units in terms of time spent on nHFT, the use of nHFT was different between the two units. At Oxford, it was used as the primary mode of non-invasive respiratory support at Oxford post-extubation, whereas at Monash nCPAP was the primary mode of non-invasive respiratory support, with nHFT being used towards the end of the infant’s admission, as a part of the local weaning strategy.
Though there were no significant differences between the two NICUs in terms of MV (both rates of infants on MV and hours that infants spent on invasive ventilation), infants at Monash remained on non-invasive ventilation (nCPAP and high flow oxygen) for a longer period of time overall than infants at Oxford. This suggests that using nHFT as the infants are convalescing and weaning off nCPAP may in fact increase the amount of time infants spend on respiratory support.
Whilst intubation at birth, surfactant administration and use of INO were all significantly associated with increased odds of developing the composite outcome, only INO remained an independent predictor of composite outcome after adjusting for birthweight (adjusted odds ratio 18.26, 95% Cl 2.36–141.34, p = 0.005). However, from a retrospective observational study, we cannot determine causality and this association may be due to the burden of pulmonary hypertension in preterm infants or the adverse effect of the treatment itself [10, 11].
Nonetheless, practices surrounding INO for preterm infants remain controversial. To date, the majority of literature suggests that INO does not improve BPD outcomes or mortality for preterm infants less than 32 weeks’ gestation [12]. Some studies, however, suggest that certain subgroups within the population of preterm infants may benefit from INO administration [13]. Currently, there are no nation-wide guidelines in either Australia or the UK and wide practice variations exist between units regarding the use of INO [14, 15]. This suggests there is a need for evidence-based, consensus guidelines to be developed in order to standardised practices between neonatal units in accordance with existing literature [3].
A major limitation of this study is that although it is a comprehensive, 26-month spanning, detailed observation following the latest AHA and ERC resuscitation guidance update (2015), it is still a retrospective observational study. One can only determine associations and not causality, thus the findings must be interpreted with caution. We also acknowledge that the presence of confounders can influence the results and we have attempted to adjust for potential confounders as best we could during the analysis phase.
Significance and future directions
The BPD incidence of 62% also highlights that this disease remains an important cause of morbidity in extremely preterm infants. The majority of previous large-scale randomised controlled trials comparing high flow therapy and nCPAP have excluded ELGANs. Thus, reliable data for this infant population remains sparse, despite extremely preterm infants being disproportionately affected by BPD.
In spite of modern neonatal advances, BPD rates remain stagnant, with some studies even suggesting an increasing incidence [16, 17, 2]. Moreover, rates of BPD can vary markedly between units, thus ongoing surveillance of BPD rates can provide useful feedback to individual units and act as a catalyst to review the unit’s respiratory management practices. Future collaborations between countries and individual units are needed to investigate variations in neonatal practices and identify practices associated with improved outcomes.
Conclusion
BPD remains a significant cause of neonatal morbidity amongst extremely preterm infants. The overall incidence of BPD for extremely preterm infants in this study was 62.20% and was similar between centres. Despite using different modes of primary non-invasive respiratory support, there was no difference in terms of the combined outcome of BPD or mortality between study sites. Independent risk predictors for the development of the combined outcome (BPD or mortality) included the use of INO and days on MV.
References
Beltempo M, Shah P, Yoon EW, Chan P, Balachandran N, et al. Le Réseau Néonatal Canadien: Annual Report 2019. Toronto ON: Canadian Neonatal Network, Maternal-Infant Care Research Centre 2020.
Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314:1039–51.
Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, te Pas A, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology 2019;115:432–50.
Roehr CC, Gröbe S, Rüdiger M, Hummler H, Nelle M, Proquitté H, et al. Delivery room management of very low birth weight infants in Germany, Austria and Switzerland_a comparison of protocols. Eur J Med Res. 2010;15:493–503.
McCarthy LK, Morley CJ, Davis PG, Kamlin CO, O’Donnell CP. Timing of interventions in the delivery room: does reality compare with neonatal resuscitation guidelines? J Pediatr. 2013;163:1553–7.e1.
Vanpee M, Walfridsson-Schultz U, Katz-Salamon M, Zupancic JA, Pursley D, Jonsson B. Resuscitation and ventilation strategies for extremely preterm infants: a comparison study between two neonatal centers in Boston and Stockholm. Acta Paediatr. 2007;96:10–6.
Shah PS, Lui K, Sjörs G, Mirea L, Reichman B, Adams M, et al. Neonatal outcomes of very low birth weight and very preterm neonates: an international comparison. J Pediatr. 2016;177:144–52.e6.
Adams M, Bassler D, Bucher HU, Roth-Kleiner M, Berger TM, Braun J, et al. Variability of Very Low Birth Weight Infant Outcome and Practice in Swiss and US Neonatal Units. Pediatrics. 2018;141:e20173436.
Lambert P. STPM2_STANDSURV: Stata module to obtain standardized survival curves after fitting an stpm2 survival model. 2018. Statistical Software Components S458289, Boston College Department of Economics, revised 19 Jun 2018.
Nakanishi H, Suenaga H, Uchiyama A, Kusuda S. Persistent pulmonary hypertension of the newborn in extremely preterm infants: a Japanese cohort study. Arch Dis Child - Fetal Neonatal Ed. 2018;103:F554.
Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, et al. Pulmonary Artery Hypertension in Formerly Premature Infants With Bronchopulmonary Dysplasia: Clinical Features and Outcomes in the Surfactant Era. Pediatrics. 2007;120:1260.
Clark RH, Ursprung RL, Walker MW, Ellsbury DL, Spitzer AR. The changing pattern of inhaled nitric oxide use in the neonatal intensive care unit. J Perinatol. 2010;30:800–4.
Cheng DR, Peart S, Tan K, Sehgal A. Nitric therapy in preterm infants: rationalised approach based on functional neonatal echocardiography. Acta Paediatr. 2016;105:165–71.
Sasi A, Sehgal A. Use of inhaled nitric oxide in preterm infants: a regional survey of practices. Heart Lung. 2014;43:347–50.
Kumar P. Use of inhaled nitric oxide in preterm infants. Pediatrics. 2014;133:164–70.
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56.
Botet F, Figueras-Aloy J, Miracle-Echegoyen X, Rodríguez-Miguélez JM, Salvia-Roiges MD, Carbonell-Estrany X. Trends in survival among extremely-low-birth-weight infants (less than 1000 g) without significant bronchopulmonary dysplasia. BMC Pediatr. 2012;12:63.
Acknowledgements
We would like to thank Prof. Neena Modi and Dr. Chris Gale (Imperial College London) for their tireless work in leading and managing the NNRD.
Author information
Authors and Affiliations
Contributions
KT and CCR conceived the study. SS and SZ collected the data. SS, KT and AE analysed the data and all authors contributed to interpretation of the data. SS wrote the first draft of the manuscript and all authors (SS, SZ, AE, KT and CCR) contributed to drafting or revising the manuscript critically for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, S., Zivanovic, S., Earnest, A. et al. Respiratory management and bronchopulmonary dysplasia in extremely preterm infants: a comparison of practice between centres in Oxford and Melbourne. J Perinatol 42, 53–57 (2022). https://doi.org/10.1038/s41372-021-01274-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-01274-5
- Springer Nature America, Inc.