Abstract
Renin–angiotensin system (RAS) has important roles in cardiovascular disease. Angiotensin II (Ang II) and angiotensin-(1–7) (Ang-(1–7)) are major effector peptides of RAS. However, the roles of Ang II type 2 receptor (AT2R) need to be further explored and the roles of Ang-(1–7) are still not very clear on vascular calcification (VC). Therefore, we hypothesized they have effects on preventing VC in vivo and in vitro. VC model is established by inorganic phosphate (IP) cultured with vascular smooth muscle cells (VSMC) for in vitro study and by 5/6 nephrectomy in mice for in vivo study. Increased calcified nodules by Alizarin Red S staining and mRNA expressions of bone morphogenetic protein-2 (BMP-2) and osteocalcin (OCN) by reverse transcription polymerase chain reaction in calcified WT VSMC were significantly inhibited in calcified AT2R overexpression (SmAT2) VSMC or after Ang-(1–7) treatment. After 5/6 nephrectomy, the ratio of positive and total area by Alizarin Red S and von Kossa staining and mRNA expressions of BMP-2 and OCN were significantly increased in ApoE/AT2R knockout mice compared with apolipoprotein E knockout mice, and which were significantly inhibited with Ang-(1–7) administration. Both AT2R and Ang-(1–7) have the effects on preventing VC induced by IP, at least in part through inhibiting BMP-2, OCN expressions, and in which Ang-(1–7) had protective roles mainly through Mas receptor rather than AT2R.
Similar content being viewed by others
Introduction
Vascular calcification (VC) is a major risk factor for cardiovascular mortality, particularly in patients with chronic renal disease (CKD) and diabetes [1]. With decreasing kidney function, the prevalence of VC increases and calcification occurs years earlier in CKD patients than in the general population [2]. When VC occurs, it indicates the possibility of worse clinical results and cardiovascular adverse events [3], but the mechanism of VC is not very clear. VC is a complex and active cell-mediated pathological process, and the exact mechanisms and interactions between these regulators remain incompletely characterized.
VC is related to the disorder of mineral metabolism in CKD, and in which serum phosphorus has become an important regulator [4]. It has been documented that hyperphosphatemia is highly associated with the extent of VC and contributes directly to high morbidity and mortality in cardiovascular disease [5]. In the mouse model of chronic renal failure (CRF), high phosphorus diet could induce calcification of vascular smooth muscle cells (VSMC) in the media of aorta [6]. Hyperphosphate also induced calcification of medial VSMC in isolated rat aortic rings [7]. In vitro experiments showed that the same level of hyperphosphate in individuals can directly promote the calcification of VSMC, leading to the transformation of VSMC from contraction phenotype to osteochondral phenotype [8]. Therefore, VSMC can undergo calcification by excessive inorganic phosphate (IP) stimulation. Although current treatment for VC is based on reducing hyperphosphatemia, new effective therapeutic strategies to prevent VC remain urgent.
In recent years, numerous studies have shown that endogenous cardiovasoactive peptides are involved in VC [9]. Renin–angiotensin system (RAS) usually has important roles in cardiovascular disease. As we known, angiotensin II (Ang II) and angiotensin-(1–7) (Ang-(1–7)) are major effector peptides of RAS. Ang II binds two receptor subtypes, type 1 receptor (AT1R) and type 2 receptor (AT2R). Ang-(1–7) can be formed mainly from Ang II through the activity of angiotensin converting enzyme 2 (ACE2) [10]. Ang-(1–7) acts through binding to the specific Mas receptor (MasR) [11]. Many reports suggested that Ang II could promote VC mainly mediated by AT1R [12,13,14]. However, both of Ang II/AT2R and ACE2/Ang-(1–7)/MasR axis work to offset the actions of Ang II via AT1R [15]. Numerous studies reported that ARB limits VC in aortic artery [16,17,18]. Moreover, blockade of AT2R by PD123319 inhibits osteogenic differentiation of human mesenchymal stem cells via inhibition of extracellular signal-regulated kinase signaling [19]. Previously, our research suggested that AT2R activation represented an endogenous protective pathway against VC, which might efficiently reduce adverse cardiovascular events in patients with CKD [20]. It suggested that AT2R play an important role in VC formation. However, on the basis of our previous research, the pathophysiologic roles of AT2R in VC need to be further explored. On the other hand, ACE2/Ang-(1–7)/MasR axis becomes the major counter-regulatory system against Ang II/AT1R at both systemic and local levels [21]. Recently, only one paper reported that Ang-(1–7) could inhibit VC in a rat model induced by vitamin D3 plus nicotine [9]. However, the effects of Ang-(1–7) on VC have not yet been very clear.
