Abstract
This randomized crossover and placebo-controlled trial evaluated the effects of daily use of sildenafil citrate (SIL, 1-month 50 mg twice daily) on penile and systemic endothelial microvascular function in hypertensive patients presenting with erectile dysfunction. The effects of SIL on arterial pressure were evaluated using ambulatory blood pressure monitoring (ABPM). Fifty patients diagnosed with primary arterial hypertension and erectile dysfunction (aged 57.4 ± 5.6 years), recruited in a tertiary public hospital, were treated with SIL (50 mg twice daily) or placebo (PLA) for two 30-day periods with a 30-day washout between them. Laser speckle contrast imaging coupled with acetylcholine skin iontophoresis was used to evaluate penile and systemic (forearm) cutaneous microvascular reactivity. SIL treatment increased penile basal microvascular flow (P = 0.002) and maximal endothelial-dependent peak response to skin iontophoresis of acetylcholine (ACh, P = 0.006). The area under the curve of microvascular vasodilation induced by ACh was also significantly increased (P = 0.02). Lastly, SIL treatment did not modify systemic microvascular reactivity. Twenty-four-hour ABPM (P = 0.0002) and daytime (P = 0.002) and nighttime (P = 0.001) mean diastolic blood pressure values were significantly reduced after SIL treatment. The scores of the Simplified International Index of Erectile Function (P < 0.0001) and the number of patients with positive responses to Sexual Encounter Profile question 3 (P < 0.0001) also increased after SIL treatment. Penile endothelium-dependent microvascular reactivity improved after continuous use of sildenafil in hypertensive patients with erectile dysfunction; the treatment also reduced blood pressure, suggesting that, in addition to improving erectile function, daily use of sildenafil could improve blood pressure control.
Similar content being viewed by others
Introduction
Erectile dysfunction (ED) is a health problem with high prevalence that is related to endothelial dysfunction [1] and to an increased risk of cardiovascular disease [2]. In fact, it is currently accepted that vascular endothelial dysfunction is the link between ED and cardiometabolic diseases, including hypertension, diabetes, and atherosclerosis [1, 3]. In this context, the widespread use of type 5 phosphodiesterase inhibitors (iPDE-5) has led more patients with ED to seek medical help, providing an excellent opportunity for the identification and correction of possible cardiovascular risk factors presented by these patients [4]. ED is strongly associated with age, with an estimated prevalence of 39% in men aged 40 years and 67% at 70 years [5]. Burchardt et al. reported a prevalence of 68.3% of ED in hypertensive men with a mean age of 62.2 years and observed that ED was considered severe in 45.2% of these patients [6]. Thus, in addition to being considered the main risk factor for the development of cardiovascular diseases, arterial hypertension appears to be an important risk factor for ED.
In the late 1990s, sildenafil citrate (SIL) was the first oral drug approved for the treatment of ED. Currently, there is no doubt about the efficacy and safety of iPDE-5 in the treatment of vasculogenic ED, including in hypertensive patients [7,8,9,10]. Nevertheless, there is still little evidence on the continuous use of SIL on blood pressure reduction. Oliver et al. were the first to test SIL in continuous use in hypertensive patients and suggested that this drug could be used as antihypertensive drug [11].
There are no reports on penile microvascular reactivity in patients with ED or on the effects of the chronic treatment with iPDE-5 on penile microvascular function. Vardi et al. were the first to describe the existence of penile endothelial dysfunction in nonhypertensive men with ED, compared with men without ED, using venous-occlusive plethysmography [12]. Using this methodology, these same authors showed that a chronic treatment with SIL (1-month 50 mg daily) improves penile endothelial function compared with a placebo (PLA) treatment [13]. It is important to mention that venous-occlusive plethysmography evaluates changes in blood flow per tissue volume independently from endothelial function [14]. On the other hand, in the present study, we evaluated exclusively the microvascular endothelium-dependent effects of chronic treatment with SIL.
We have recently demonstrated the usefulness of a laser-based noninvasive technique, known as laser speckle contrast imaging (LSCI), on the evaluation of penile microvascular endothelial reactivity [15]. Using LSCI, we showed that the acute oral administration of SIL (100 mg) significantly increases penile microvascular vasodilation in both normotensive and hypertensive individuals [16].
The primary objective of the present randomized crossover PLA-controlled trial was to evaluate the effects of SIL (1-month 50 mg twice daily) on penile and systemic endothelial microvascular function in hypertensive patients presenting with ED. The secondary objective of the study was to determine the chronic effects of SIL on arterial pressure, using ambulatory blood pressure monitoring (ABPM).
Methods
Study design
This study was performed in accordance with the Declaration of Helsinki (revised in 2013) and approved by the Institutional Review Board of the National Institute of Cardiology, at the Ministry of Health in Brazil (http://plataformabrasil.saude.gov.br) with protocol number CAEE 17663813.4.0000.5272 and was registered on clinicaltrials.gov with the number NCT02620995. Written informed consent was obtained before inclusion.
