Abstract
Advanced glycation end products (AGEs) are involved in several pathophysiologic processes in vascular diseases, including progressive loss of elasticity of the vessel wall (arterial stiffness). Circulating soluble receptors for AGEs (sRAGE) act as a decoy and counterbalanced the harmful properties of AGEs as the natural protective factor. We compared the role of circulating or skin-deposed AGEs and sRAGE regarding the natural course of arterial stiffening. In a prospective cohort study, we longitudinally followed 536 general population-based subjects (subsample of Czech post-MONICA study). Aortic pulse-wave velocity (PWV) was measured twice (at baseline and after ~8 years of follow-up) using a SphygmoCor device (AtCor Medical Ltd), and the intraindividual change in PWV per year (∆PWV/year) was calculated. Concentrations of sRAGE and carboxymethyl lysine (circulating AGEs) were assessed at the follow-up visit by ELISA, while skin AGEs were measured using the autofluorescence-based device AGE Reader. Using multiple regressions, we found significant association between ∆PWV/year as a dependent variable, and both, sRAGE and skin AGEs as independent ones (each on its own model). However, the closest associations to ∆PWV/year were found for the ratio of these two factors (skin AGEs/sRAGE) [β coeff = 0.0747 (SE 0.0189), p < 0.0001]. In a categorized manner, subjects with skin AGEs/sRAGE ratio ≥ 3.3 showed about twofold higher risk having ΔPWV/year ≥ 0.2 m/s [adjusted odds ratio was 2.09 (95% CI: 1.35–3.22), p = 0.001]. In contrast, neither circulating AGEs nor circulating AGEs/sRAGE showed any significant relation to ΔPWV/year. In conclusion, skin AGEs/sRAGE ratio seems to be a more sensitive biomarker of vascular aging than these single factors themselves or circulation status of AGEs.
Similar content being viewed by others
Introduction
Progressive stiffening of the arterial wall is the most typical phenotype of vascular aging. The pathophysiology of this process (collectively known as “arteriosclerosis”) involves several parallel mechanisms [1], from mechanical damage and loss of elasticity, caused by fragmentation of elastin fibers, to calcification of arterial media, and infiltration by other compounds. Generally, dynamic of arterial stiffening may substantially vary across different individuals, and “vascular age” is not completely equal to “calendar age” for each of us.
Advanced glycation end products (AGEs) represent heterogeneous groups of compounds that arise from nonenzymatic reactions between reducing sugars and biological proteins or lipids [2, 3]. AGEs develop even in euglycemia, but their formation is accelerated in hyperglycemic state, oxidative stress, or prolonged lipid/protein turnover [4]. AGEs interact with their specific membrane receptor (RAGE), and via nuclear factor kappa-B gene expression, activate several other pathophysiologic cascades (release of inflammatory cytokines, increase in oxidative stress, etc.). Clinically, AGEs potentiate multiple microvascular and macrovascular complications, and RAGE is expressed on cardiomyocytes, smooth muscle cells, endothelial cells, and in several other tissues [5, 6]. Rather specific is the deleterious effect of AGEs on the levels of the vessel wall, where AGE–RAGE interaction culminates into cross-linking of collagen fibers. These cross-links lead not only to mechanical reinforcement of collagen fibers, but because of higher resistance to enzymatic proteolysis, increase the relative collagen content in the arterial wall; these processes collectively lead to increased arterial stiffness again [7, 8].
However, a pathophysiologic situation on the levels of AGE–RAGE interaction (“AGE–RAGE axis”) is much more complicated [4]. First of all, there are four different species of RAGE: full-length membrane RAGE (generally responsible for the harmful consequences of AGEs), membrane N-truncated RAGE (with a still unknown role), and two circulating C-truncated isoforms, esRAGE and sRAGE. These circulating receptors act as a decoy, competing with full-length RAGE for binding of the ligand (AGEs) [9]. Thus, these circulating isoforms represent natural defense against the deleterious effects of AGEs. Another problem is the interpretation of the results regarding circulating RAGE species, because two contradictory scenarios were described: low concentrations of sRAGE or esRAGE were associated with increased cardiovascular risk (in line with their presumed pathophysiological role) [10], but also high levels of sRAGE were identified as a mortality indicator in acutely ill patients (perhaps the physiological response to acute load of AGEs during stress conditions) [11].