Therefore, we hypothesized they have effects on preventing VC in vivo and in vitro, which could be potential targets for VC therapy. This study is to investigate the roles of AT2R and Ang-(1–7) on VC induced by IP in vivo and in vitro. VC model is established by IP cultured with VSMC for in vitro study and by 5/6 nephrectomy in mice for in vivo study. We used wild-type (WT) mice, AT2R knockout (AT2KO) mice, AT2R overexpression (SmAT2) mice, and mice with or without Ang-(1–7) treatment, to find potential targets for VC therapy.
Materials and methods
Animals
All kinds of mice were obtained from Jackson Laboratory (Bar Harbor, ME), bred and housed in a room in which lighting was controlled (12-h light/dark cycle), and the room temperature was kept at 25 °C. WT and AT2R overexpression (SmAT2) mice based on C57BL/6J strain were given a standard diet (MF; Oriental Yeast, Tokyo, Japan) and water ad libitum. Apolipoprotein E knockout (ApoE) and ApoE/AT2R knockout (ApoE/AT2KO) mice based on C57BL/6J strain were given a high-cholesterol diet (HCD) consisting of 1.25% cholesterol and 10% coconut oil (MF; Oriental Yeast, Tokyo, Japan) and water ad libitum. Male mice at 8 weeks of age were used in this experiment and randomly assigned to each group without blinding. Each experiment was replicated for at least three times both for technical and biological replicates. All experiments were performed in compliance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Animal Ethics Committee of Dalian Medical University.
Cell culture
VSMCs were isolated from the thoracic aorta of WT and SmAT2 mice as previously described [22]. Cells were cultured on 100 mm dishes in Dulbecco’s Modified Eagle Medium (Life Technologies, Gaithersburg, Md) supplemented with 10% fetal bovine serum (FBS) and antibiotics at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. Some cells were cultured in calcified medium containing 5% FBS with or without 10 mM β-glycerophosphate (Sigma, G9422) and 0.25 mM ascorbic acid (Sigma, A7506) for 14 days. In some experiments, the cells were stimulated with Ang-(1–7) (Peptide Institute, Osaka, Japan) at 100 nM for 14 days. The medium was changed every 3 days with new medium containing freshly prepared reagents.
Chronic renal failure (CRF) model (5/6 nephrectomy)
Under anesthesia, a two-step procedure operation of 5/6 nephrectomy was performed in ApoE and ApoE/AT2KO mice as previously described [23]. In some experiments, Ang-(1–7) was administered intraperitoneally at 0.5 mg/kg/day using an osmotic mini pump (Alzet model 1002, Durect Corp., Cupertino, California, USA). In the control group, saline was administered with an osmotic mini pump. Serum samples were prepared from mice before and 2 weeks after 5/6 nephrectomy. Serum blood urea nitrogen (BUN), creatinine (Cre), and IP were determined by DRI-CHEM 7000 V (Fujifilm Co, Tokyo, Japan). Samples of aortic valves and aorta from the mice were obtained 6 weeks after operation.
Alizarin Red S and von Kossa staining
Calcium deposition was stained using 2% Alizarin red (Sigma). Briefly, cultured VSMC were fixed by 10% formalin for 15 min and washed twice with distilled water. After adding Alizarin red solution, cells were stained for 15 min at room temperature. Excess stain was washed twice with distilled water. The cells were photographed at ×100 magnification of a microscopy (Leica Microsystems) and a computer software system (Nikon, ACT-2U). Alizarin red stain was quantified by extracting the stain with 10% cetylpyridinium chloride (Sigma-Aldrich, USA) and measuring the absorbance at 540 nm.