We selected for this study sexually active men, aged 49–70 years, with diagnoses of primary systemic arterial hypertension and ED, treated at the outpatient clinic of the National Institute of Cardiology of Rio de Janeiro, a tertiary public hospital, or who were referred from other units by their attending physicians, during a 36-month period (from 2015 to 2018). Inclusion criteria were patients with blood pressure <160/100 mmHg under drug treatment and with a score ≤21 in the IIEF-5 questionnaire, that is, those diagnosed with ED. Exclusion criteria were as follows: patients using nitrates, alpha-adrenergic blockers and beta-blockers; intolerance to sildenafil; diagnosis of ED of causes other than vasculogenic etiology such as pelvic surgeries or trauma and prostate diseases; and report of use of any iPDE-5 within 30 days prior to inclusion in the study. We also excluded patients with diabetes and with diagnosis of neurological or psychiatric diseases, heart disease, nephropathy or decompensated liver disease, and those with thyroid or pituitary gland disorders.
This is a randomized, double-blind, prospective and crossover study, where hypertensive patients were treated with SIL (50 mg twice daily by oral intake) or PLA in two 30-day periods with a 30-day washout between them. Considering that sildenafil has a terminal half-life of 3–5 h [17], there is no possibility of a carryover effect with the washout period used in the present study. The randomization was simple, in random block sizes (8–12) and based on a table of random numbers generated online using OpenEpi [18] (available on www.OpenEpi.com); the numbers were blinded from all researchers except for one who encoded the flasks of treatments and had no contact with the center at which the study was conducted. Sildenafil and PLA flasks and capsules were blinded for appearance and color. Before randomization and after the end of each treatment step, the hypertensive patients were evaluated through anamnesis, physical examination, and blood and urine collection. Erectile function, ABPM, and skin laser-based microvascular flowmetry were also evaluated in all steps of the study. The flow chart of inclusion of participants is described in Supplementary Table 1.
The primary outcome of the present study was penile endothelial-dependent microvascular vasodilation after a chronic sildenafil treatment, which was analyzed using multiple microvascular parameters (related parameters), including cutaneous vascular conductance (CVC), area under the curve, and maximum vasodilation during skin iontophoresis of acetylcholine. The secondary and related outcomes of the study were the effects of a chronic sildenafil treatment on arterial pressure (office blood pressure and ambulatory blood pressure measurements) and erectile function (evaluated using the Simplified International Index of Erectile Function (IIEF-5) and the Sexual Encounter Profile, as described below).
Evaluation of erectile function
To evaluate erectile function, the IIEF-5 was used [19, 20]. ED was diagnosed when the IIEF-5 score was ≤21. The patients also responded to sexual encounter profile question 3 (SEP3 question), as described previously [21]. SEP3 consists of a direct question about the quality of the patient’s erection, that is, if “the quality of the erection was enough to complete the sexual intercourse in a satisfactory manner.” These evaluations of erectile function were performed in each step of the treatment for prospective comparison.
Evaluation of blood pressure
The blood pressure measurements were obtained with a validated and calibrated automatic sphygmomanometer (Omron M7, Omron Healthcare, Hoofddorp, The Netherlands) using the recommendations of the 7th Brazilian guidelines for arterial hypertension [22]. After 5 min of rest in the supine position, blood pressure measurements were taken in both arms and the arm with the highest arterial pressure value was considered for later evaluations. Then, the orthostatic blood pressure was evaluated and finally three new measurements were performed, with the patient sitting, with a 1-min interval between them, in the selected arm. The mean of the last two measures was considered for further analysis.
The assessment of 24-h blood pressure values was performed by ABPM using Spacelabs model 90121(Spacelabs Healthcare, St Snoqualmie, WA 98065, USA).
Evaluation of systemic and penile microcirculatory flow and reactivity
The microcirculatory tests were performed in an undisturbed quiet room with a defined stable temperature (23 ± 1 °C) after a 20-min rest period in the supine position. The room temperature was monitored and adjusted if necessary, using air conditioning, as the outside temperature was usually >25 °C. Skin iontophoresis was first performed on the forearm and after on the penis, ~15 min after the forearm procedure because it was technically impossible to perform both procedures simultaneously.
Microvascular reactivity was evaluated using an LSCI system with a laser wavelength of 785 nm (PeriCam PSI system, Perimed, Järfälla, Sweden), which enabled us to perform noninvasive and continuous measurements of cutaneous microvascular perfusion changes, which were measured in arbitrary perfusion units, APU. The images were analyzed using the indicated software (PIMSoft, Perimed, Järfälla, Sweden).