The association between arterial stiffness (quantified using pulse-wave velocity, PWV) and several components of the AGE–RAGE axis (circulating AGEs, sRAGE, or esRAGE) was times reported previously [12,13,14,15], but with the exception of our previous recent prospective follow-up study [16], all had a cross-sectional design. Moreover, due to the above-mentioned complexity of AGE–RAGE interaction, a recent summary report [17] advocates to use a ratio between a ligand (AGEs) and its circulating receptor (esRAGE or sRAGE), instead of single factors. In the present analysis, we would like to test this concept using a prospective design, and in the context of a natural course of arterial stiffening (individual increase in aortic PWV during about 8 years) as the dynamic outcome.
Methods
The study protocol was approved by the local Ethics Committee of the University Hospital in Pilsen, and written informed consent was obtained from all participants of the study. All procedures performed were in accordance with the principles of Good Clinical Practice, formulated in the 1964 Declaration of Helsinki and its later amendments.
Design and study population
The analysis represents a prospective cohort study of a general population-based sample (Fig. 1). The initial study sample consisted of 1103 individuals examined as a part of Czech post-MONICA study in 2008. A random 1% sample of Pilsen region residents aged 25–75 years were selected from General Health Insurance Registry—all details are reported elsewhere [15, 18]. From this sample (examined in 2008), over time, 115 subjects had moved and 87 deceased; therefore, 901 individuals were eventually invited to attend clinical examination.
A total of 632 subjects responded to our invitation (70.1% response rate) to attend the second visit (follow-up examination) after ≈8 years or more. However, PWV measurements or AGE–RAGE axis parameters were unavailable or lack appropriate quality in 96 subjects, and they had to be excluded from the final analysis. Thus, the total analyzed population consisted of 536 subjects who have two PWV measurements ≈8 years apart available (i.e., from baseline examination in 2008 and from follow-up visit in 2016/17).
Clinical examination and laboratory analysis
We obtained information on each subject’s personal characteristics, personal and family history of coronary artery disease, lifestyle (smoking, drinking, exercise habits, etc.), and pharmacotherapy. Height and weight were measured using a SECA 767 (SECA Gmbh & co, Hamburg, Germany) scale with a telescopic measuring rod. Body mass index (BMI) was calculated as body weight (kg)/height2 (m2). Blood pressure (BP) was measured using auscultatory technique with standard mercury sphygmomanometers at baseline, and with auscultatory-controlled electronic NISSEI DM-3000 device (NISSEI, Tokyo, Japan) at follow-up. Appropriate-size arm cuffs were used. Three consecutive BP measurements were taken in a patient in sitting position on the right arm; the average BP was determined as the average of the second and third measurements.
The properties of large arteries were measured based on a 2006 Expert Consensus Document [19]. Arterial measurements were obtained after 15 min of rest in the supine position in a quiet room, using a SphygmoCor MM3 device (AtCor Medical Ltd, Sydney, NSW, Australia). Aortic PWV was measured between carotid and femoral arteries. It was calibrated by measuring BP on the contralateral arm before the recordings. The distance between the carotid pulsation and jugular fossa, and between the jugular fossa and femoral recording site, was measured on the body surface. The travel distance along the aorta was calculated by subtracting these two distances. Registrations of the pulse waves were ECG-gated, so we could calculate the time shift between the appearance of the wave at the first and second sites. PWV was calculated as the ratio of the travel distance (m) and the transit time (s), and the average of two recordings was used for analysis. Two recordings were usually performed, but another recording was done if the difference between recordings was greater than 0.5 m/s.