Formalin-fixed and paraffin-embedded sections of aortic valves of mice were prepared. The sections were stained with Alizarin Red S and von Kossa stainings. For Alizarin Red S staining, the protocol is same as above. For von Kossa staining, the sections were fixed with 4% paraformaldehyde, incubated with 2% silver nitrate solution, and then placed under ultraviolet light for 30 min. Uncombined silver was removed with 5% sodium thiosulfate for 5 min and stained with nuclear fast red for 3 min after washing with phosphate-buffered saline three times. Photos were examined with a Zeiss Axioskop2 microscope equipped with a computer-based imaging system (Carl Zeiss, Oberkochen, Germany). Quantitative analysis was calculated by the ratio of positive area and total area in aortic valves using computer imaging software (Densitograph).
Real-time reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from the cultured VSMC or the aortas of mice using an RNA extraction kit (Sepasol-RNA I Super; Nacalai Tesque, Kyoto, Japan). RT-PCR was performed with a SYBR Premix Ex Taq kit (Takara Bio, Shiga, Japan) and Thermal Cycler Dice Real Time System (TP800; Takara Bio, Shiga, Japan). The sequences of the RT-PCR primers used in this study were as follows: bone morphogenetic protein-2 (BMP-2), 5′-ACCCCCAGCAAGGACGTCGT-3′ (forward) and 5′-TGGAAGCTGCTGCGCACGGTGTT-3′ (reverse); runt-related transcription factor 2 (RUNX2), 5′-CCGAACTGGTCCGCACCGAC-3′ (forward) and 5′-CTTGAAGGCCACGGGCAGGG-3′ (reverse); osteocalcin (OCN), 5′-AGCAGCTTGGCCGAGACCTA-3′ (forward) and 5′-TGAGGCTCCAAGGTAGCGCC-3′ (reverse); and GAPDH, 5′-ATGTAGGCCATGAGGTCCAC-3′ (forward) and 5′-TGCGACTTCAACAGCAACTC-3′ (reverse).
Statistical analysis
All values are expressed as mean ± SEM in the text and figures. Statistical analyses were performed by Statcel-3 (OMS, Saitama, Japan). Data were evaluated by analysis of variance. The variation of data between all groups was similar. If a statistically significant effect was found, we performed a Tukey–Kramer post hoc test for multiple comparisons. A difference of p < 0.05 was considered statistically significant.
Results
Effects of AT2R on aortic calcification and bone-related genes
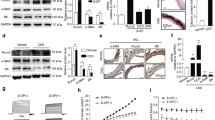
Alizarin Red S staining results showed that compared with WT group, the number of calcified nodules was increased, and the OD value was significantly increased in WT + IP group (Fig. 1a), indicating that IP can lead to the formation of VSMC calcification. However, there were no significant differences in the number of calcified nodules and the OD value between SmAT2 and SmAT2 + IP groups (Fig. 1a). Compared with WT + IP group, SmAT2 + IP group showed less calcified nodules and smaller OD value (p < 0.05) (Fig. 1a). This evidence suggested that AT2R overexpression could prevent the formation of VSMC calcification. RT-PCR results of cultured VSMCs showed that the mRNA expressions of BMP-2, RUNX2, and OCN were significantly increased in WT + IP group compared with those in WT group (Fig. 1b), indicating that IP could lead to the increased expressions of bone-related genes in calcification. Compared with WT + IP group, the increased mRNA expressions of BMP-2 and OCN were significantly decreased in SmAT2 + IP group (Fig. 1b). This evidence suggested that AT2R overexpression could attenuate the expressions of bone-related genes in calcification.
Values are expressed as mean ± SEM. a Calcified nodule is evaluated by Alizarin Red S staining (original magnification ×100). Quantitative analysis of Alizarin Red S staining is shown as the OD value (n = 3 for WT group; n = 4 for the other groups). **p < 0.01 vs. WT. #p < 0.05 vs. SmWT. b mRNA expressions of BMP-2, RUNX2 and OCN were determined by RT-PCR (n = 4 for WT group; n = 6 for the other groups). *p < 0.05, **p < 0.01 vs. WT. #p < 0.05 vs. SmWT. &p < 0.05, &&p < 0.01 vs. WT + IP.