High-frame-rate LSCI is a recently marketed noncontact technique based on the analysis of the variations in speckle contrast, which allows measurements of fast changes in skin microvascular blood flow over wide skin areas, with very good interday reproducibility [23]. In fact, LSCI measures skin perfusion over wide areas (up to 100 cm2) with a high frequency (up to 100 images/s). LSCI thus presents the advantages of good temporal and spatial resolutions [24].
One skin site on the ventral surface of the forearm was randomly chosen for the recordings. Hair, broken skin, areas of skin pigmentation and visible veins were avoided, and two drug-delivery electrodes were installed using adhesive disks (LI 611, Perimed, Järfälla, Sweden). The following two measurement areas were identified: a measurement area within the electrode (ACh) and another measurement area (baseline control) adjacent to the electrode. A vacuum cushion (AB Germa, Kristianstad, Sweden) was used to minimize recording artifacts generated by arm movements. ACh 2% w/v (Sigma Chemical CO, St. Louis, USA) iontophoresis was performed using a micropharmacology system (PF 751 PeriIont USB Power Supply, Perimed, Sweden) using increasing anodal currents of 30, 60, 90, 120, 150, and 180 μA, which were administered for 10-s intervals spaced 1 min apart. The total charges for the above currents were 0.3, 0.6, 0.9, 1.2, 1.5, and 1.8 mC, respectively. The dispersive electrode was attached ~15 cm from the electrophoresis chamber. The pharmacological test results were expressed as peak values representing the maximal vasodilation observed after the highest ACh dose. Skin blood flow measurements in APU were divided by mean arterial pressure values to yield CVC in APU/mmHg.
Finally, to evaluate penile skin microcirculation, the electrode was positioned on the base of the penis to perform iontophoretic ACh delivery, as described above and in previous works [15, 16]. In this case, the dispersive electrode was attached to the thigh of each research subject. We used a fenestrated drape with a round opening in the center to lessen the effects of movement on the penile microvascular blood flow recordings. The penis was introduced through the opening of the drape and fixed to the drape using medical pressure-sensitive adhesive tape (Henkel Adhesive North America, Scottsdale, AZ, USA). This tape features a hypoallergenic adhesive that is designed to hold firmly to the skin and dressing materials but can be easily removed without damaging the skin. The recordings of penile microvascular flow were always performed on the flaccid penis.
Statistical analysis
The prospective analysis of statistical power was based on previous study data from our research group, using the microvascular flowmetry technique described above [16]. In that study, we observed a 30% increase (0.18 APU/mmHg) in penile microvascular conductance induced by cutaneous acetylcholine iontophoresis after a single dose of 100 mg sildenafil, from 0.59 to 0.77 APU/mmHg. Assuming that in the PLA group there will be no variation in measurements, then the expected difference between the increments will be 30% (based on within subject measurements), which is consistent with the design of the current crossover study. Consequently, using a statistical power of 80% at significance level of 5%, and an effect size of ~0.62 (predictable difference of 0.18 APU/mmHg using a standard deviation of 0.29 APU/mmHg), the sample size was of 23 patients for each arm of the study, with the total of 46 patients.
The results are presented as the mean ± SD. Variables without a Gaussian distribution, which was determined with the Shapiro–Wilk normality test, are presented as the median (25th–75th percentile). Comparisons of parameters obtained in single measurements before and after treatment with PLA or sildenafil were performed using two-way ANOVA followed by multiple comparisons (Sidak’s multiple comparisons test), where we considered the interactions of time (pre- and post-treatment) and treatment (PLA or sildenafil). Statistical analyses of values obtained with repeated measurements (effects of increasing currents of Ach iontophoresis on vascular conductance) were performed using one-way ANOVA for repeated measures followed by Tukey’s multiple comparisons test. The effect size calculation was performed according to Cohen’s d [25] and considered as small (0.20), medium (0.50), or large (>0.80) effect. P values < 0.05 were considered statistically significant. The statistical package used for the statistical analyses is Prism version 7.0 (GraphPad Software Inc., La Jolla, CA, USA).
Results
Clinical characteristics of the study subjects
The clinical characteristics of the hypertensive patients (n = 50) are presented in Table 1. The values of the IIEF-5 score [18 (16–20)] were inferior to 21 and, thus, characterized ED. Supplementary Table 2 depicts the antihypertensive treatment of the patients during the study period. Most hypertensive patients were taking renin-angiotensin system blockers (88%), including angiotensin receptor blockers or angiotensin converting enzyme inhibitors; calcium channel blockers were used by 52% of the patients, diuretics by 48% and peripheral vasodilators by 6%. Patients who were taking alpha-blockers and beta-blockers were excluded from the study. The mean number of antihypertensive drugs used per patient was 1.9. Thirty-two percent of the hypertensive patients were under treatment with statins. Supplementary Table 3 describes the main side effects observed during PLA or sildenafil treatments.