Skin AGEs were quantified by AGE Reader© device (Diagnoptics Technologies B.V., Groningen, The Netherlands), a noninvasive technology that uses ultraviolet light to excite autofluorescence of specific AGEs (pentosidine, Nε-carboxymethyl lysine, and Nε-carboxyethyl lysine) in human skin [20]. We performed three recordings in each subject, and the average was used for analysis (the intraindividual variability of measurements was very low, about 4.4% on average).
Venous blood samples were drawn after at least 12 h of fasting, and all laboratory examinations were realized in series from aliquots stored at −80 °C. We assessed the total and HDL cholesterol, triglycerides, serum creatinine, and glucose levels from serum samples using a Cobas Mira/ROCHE analyzer (ROCHE Diagnostics, Basel, Switzerland) and commercially available kits of the same provenience. LDL cholesterol was calculated using the Friedewald equation, i.e., LDL = total cholesterol − HDL − (TG/2.22). Estimated glomerular filtration (eGFR) was assessed by CKD-EPI formula (using serum creatinine, age, and gender) [21]. The concentration of sRAGE was quantified by ELISA using a Human RAGE Quantikine ELISA Kit (R&D Systems Inc., Minneapolis, MN, USA). Circulating AGEs was quantified as serum carboxymethyl lysine (CML) using commercial ELISA kits (Cusabio Technology LLC, Houston, TX, USA).
Data analysis
As the main indicator of age-dependent progression of arterial stiffness, we used intraindividual change of aortic PWV between baseline and follow-up visit, standardized for 1 year of follow-up (ΔPWV/year [m/s]); in other words, follow-up visit PWV minus baseline PWV divided by the exact time between these visits in days times 365. Five AGE–RAGE axis parameters registered at the follow-up visit, i.e., sRAGE, circulating AGEs, skin AGE, circulating AGE to sRAGE ratio (times 1000), and skin AGE to sRAGE ratio (times 1000) were used as the main independent variables. As covariates, we tested conventional confounders (age, gender… ), the main cardiovascular risk factors, and usual treatments (also those registered at the follow-up visit), and if necessary, dichotomized them into binary variables with cutoff points based on the 6th European Guidelines for Cardiovascular Prevention [22]. Moreover, hypertension was defined as systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg, and/or treatment with antihypertensive medication; overt diabetes as fasting glucose ≥7.0 mmol/L and/or use of antidiabetic medication; impaired renal function as eGFR <60 mL/min/1.73 m2 (Grade 3 or less by The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative) [23]; abnormal HbA1c as ≥42 mmol/mol. Furthermore, all five AGE–RAGE axis parameters were divided into quartiles as follows: <762, 762–997.9, 998–1338.9, and ≥1339 (sRAGE, pg/mL); <110, 110–180.2, 180.3–277.9, and ≥278 (circulating AGEs, pg/mL); <2.1, 2.11–2.4, 2.41–2.8, and ≥2.8 (skin AGEs, autofluorescence units); <94.3, 94.3–179.1, 179.2–308.4, and ≥308.5 (circulating AGEs/RAGE ratio × 1000); <1.658, 1.659–2.396, 2.397–3.288, and ≥3.289 (skin AGEs/sRAGE ratio × 1000), respectively. HbA1c was divided into quartiles as follows: <35, 35–37.9, 38–40.9, and ≥41 mmol/mol.
Accelerated arterial stiffening was arbitrarily set as ∆PWV/year ≥ 0.1 m/s (which is approximately the expected “natural”, age-dependent progression of arterial stiffness, i.e., 1 m/s per age decade [24, 25]) and ∆PWV/year ≥ 0.2 m/s (twofold higher increase than should be expected).
For statistical analyses, STATISTICA 8 (StatSoft Inc., Tulsa, OK, USA) and STATA 8 (STATA Corp. LP, College Station, TX, USA) software packages were used, and generally conventional statistical methods were applied (see the relevant section of “Results”). Power calculations revealed that our population of subjects is sufficiently large to detect differences in primary estimate (ΔPWV/year), with a 5% relative precision level; all variables were tested for normality of distribution, and the appropriate statistical test was chosen. Statistical significance was considered present at P value of 0.05.