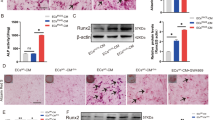
Biochemical analysis of serum samples showed that the levels of BUN and Cre in ApoE mice were significantly higher after operation than those before, and the levels of BUN, Cre, and IP in ApoE/AT2KO mice were also significantly higher after operation than those before (Fig. 2a). This evidence suggested that in this study, CRF model was successfully established. Moreover, after operation, serum BUN and IP levels in ApoE/AT2KO mice were significantly higher than those in ApoE mice (Fig. 2a), indicating that AT2KO could result in CRF more severe. For Alizarin Red S and von Kossa stainings, the ratio of positive and total area in ApoE mice did not change significantly after operation, and it in ApoE/AT2KO mice was significantly increased after operation (Fig. 2a). Moreover, after operation, the ratio of positive and total area in ApoE/AT2KO mice was significantly increased than that in ApoE mice only for von Kossa staining (Fig. 2a). These data indicated that AT2KO could increase the calcification of aortic valves in CRF mice. RT-PCR results of aortic samples showed that after operation the mRNA expressions of BMP-2, RUNX2, and OCN were not changed in ApoE mice, while the mRNA expressions of BMP-2 and OCN were significantly increased in ApoE/AT2KO mice (Fig. 2c). Moreover, after operation the mRNA expressions of BMP-2 were significantly increased in ApoE/AT2KO mice compared with ApoE mice (Fig. 2c). This evidence suggested that AT2KO increase the expression of bone-related genes, which might lead to the formation of aortic calcification more serious.
Values are expressed as mean ± SEM. a The levels of serum BUN, Cre, and IP were determined (n = 4 for ApoE + CRF group; n = 3 for the other groups). **p < 0.01 vs. ApoE. #p < 0.05, ##p < 0.01 vs. ApoE/AT2KO. &p < 0.05, &&p < 0.01 vs. ApoE + CRF. CON: WT. IP: WT + IP, IP + Ang-(1–7), WT + IP + Ang-(1–7). b The calcification of aortic valves is evaluated by Alizarin Red S and von Kossa stainings (original magnifications ×200 and ×400). Quantitative analysis of positive area (percentage of total area) in aortic valves (n = 3 for Alizarin Red S staining; n = 4 for von Kossa staining). #p < 0.05, ##p < 0.01 vs. ApoE/AT2KO. &p < 0.05 vs. ApoE + CRF. c mRNA expressions of BMP-2, RUNX2, and OCN were determined by RT-PCR (n = 4 for ApoE group; n = 6 for the other groups). *p < 0.05, **p < 0.01 vs. ApoE. #p < 0.05, ##p < 0.01 vs. ApoE/AT2KO. &p < 0.05, &&p < 0.01 vs. ApoE + CRF.
Effects of Ang-(1–7) on aortic calcification and bone-related genes
Alizarin Red S staining results showed that compared with the IP group, the number of calcified nodules and the OD value were significantly decreased in IP + Ang-(1–7) group (Fig. 3a), which proved that Ang-(1–7) treatment could also prevent the formation of VSMC calcification. RT-PCR results showed that compared with IP group, the increased mRNA expressions of BMP-2, RUNX2, and OCN were significantly decreased in IP + Ang-(1–7) group (Fig. 3b), which proved that Ang-(1–7) treatment could attenuate the expressions of bone-related genes in calcification.
Values are expressed as mean ± SEM. a Calcified nodule is evaluated by Alizarin Red S staining (original magnification ×100). Quantitative analysis of Alizarin Red S staining is shown as the OD value (n = 3 for CON group; n = 4 for the other groups). *p < 0.05, **p < 0.01 vs. CON. ##p < 0.01 vs. IP. b mRNA expressions of BMP-2, RUNX2, and OCN were determined by RT-PCR (n = 4 for CON group; n = 6 for the other groups). *p < 0.05, **p < 0.01 vs. CON. #p < 0.05 vs. IP.
Biochemical analysis of serum samples showed that after operation the increased levels of BUN, Cre, and IP in ApoE mice were significantly lower after Ang-(1–7) treatment (Fig. 4a). Moreover, after operation the increased levels of BUN and IP in ApoE/AT2KO mice were significantly lower after Ang-(1–7) treatment (Fig. 5a). This evidence suggested that Ang-(1–7) treatment could relieve CRF to some extent. For Alizarin Red S and von Kossa stainings, after operation the ratio of positive and total area in ApoE mice did not change significantly after Ang-(1–7) treatment (Fig. 4b). Moreover, after operation the increased ratio of positive and total area in ApoE/AT2KO mice was significantly lower after Ang-(1–7) treatment for Alizarin Red S staining, and there was no significant difference for von Kossa staining (Fig. 5b). These data indicated that Ang-(1–7) treatment could decrease the calcification of aortic valves in CRF mice. RT-PCR results showed that after operation the mRNA expressions of BMP-2 and RUNX2 in ApoE mice were significantly decreased after Ang-(1–7) treatment (Fig. 4c), indicating that Ang-(1–7) treatment decrease the expressions of bone-related genes in calcification, which might improve the formation of aortic calcification. However, after operation the mRNA expressions of BMP-2, RUNX2, and OCN in ApoE/AT2KO mice were not changed after Ang-(1–7) treatment (Fig. 5c).