Effects of sildenafil on erectile function
Data on erectile function, which was considered as a secondary outcome of the study, are depicted in Fig. 1. The score of the IIEF-5 significantly increased after 1 month of sildenafil oral treatment from [18 (16–20)] before vs. [21.5 (20–23)] after treatment (P < 0.0001; Fig. 1a). Likewise, the number of patients responding “yes” to SEP-3 also increased significantly from 66 to 98% after sildenafil treatment (P < 0.0001; Fig. 1b). Treatment with PLA did not induce any significant effect on erectile function (Fig. 1a, b).
Effects of the chronic treatment with sildenafil (SIL) or placebo (PLA) on the scores of the International Index of Erectile Function (IIEF) (a) and on the number of patients responding “yes” to the Sexual Encounter Profile question 3(SEP-3) (b) of hypertensive patients (n = 50). The results are shown as the median (25th–75th percentiles) for the IIEF and as the number of patients (N) for SEP-3. Statistical analyses of the IIEF data were performed using two-way ANOVA followed by multiple comparisons (Sidak’s multiple comparisons test), where we considered the interactions of time (pre- and post-treatment) and treatment (placebo or sildenafil). Statistical analyses of the SEP-3 data were performed using Fisher’s exact test. PRE pretreatment, POST posttreatment.
We also compared IIEF-5 scores between patients who were being treated with diuretics (n = 24) or not (n = 26). IIEF-5 scores were not different between patients treated with diuretics [17 (15–19)] and patients not treated with diuretics [17.5 (14.5–20); P = 0.85]. Then, we evaluated each group before and after SIL or PLA treatment. In the group of patients who were not treated with diuretics, IIEF-5 scores before and after PLA were of 18 (15.25–20.25) and 19.5 (17.75–22), respectively (P = 0.20) and IIEF-5 scores before and after SIL treatment were of 18 (16–20.25) and 22 (19.75–24) respectively (P = 0.0001). In the group of patients treated with diuretics, IIEF-5 scores before and after PLA were of 17 (14.25–19) and 17.5 (14.25–18.75) respectively (P = 0.94) and before and after SIL treatment were of 17.5 (16–19.75) and 21 (19.25–22) respectively (P = 0.002).
Evaluation of skin microvascular flow and reactivity
Penile and systemic microvascular flow and reactivity
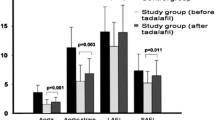
As expected, treatment with PLA did not induce any effect on penile microvascular reactivity of hypertensive patients (Fig. 2a and 3). On the other hand, SIL treatment induced significant increases in penile microvascular vasodilation induced by skin ACh iontophoresis (Figs. 2 and 3, Table 2), which was the primary outcome of the study. The effect sizes are reported on Table 2 and considered to be situated in the medium range. Figure 2b illustrates the effects of increasing currents of iontophoresis of ACh on penile endothelial-dependent microvascular reactivity, showing that vasodilation was significantly higher throughout the entire dose–response curve after SIL treatment, when compared with values obtained before treatment (baseline values). On the other hand, treatment with PLA did not induce any change in penile microvascular reactivity, when compared with values obtained before treatment (baseline values, Fig. 2a).
a Baseline cutaneous microvascular conductance (CVC); b Peak effects of cutaneous acetylcholine iontophoresis on penile CVC; c area under the curve of microvascular vasodilation induced by skin penile acetylcholine iontophoresis. CVC is expressed as arbitrary perfusion units (APU) divided by mean arterial pressure, in mmHg. The results are shown as the median (25th–75th percentiles). Statistical analyses were performed using two-way ANOVA followed by multiple comparisons (Sidak’s multiple comparisons test), where we considered the interactions of time (pre- and post-treatment) and treatment (placebo or sildenafil). PRE pretreatment; POST posttreatment.
The vasodilator effects of SIL treatment in penile microvascular reactivity of hypertensive patients were also demonstrated by the increase in single measurements of penile basal microvascular flow from [0.39 (0.32–0.46)] to [0.44 (0.36–0.54)] APU/mmHg, (P = 0.002; Fig. 3a) and by a significant increase in single measurements of maximal endothelial-dependent peak response to skin iontophoresis of ACh from [0.49 (0.34–0.63)] to [0.61 (0.46–0.88)] APU/mmHg, (P = 0.006; Fig. 3b). Finally, the single measurements of the area under the curve of microvascular vasodilation induced by ACh was also significantly increased from [23,301 (15,686–27,189)] to 26,706 ± 9387 APU/s, (P = 0.02; Fig. 3c). During the PLA treatment, no significant differences were observed in any of the abovementioned microvascular parameters (Fig. 3a–c).
The effects of PLA and SIL treatments on systemic microvascular reactivity, which were considered as a secondary outcome of the study, are depicted in Table 3. There were no statistically significant differences in systemic microvascular vasodilation during treatment with PLA or SIL. The effect sizes are reported on Table 3 and considered to be not relevant.