Values are expressed as mean ± SEM. a The levels of serum BUN, Cre, and IP were determined (n = 4 for ApoE + CRF group; n = 3 for the other groups). *p < 0.05, **p < 0.01 vs. ApoE. #p < 0.05, ##p < 0.01 vs. ApoE + CRF. b The calcification of aortic valves is evaluated by Alizarin Red S and von Kossa stainings (original magnification ×200 and ×400). Quantitative analysis of positive area (percentage of total area) in aortic valves (n = 3 for Alizarin Red S staining; n = 4 for von Kossa staining). c mRNA expressions of BMP-2, RUNX2, and OCN were determined by RT-PCR (n = 4 for ApoE group; n = 6 for the other groups). *p < 0.01, **p < 0.01 vs. ApoE. #p < 0.05 vs. ApoE + CRF.
Values are expressed as mean ± SEM. a The levels of serum BUN, Cre, and IP were determined (n = 3 for each group). **p < 0.01 vs. ApoE/AT2KO. #p < 0.05 vs. ApoE/AT2KO + CRF. b The calcification of aortic valves is evaluated by Alizarin Red S and von Kossa stainings (original magnification ×200 and ×400). Quantitative analysis of positive area (percentage of total area) in aortic valves (n = 3 for Alizarin Red S staining; n = 4 for von Kossa staining). *p < 0.01, **p < 0.01 vs. ApoE/AT2KO. ##p < 0.01 vs. ApoE/AT2KO + CRF. c mRNA expressions of BMP-2, RUNX2, and OCN were determined by RT-PCR (n = 6 for each group). *p < 0.05, **p < 0.01 vs. ApoE/AT2KO.
Discussion
In this study, VSMC calcification in vitro in WT + IP group was significantly increased compared with WT group, which was significantly inhibited in SmAT2 + IP group. It suggested that AT2R overexpression could prevent VSMC calcification. Aortic calcification in vivo in ApoE/AT2KO + CRF group was significantly increased compared with ApoE/AT2KO and ApoE + CRF groups. It indicated that AT2KO could increase susceptibility to aortic calcification with increased levels of IP in CRF mice. Moreover, VSMC calcification in vitro in IP group was significantly lower after Ang-(1–7) treatment, which proved that Ang-(1–7) treatment could also prevent VSMC calcification. Aortic calcification in vivo in ApoE/AT2KO + CRF was significantly lower after Ang-(1–7) administration, which indicated that Ang-(1–7) administration could decrease aortic calcification with decreased levels of IP in CRF mice, which works mainly through MasR rather than AT2R.
VC is one of the strong predictors of cardiovascular mortality and morbidity in CKD patients, so the prevention and treatment of VC are crucial [24]. However, to date, no treatment strategy has been demonstrated to prevent or completely reverse VC. Experimental findings confirmed an acceleration of atherosclerosis, which seems to start very early in the course of CKD and is characterized by marked medial calcification [25]. In this study, after 5/6 nephrectomy, the increased levels of serum BUN and Cre are the main characteristics of CKD (Fig. 2a), suggesting that the CKD model was successfully established. VC is related to the disorder of serum phosphorus in CKD [4]. Hyperphosphate induced calcification of VSMC in isolated rat aortic rings [7] and in in vitro study [8]. Phosphate also directly induces phenotypic changes in VSMCs from a contractile phenotype into an osteogenic phenotype [26]. Exposure of VSMC to calcified level of phosphate, akin to what may occur in patients with CKD, generates matrix vesicles and exosomes that initiate the mineralization process and induce calcification in the extracellular matrix surrounding VSMC [27]. These changes occur early after exposure to high phosphate level and continue to accumulate over time with increasing phosphate concentration. This study is consistent with the above research that the formation of VSMC calcification was significantly increased in WT + IP group compared with WT group (Fig. 1), indicating that IP in vitro study could lead to VSMC calcification. Serum IP level and the formation of aortic calcification were significantly increased in ApoE/AT2KO + CRF group compared with ApoE/AT2KO group (Fig. 2), indicating that IP in vivo study could lead to aortic calcification.
Previously, our research suggested that AT2R activation represented an endogenous protective pathway against VC [20]. In that study, we generated a CKD-induced VC model induced by an adenine and high-phosphate diet in mice. In many previous reports, CKD and VC have been induced by a high fat diet and partial nephrectomy in apoE−/−KO mice in vivo [28]. On the basis of our previous research, the pathophysiologic roles of AT2R in VC have been further explored by 5/6 nephrectomy in ApoE mice given with an HCD. Osteogenic markers that play an important role in mineralization of ectopic sites have recently been identified and linked to VC [29]. Here, we additionally tested the mRNA expressions of BMP-2 and OCN because they are also important bone-related genes in calcification. The mRNA expression of RUNX2 was significantly increased in SmAT2 VSMC compared with WT VSMC (Fig. 1b). Moreover, the mRNA expression of RUNX2 was not changed in ApoE/AT2KO mice before, after operation and even after Ang-(1–7) treatment (Fig. 5c). This evidence suggested that RUNX2 expression can be affected by overexpressing or lacking AT2R. When the AT2R is overexpressed or knocked out, the expression of RUNX2 could not really reflect the degree of VC. Calcium accumulation in the aortic valve is a hallmark of aortic sclerosis and aortic stenosis [30]. This time we examined the formation of calcification in the aortic valves, which could more directly reflect the formation of VC in mice instead of aortic rings. Compared with the in vitro experiments in our previous studies, AT2KO mice were not studied in this study because primary cultured VSMC expressed only AT1R but not AT2R [31].
On the other hand, ACE2/Ang-(1–7)/MasR axis becomes the major counter-regulatory system against Ang II/AT1R at both systemic and local levels [21]. Recently, a paper from Peptides reported that Ang-(1–7) could inhibit VC in a rat model induced by vitamin D3 plus nicotine [9]. In that study, the rat VC model was created with a high dose of vitamin D3 plus nicotine. However, the effects of Ang-(1–7) on VC have not yet been very clear. VC is highly correlated with cardiovascular morbidity and mortality in chronic kidney disease [32]. Here, we investigated the effect of Ang-(1–7) on VC using gene-modified mice. The VC model is established by IP cultured with VSMC for in vitro study and by 5/6 nephrectomy in mice with increased levels of IP for in vivo study. In addition, that study showed that the formation of aortic calcification is not very obvious in morphology, and there is no statistical analysis. In the experiment, it is also very difficult to cut sections of different positions on different aortas for staining. In this study, we investigated the formation of calcification in the aortic valves instead of the aorta by Alizarin Red S and von Kossa stainings with statistical analysis.
Numerous reports suggested that Ang II could promote VC mainly mediated by AT1R [12,13,14]. As we known both of the Ang II/AT2R and ACE2/Ang-(1–7)/MasR pathways work to offset the actions of Ang II via AT1R [15], so they may play important roles in VC formation. Receptor binding ability of AT1R and AT2R with Ang II indicates the activity of receptors. Compared with WT VSMC, the receptor binding of AT2R with Ang II was significantly increased in SmAT2 VSMC (Supplementary Fig. 1), and indicating that AT2R overexpression (more AT2R activated by endogenous Ang II) could prevent VSMC calcification. Moreover, the AT1R mRNA expression has a decrease tendency in SmAT2 VSMC compared with WT VSMC after IP stimulation (Supplementary Fig. 2a), and the AT1R mRNA expression was significantly increased in ApoE/AT2KO mice compared with ApoE mice after operation (Supplementary Fig. 3a). Therefore, AT2R has protective roles in the formation of VC induced by IP at least in part by inhibiting the effects of AT1R. At the same time, we found that both after IP stimulation in vitro and after operation in vivo, the increased mRNA expression of AT1R was further increased after Ang-(1–7) treatment (Supplementary Figs. 2b and 3b). This may be because exogenous administration of Ang-(1–7) reduces the endogenous Ang-(1–7) produced by Ang II, resulting in a negative feedback, which increases the roles of Ang II on AT1R. Moreover, both after IP stimulation in vitro and after operation in vivo, the mRNA expressions of MasR and ACE2 were upregulated (Supplementary Figs. 2b and 3b), which may be because of the response of MasR and ACE2 to the VC formation. The increased mRNA expression of ACE2 induced by IP was decreased after Ang-(1–7) treatment (Supplementary Figs. 2b and 3b). This may be because exogenous administration of Ang-(1–7) reduces the endogenous Ang-(1–7) produced by Ang II through ACE2, resulting in inhibiting the product of ACE2.