Effects of sildenafil on arterial blood pressure
Effects of chronic sildenafil treatment on arterial pressure were considered as a secondary outcome of the study. Sildenafil treatment induced significant reductions of the office systolic blood pressure from 137.1 ± 12.7 to 132.1 ± 15.8 mmHg (P = 0.02; Fig. 4a) and diastolic blood pressure from 86.8 ± 7.5 to 81.4 ± 9.4 mmHg (P < 0.0001; Fig. 4b). Treatment with PLA did not modify neither systolic (P = 0.39) nor diastolic (P = 0.79) office blood pressures (Fig. 4a, b). Figure 5 shows the effects of the 30-period treatments with SIL or PLA on blood pressure evaluated using ABPM. Treatment with PLA or SIL did not modify ABPM results concerning systolic blood pressure (Fig. 5a, c, e). On the other hand, 24-h (83.5 ± 8.9 vs. 79.9 ± 8.2 mmHg, P = 0.0002; Fig. 5b), daytime (85.5 ± 9.6 vs. 85.4 ± 8.5 mmHg, P = 0.002; Fig. 5d) and nighttime (73.2 ± 9.7 vs. 69.4 ± 8.9 mmHg, P = 0.001; Fig. 5f) diastolic blood pressure values were significantly reduced after sildenafil treatment. Treatment with PLA did not modify 24-h (P = 0.51; Fig. 5b), daytime (P = 0.47; Fig. 5d) or nighttime (P = 0.99; Fig. 5f) diastolic blood pressure values. It is important to note that effects of SIL on arterial pressure are considered as secondary outcomes of the study.
a Office systolic blood pressure (SBP) and b Office diastolic blood pressure (DBP). The results are shown as the mean ± SD. Statistical analyses of the office blood pressure data were performed using two-way ANOVA followed by multiple comparisons (Sidak’s multiple comparisons test), where we considered the interactions of time (pre- and post-treatment) and treatment (placebo or sildenafil). PRE pretreatment, POST posttreatment.
a 24-h ABPM systolic blood pressure (SBP); b 24-h ABPM diastolic blood pressure (DBP); c Daytime ABPM SBP; d Daytime ABPM DBP; e Nighttime ABPM SBP and f Nighttime ABPM DBP. The results are shown as the mean ± SD. Statistical analyses of the office blood pressure data were performed using two-way ANOVA followed by multiple comparisons (Sidak’s multiple comparisons test), where we considered the interactions of time (pre- and post-treatment) and treatment (placebo or sildenafil). PRE pretreatment, POST posttreatment.
Discussion
Safety and tolerability of iPDE-5 in patients with cardiovascular diseases, including primary arterial hypertension, is well documented in the literature [7,8,9]. Likewise, it has been shown that the chronic use of iPDE-5 improves the erectile function of patients with vasculogenic ED [11]. Nevertheless, to the best of our knowledge, the present study is the first one that evaluated penile microvascular endothelial function before and after continuous use of iPDE-5 in hypertensive patients.
The assessment of penile endothelial function is relevant not only in the diagnosis but also for the follow up of the treatment of vasculogenic ED. The present study demonstrates that overall penile microvascular vasodilatory response was clearly improved after 30-day use of SIL, compared with PLA administration. This is further made evident by the significant increase in the area under the curve of endothelium-dependent microvascular vasodilation induced by acetylcholine skin iontophoresis. Moreover, basal and maximum microvascular penile blood flow, are markedly increased by SIL treatment. Thus, in addition to the increase in penile microvascular flow, SIL treatment improves penile endothelium-dependent microvascular reactivity. In contrast, SIL treatment did not modify systemic microvascular reactivity, as evaluated in the forearm skin. It has already been demonstrated that the evaluation of systemic microvascular endothelial function does not reflect penile endothelial function in patients presenting with ED [12]. In addition, our results give support for the existence of a selective penile vascular endothelial dysfunction in men with ED, despite their similarity in cardiovascular profile and in systemic hemodynamics with men without ED. Moreover, the inhibition of PDE-5, an enzyme considered to be specific for the vascular smooth muscle of the penis [26], is known to increase nitric oxide (NO) bioavailability in the penis and its supplying vasculature, resulting in vasodilation and increased blood flow [7]. Another hypothesis that could explain the dissimilar response of the penile vascular endothelium in relation to the systemic circulation, is that the sensitivity to NO differs between these vascular beds. Even though NO plays an important role in the regulation of basal blood flow of the systemic circulation [27], basal production of NO is low in the penile endothelium, since it is not relevant for maintaining the basic tonus of this organ [28]. However, in response to sildenafil, after sexual stimulation, an expressive acute release of NO may occur in the cavernous body [29]. Thus, it would be likely that in basal conditions, NO could decrease the expression of endothelial NO synthase in a negative feedback loop in penile circulation [30], in a way that a vascular bed that is exposed to NO only intermittently, like the cavernous body, could have an increased response of NO release during sexual stimulation in the course of chronic treatment with sildenafil. Finally, using venoocclusive strain gauge plethysmography, Vardi et al. [13] had already shown that a chronic treatment with SIL (1-month 50 mg daily) improves penile, but not systemic endothelial function, compared with a PLA treatment.