Ang-(1–7) is considered to be the specific ligand of MasR [11]. However, Ang-(1–7) also showed significant binding to AT2R, and the role of Ang-(1–7) mediated by AT2R was also observed [33]. There are some relevant reports in the previous studies. Ang-(1–7) lowered blood pressure in normal and hypertensive adult rats, which was blocked by AT2R antagonist PD123319 [34], which suggested that Ang-(1–7) can bind both AT2R and its own MasR to reduce blood pressure. Moreover, the effect of Ang-(1–7) on increasing perfusion pressure was not observed in the isolated mouse heart after exposure to PD123319 [35]. Therefore, we attempt to study whether the effects of Ang-(1–7) on VC are related to AT2R using AT2KO mice. In this study, the increased calcification of aortic valves in ApoE/AT2KO mice after operation was significantly lower after Ang-(1–7) treatment (Fig. 5b). These data indicated that Ang-(1–7) decreased the calcification of aortic valves in CRF mice, which works mainly through MasR rather than AT2R.
In conclusion, our results provide evidence of the effects of AT2R and Ang-(1–7) in preventing VC induced by IP, at least in part through changing BMP-2 and OCN expressions, and in which Ang-(1–7) had the protective roles mainly through MasR rather than AT2R. Our data also provide a novel insight into the possibility that anti-VC treatment by the effects of AT2R and Ang-(1–7) could be effective in the preventing the process of VC and even reversing VC.
Summary table
What is known about this topic
-
Hyperphosphate induced VC.
-
We have reported AT2R activation preventing VC induced by IP.
-
Ang-(1–7) inhibited VC induced by vitamin D3 plus nicotine.
What this study adds
-
AT2R and Ang-(1–7) prevented VC induced by IP, at least in part through changing BMP-2 and OCN expressions.
-
Ang-(1–7) had the protective roles mainly through MasR rather than AT2R.
References
Guerin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transpl. 2000;15:1014–21.
Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–83.
Eggen DA, Strong JP, McGill HC Jr. Coronary calcification. Relationship to clinically significant coronary lesions and race, sex, and topographic distribution. Circulation. 1965;32:948–55.
Moorthi RN, Moe SM. CKD-mineral and bone disorder: core curriculum 2011. Am J Kidney Dis. 2011;58:1022–36.
Alfrey AC, Ibels LS. Role of phosphate and pyrophosphate in soft tissue calcification. Adv Exp Med Biol. 1978;103:187–93.
Montezano AC, Zimmerman D, Yusuf H, Burger D, Chignalia AZ, Wadhera V, et al. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension. 2010;56:453–62.
Larsson TE, Olauson H, Hagstrom E, Ingelsson E, Arnlov J, Lind L, et al. Conjoint effects of serum calcium and phosphate on risk of total, cardiovascular, and noncardiovascular mortality in the community. Arterioscler Thromb Vasc Biol. 2010;30:333–9.
Kendrick J, Chonchol M. The role of phosphorus in the development and progression of vascular calcification. Am J Kidney Dis. 2011;58:826–34.
Sui YB, Chang JR, Chen WJ, Zhao L, Zhang BH, Yu YR, et al. Angiotensin-(1-7) inhibits vascular calcification in rats. Peptides. 2013;42:25–34.
Santos RA, Campagnole-Santos MJ, Andrade SP. Angiotensin-(1-7): an update. Regul Pept. 2000;91:45–62.
Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100:8258–63.
Zhang Z, Wang M, Fan XH, Chen JH, Guan YY, Tang YB. Upregulation of TRPM7 channels by angiotensin II triggers phenotypic switching of vascular smooth muscle cells of ascending aorta. Circ Res. 2012;111:1137–46.