Regarding the effects of SIL treatment on blood pressure, the present study showed that both systolic and diastolic office blood pressure values were significantly reduced after 1-month treatment, while treatment with PLA had no significant effects. Moreover, the analysis of the ambulatory blood pressure monitoring (ABPM) recordings showed significant reductions of diastolic, but not systolic, 24-h, daytime and nighttime values after SIL chronic treatment. Treatment with PLA did not induce any significant effect on blood pressure measure using ABPM. Thus, it is conceivable that the continuous use of iPDE-5 in hypertensive patients with ED might act synergistically with antihypertensive drugs, contributing to a better control of blood pressure, while improving erectile function. In the present study, the blood pressure reduction after chronic use of SIL, as recorded by ABPM, was lower than the values described in previous trials where the acute effects of SIL were evaluated (one hour after a single dose, for instance) [31, 32]. However, as it has been well documented in the literature, it is important to note that even a small reduction on blood pressure would be able to promote significant reductions in cardiovascular risk [33, 34].
As expected, erectile function was clearly improved after treatment with SIL, as evaluated by both the IIEF-5 questionnaire and SEP3 response. IIEF-5 is a simplified version of the IIEF questionnaire, consisting of only five items, which has been shown to be a practical tool for the diagnosis and classification of ED [20]. Median IIEF-5 score before SIL treatment was of 18, thus characterizing ED (scores ≤ 21), while after 1-month SIL treatment the median score improved to 21.5. At this point, it is important to mention the documented existence of an association between ED and coronary artery disease (CAD) [35,36,37,38]. In fact, mild to severe ED in hypertensive patients, as measured by the IIEF-5 index, is independently associated with CAD [39]. Chronic treatment with SIL also markedly increased SEP3 positive responses from 66 to 98%, indicating that virtually all hypertensive patients obtained successful sexual intercourse after SIL treatment.
In the present study, most hypertensive patients were being treated with antihypertensive drugs that supposedly do not interfere with erectile function (calcium channel blockers, 52% of patients) [40] or that improve endothelial function and, almost certainly, erectile function (angiotensin II receptor blockers, 66% of patients, and angiotensin-converting enzyme inhibitors, 22% of patients) [41, 42]. Patients receiving β-blockers were excluded from the study, because antihypertensive drugs blocking the sympathetic nervous system, including β-blockers, have been frequently linked to ED [42, 43]. In fact, several studies suggest that using diuretics and β-blockers other than nebivolol [44] commonly results in ED in hypertensive men [45, 46]. It is also worth mentioning that, in the present study, 48% of the patients were being treated with diuretics for medical reasons. Nevertheless, in the subgroup of patients treated with diuretics, IIEF-5 scores were equivalent to those measured in the group of patients treated with diuretics. Thus, it is reasonable to conclude that antihypertensive drugs, including diuretics, used to treat hypertensive patients in our study, had no influence on erectile function.
In the present study, 36% of the patients did not present any undesirable effect during SIL treatment, against 88% in the PLA group. The most common side effect was headache (46%), followed by stuffy nose (18%), skin flushing (14%) and muscle pain (14%). In most cases, the side effects were transient and did not require medical treatment. While the first three side effects are clearly related to the vasodilator properties of SIL, muscle pain has been related to the inhibition of type 11 PDE [47].
In conclusion, the main findings of this study are as follows: (i) Penile endothelium-dependent microvascular reactivity and erectile function improved after daily use of sildenafil in hypertensive patients with ED; this was the primary endpoint of the study; (ii) Concerning the secondary outcomes of the study, the treatment also reduced blood pressure, suggesting that, in addition to improving erectile function, treatment with sildenafil could improve blood pressure control; (iii) Laser-based methods may well be valuable noninvasive tools for the evaluation of penile microvascular responses to drug treatment in patients presenting with cardiovascular diseases.
Summary table
What is known about topic
-
Erectile dysfunction (ED) is a health problem with high prevalence that is related to endothelial dysfunction
-
Sildenafil is effective and safe in the treatment of vasculogenic ED, including hypertensive patients
-
Laser-based methods are valuable noninvasive tools for the evaluation of penile microvascular function
What this study adds
-
Penile microvascular baseline flow improves after 1-month twice-daily use of sildenafil in hypertensive patients with ED
-
Penile endothelial-dependent microvascular reactivity also improves with sildenafil treatment
-
Besides improving erectile function, sildenafil also reduces arterial blood pressure in hypertensive patients
Change history
18 June 2020
The original HTML version of this Article was updated shortly after publication in order to change all the figures from colour to black & white as per the authors’ request.