Feng W, Zhang K, Liu Y, Chen J, Cai Q, Zhang Y, et al. Apocynin attenuates angiotensin II-induced vascular smooth muscle cells osteogenic switching via suppressing extracellular signal-regulated kinase 1/2. Oncotarget. 2016;7:83588–600.
Jia G, Stormont RM, Gangahar DM, Agrawal DK. Role of matrix Gla protein in angiotensin II-induced exacerbation of vascular calcification. Am J Physiol Heart Circ Physiol. 2012;303:H523–32.
Carey RM. Update on angiotensin AT2 receptors. Curr Opin Nephrol Hypertens. 2017;26:91–6.
Ameer OZ, Butlin M, Kaschina E, Sommerfeld M, Avolio AP, Phillips JK. Long-term angiotensin II receptor blockade limits hypertension, aortic dysfunction, and structural remodeling in a rat model of chronic kidney disease. J Vasc Res. 2016;53:216–29.
Li M, Wu P, Shao J, Ke Z, Li D, Wu J. Losartan inhibits vascular calcification by suppressing the BMP2 and Runx2 expression in rats in vivo. Cardiovasc Toxicol. 2016;16:172–81.
Armstrong ZB, Boughner DR, Drangova M, Rogers KA. Angiotensin II type 1 receptor blocker inhibits arterial calcification in a pre-clinical model. Cardiovasc Res. 2011;90:165–70.
Matsushita K, Wu Y, Pratt RE, Dzau VJ. Blockade of angiotensin II type 2 receptor by PD123319 inhibits osteogenic differentiation of human mesenchymal stem cells via inhibition of extracellular signal-regulated kinase signaling. J Am Soc Hypertens. 2015;9:517–25.
Kukida M, Mogi M, Kan-No H, Tsukuda K, Bai HY, Shan BS, et al. AT2 receptor stimulation inhibits phosphate-induced vascular calcification. Kidney Int. 2019;95:138–48.
Ferreira AJ, Santos RA, Bradford CN, Mecca AP, Sumners C, Katovich MJ, et al. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension. 2010;55:207–13.
Chen Y, Kelm RJ Jr., Budd RC, Sobel BE, Schneider DJ. Inhibition of apoptosis and caspase-3 in vascular smooth muscle cells by plasminogen activator inhibitor type-1. J Cell Biochem. 2004;92:178–88.
Gava AL, Freitas FP, Balarini CM, Vasquez EC, Meyrelles SS. Effects of 5/6 nephrectomy on renal function and blood pressure in mice. Int J Physiol Pathophysiol Pharm. 2012;4:167–73.
Palit S, Kendrick J. Vascular calcification in chronic kidney disease: role of disordered mineral metabolism. Curr Pharm Des. 2014;20:5829–33.
Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1599–605.
Shioi A, Nishizawa Y, Jono S, Koyama H, Hosoi M, Morii H. Beta-glycerophosphate accelerates calcification in cultured bovine vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1995;15:2003–9.
Giachelli CM, Speer MY, Li X, Rajachar RM, Yang H. Regulation of vascular calcification: roles of phosphate and osteopontin. Circ Res. 2005;96:717–22.
Shobeiri N, Adams MA, Holden RM. Vascular calcification in animal models of CKD: a review. Am J Nephrol. 2010;31:471–81.
Stompor T. An overview of the pathophysiology of vascular calcification in chronic kidney disease. Perit Dial Int. 2007;27 (Suppl 2):S215–22.
O’Brien KD, Probstfield JL, Caulfield MT, Nasir K, Takasu J, Shavelle DM, et al. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch Intern Med. 2005;165:858–62.
Rajamannan NM, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation. 2005;112 (Suppl 9):I229–34.
Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–42.
Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci. 2011;121:297–303.
Walters PE, Gaspari TA, Widdop RE. Angiotensin-(1-7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertension. 2005;45:960–6.
Bosnyak S, Widdop RE, Denton KM, Jones ES. Differential mechanisms of ang (1-7)-mediated vasodepressor effect in adult and aged candesartan-treated rats. Int J Hypertension. 2012;2012:192567.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant Number 81570391).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bai, HY., Shan, BS. & Jiang, YN. The protective effects of renin–angiotensin system componts on vascular calcification. J Hum Hypertens 35, 410–418 (2021). https://doi.org/10.1038/s41371-020-0347-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-020-0347-z
- Springer Nature Limited