References
Solomon H, Man JW, Jackson G. Erectile dysfunction and the cardiovascular patient: endothelial dysfunction is the common denominator. Heart. 2003;89:251–3.
Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294:2996–3002.
Fonseca V, Jawa A. Endothelial and erectile dysfunction, diabetes mellitus, and the metabolic syndrome: common pathways and treatments? Am J Cardiol. 2005;96:13M–18M.
Kloner RA, Jarow JP. Erectile dysfunction and sildenafil citrate and cardiologists. Am J Cardiol. 1999;83:576–82. A577
Bortolotti A, Parazzini F, Colli E, Landoni M. The epidemiology of erectile dysfunction and its risk factors. Int J Androl. 1997;20:323–34.
Burchardt M, Burchardt T, Baer L, Kiss AJ, Pawar RV, Shabsigh A, et al. Hypertension is associated with severe erectile dysfunction. J Urol. 2000;164:1188–91.
Chrysant SG. Effectiveness and safety of phosphodiesterase 5 inhibitors in patients with cardiovascular disease and hypertension. Curr Hypertens Rep. 2013;15:475–83.
Kloner RA, Brown M, Prisant LM, Collins M. Effect of sildenafil in patients with erectile dysfunction taking antihypertensive therapy. Sildenafil Study Group. Am J Hypertens. 2001;14:70–73.
Bohm M, Burkart M, Baumann G. Sildenafil is well tolerated by erectile dysfunction patients taking antihypertensive medications, including those on multidrug regimens. Curr Drug Saf. 2007;2:5–8.
Nehra A, Jackson G, Miner M, Billups KL, Burnett AL, Buvat J, et al. The Princeton III Consensus recommendations for the management of erectile dysfunction and cardiovascular disease. Mayo Clin Proc. 2012;87:766–78.
Oliver JJ, Melville VP, Webb DJ. Effect of regular phosphodiesterase type 5 inhibition in hypertension. Hypertension. 2006;48:622–7.
Vardi Y, Dayan L, Apple B, Gruenwald I, Ofer Y, Jacob G. Penile and systemic endothelial function in men with and without erectile dysfunction. Eur Urol. 2009;55:979–85.
Vardi Y, Appel B, Ofer Y, Greunwald I, Dayan L, Jacob G. Effect of chronic sildenafil treatment on penile endothelial function: a randomized, double-blind, placebo controlled study. J Urol. 2009;182:2850–5.
Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharm. 2001;52:631–46.
Verri V, Brandao A, Tibirica E. The evaluation of penile microvascular endothelial function using laser speckle contrast imaging in healthy volunteers. Microvasc Res. 2015;99:96–101.
Verri V, Brandao AA, Tibirica E. Penile microvascular endothelial function in hypertensive patients: effects of acute type 5 phosphodiesterase inhibition. Braz J Med Biol Res. 2018;51:e6601.
Eardley I, Ellis P, Boolell M, Wulff M. Onset and duration of action of sildenafil for the treatment of erectile dysfunction. Br J Clin Pharm. 2002;53(Suppl 1):61S–65S.
Sullivan KM, Dean A, Soe MM. OpenEpi: a web-based epidemiologic and statistical calculator for public health. Public Health Rep. 2009;124:471–4.
Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30.
Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–26.
Javaroni V, Queiroz-Miguez M, Abreu-Casanova M, Oigman W, Neves MF. Brachial flow-mediated dilation correlates with vardenafil response in hypertensive men with vasculogenic erectile dysfunction. Urology. 2011;78:368–74.
Malachias MV. 7th Brazilian guideline of arterial hypertension: presentation. Arq Bras Cardiol. 2016;107:0.
Roustit M, Millet C, Blaise S, Dufournet B, Cracowski JL. Excellent reproducibility of laser speckle contrast imaging to assess skin microvascular reactivity. Microvasc Res. 2010;80:505–11.
Roustit M, Cracowski JL. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharm Sci. 2013;34:373–84.
Cohen J. Statistical power analysis for the behavioral sciences. 1988.
Wallis RM, Corbin JD, Francis SH, Ellis P. Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am J Cardiol. Springer; Berlin Heidelberg. 1999;83:3C–12C.
Spieker LE, Flammer AJ, Luscher TF. The vascular endothelium in hypertension. Handbook of Experimental Pharmacology. 2006: 249–83.
Simonsen U, Garcia-Sacristan A, Prieto D. Penile arteries and erection. J Vasc Res. 2002;39:283–303.
Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res. 2008;20:17–29.
Vaziri ND, Wang XQ. cGMP-mediated negative-feedback regulation of endothelial nitric oxide synthase expression by nitric oxide. Hypertension. 1999;34:1237–41.
Mahmud A, Hennessy M, Feely J. Effect of sildenafil on blood pressure and arterial wave reflection in treated hypertensive men. J Hum Hypertens. 2001;15:707–13.
Vlachopoulos C, Hirata K, O’Rourke MF. Effect of sildenafil on arterial stiffness and wave reflection. Vasc Med. 2003;8:243–8.
Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–38.
Cholesterol, diastolic blood pressure, and stroke: 13,000 strokes in 450,000 people in 45 prospective cohorts. Prospective studies collaboration. Lancet. 1995;346:1647–53.
Elesber AA, Solomon H, Lennon RJ, Mathew V, Prasad A, Pumper G, et al. Coronary endothelial dysfunction is associated with erectile dysfunction and elevated asymmetric dimethylarginine in patients with early atherosclerosis. Eur Heart J. 2006;27:824–31.
Ponholzer A, Temml C, Obermayr R, Wehrberger C, Madersbacher S. Is erectile dysfunction an indicator for increased risk of coronary heart disease and stroke? Eur Urol. 2005;48:512–8. discussion 517-8
Montorsi F, Briganti A, Salonia A, Rigatti P, Margonato A, Macchi A, et al. Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur Urol. 2003;44:360–4. discussion 364-5
Kloner R. Erectile dysfunction and hypertension. Int J Impot Res. 2006;19:296.
Cordeiro AC, Mizzaci CC, Fernandes RM, Araujo-Junior AG, Cardoso PO, Dutra LV, et al. Simplified International Index of Erectile Function (IIEF-5) and coronary artery disease in hypertensive patients. Arq Bras Cardiol. 2012;99:924–30.
Baumhakel M, Schlimmer N, Kratz M, Hackett G, Jackson G, Bohm M. Cardiovascular risk, drugs and erectile function-a systematic analysis. Int J Clin Pract. 2011;65:289–98.
Javaroni V, Neves MF. Erectile dysfunction and hypertension: impact on cardiovascular risk and treatment. Int J Hypertens. 2012;2012:627278.
Fogari R, Preti P, Derosa G, Marasi G, Zoppi A, Rinaldi A, et al. Effect of antihypertensive treatment with valsartan or atenolol on sexual activity and plasma testosterone in hypertensive men. Eur J Clin Pharm. 2002;58:177–80.
Fogari R, Zoppi A, Poletti L, Marasi G, Mugellini A, Corradi L. Sexual activity in hypertensive men treated with valsartan or carvedilol: a crossover study. Am J Hypertens. 2001;14:27–31.
Brixius K, Middeke M, Lichtenthal A, Jahn E, Schwinger RH. Nitric oxide, erectile dysfunction and beta-blocker treatment (MR NOED study): benefit of nebivolol versus metoprolol in hypertensive men. Clin Exp Pharm Physiol. 2007;34:327–31.
Wassertheil-Smoller S, Blaufox MD, Oberman A, Davis BR, Swencionis C, Knerr MO, et al. Effect of antihypertensives on sexual function and quality of life: the TAIM study. Ann Intern Med. 1991;114:613–20.
Viigimaa M, Doumas M, Vlachopoulos C, Anyfanti P, Wolf J, Narkiewicz K, et al. Hypertension and sexual dysfunction: time to act. J Hypertens. 2011;29:403–7.
Reffelmann T, Kloner RA. Pharmacotherapy of erectile dysfunction: focus on cardiovascular safety. Exp Opin Drug Saf. 2005;4:531–40.
Acknowledgements
The authors wish to thank Marcio Marinho Gonzalez for his excellent technical assistance.
Funding
CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, ET grant # 305234/2017-0), FAPERJ (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro, ET grant # E-26/202.822/2018).
Author information
Authors and Affiliations
Contributions
VV and ET conceived and designed the study. VV and ET analyzed the data and interpreted the results of the experiments. ET drafted the manuscript. VV, AA and ET edited and revised the manuscript. All of the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Part of the present work was presented during the 26th European Meeting on Hypertension and Cardiovascular Protection (2016), during the European Society of Cardiology Congress (2017) and during the 29th European Meeting on Hypertension and Cardiovascular Protection (2019).
Supplementary information
Rights and permissions
About this article
Cite this article
Verri, V., Nascimento, A.R., Brandao, A.A. et al. Effects of chronic type 5 phosphodiesterase inhibition on penile microvascular reactivity in hypertensive patients with erectile dysfunction: a randomized crossover placebo-controlled trial. J Hum Hypertens 35, 360–370 (2021). https://doi.org/10.1038/s41371-020-0343-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-020-0343-3
- Springer Nature Limited
This article is cited by
-
Conservative Non-surgical Options for Erectile Dysfunction
Current Urology Reports (2023)