Results
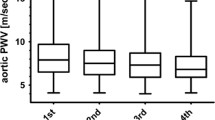
The study cohort consisted of 536 subjects (237 males and 299 females), with a median follow-up of 2918 (IQR: 2783–3612) days. The median intraindividual increase in PWV between baseline and follow-up visit (∆PWV) was 1.28 (IQR: 0.40–2.50) m/s, standardized as 0.16 (0.05–0.31) m/s per year. Detailed baseline characteristics are listed in Table 1.
Individual course of PWV and AGE–RAGE axis parameters
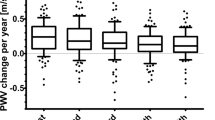
A series of analysis was performed to assess the association between each of five AGE–RAGE axis parameters at the follow-up visit, ∆PWV/year, and already-known covariates of arterial stiffness and conventional factors. Univariate analysis using Spearman’s rank correlation revealed the significant association between ∆PWV/year on one side and sRAGE (r = −0.169, P = 0.0001), skin AGEs (r = 0.142, P = 0.0010), and skin AGEs/sRAGE ratio (r = 0.207, p < 0.0001) on the other. In contrast, no such association was observed with respect to circulating AGEs (r = 0.023, p = 0.593), and a rather weak relation between ∆PWV/year and circulating AGEs/sRAGE ratio (r = 0.087, p = 0.0436). Similar trends were observed also in a semicontinuous manner, if five tested AGE–RAGE axis parameters were dichotomized into quartiles. We found significant inverse trend in ∆PWV/year across sRAGE quartiles, and a positive trend across skin AGEs or skin AGEs/sRAGE quartiles (Fig. 2). However, no apparent differences across circulating AGEs and circulating AGEs/sRAGE quartiles were found (p = 0.819 and p = 0.484, respectively, not in Fig. 2).
As the next step, we performed a series of multiple linear regression to test the association between ∆PWV/year and each of AGE–RAGE axis parameters in the context of potential covariates (conventional risk factors, reported pharmacotherapy, or other characteristics known to be associated with arterial stiffness) in Table 2. Independent association remained after complex adjustment for sRAGE (model A), skin AGEs (model C), and their ratio (model E). Again, no associations were observed between ∆PWV/year and circulating AGEs (model B) or circulating AGEs/sRAGE ratio (model D). All these regressions were performed either with fasting glucose or with HbA1c as covariates, with nearly identical results, regarding AGE–RAGE axis components.
Finally, we included all five explored parameters into one stepwise regression model (Table 2, model F). Both sRAGE and skin AGEs/sRAGE ratio jointly entered the regression model (along with current smoking, mean arterial pressure, and treatment with antihypertensives) and independent covariates of ∆PWV/year. However, with mandatory inclusion of age and gender in the same model, only skin AGEs/sRAGE ratio remained significant covariates of ∆PWV/year (not in the table).
Predictive power of different AGE–RAGE axis parameters to accelerated age-dependent stiffening
For the purpose of this analysis, we used the ∆PWV/year, arbitrarily dichotomized into two categories (≥0.2 and ≥0.1 m/s) as “accelerated arterial stiffening” (dependent variable) and multivariate logistic regression was applied (Table 3).
In semicontinuous arrangement (model A) and after adjustment for potential covariates (see footnote of Table 3), an increase in sRAGE amounting to one quartile was associated with about 21% lower relative risk of accelerated arterial stiffening, defined as ∆PWV/year ≥ 0.2 m/s, or 17% lower risk, if defined as ∆PWV/year ≥ 0.1 m/s (both statistically significant). Similarly, an increase in skin AGEs/sRAGE ratio by one quartile resulted in 35% or 29% higher risk of ∆PWV/year ≥ 0.2 or ≥0.1 m/s, respectively. In contrast, neither circulating AGEs nor circulating AGEs/sRAGE ratio did not show similar predictive potential, and only marginal power was observed in these terms for skin AGEs (Table 3, model A).
As a next step, we performed the analysis in fully categorized arrangement (Table 3, model B), i.e., 1st quartile versus 2nd–4th for sRAGE, while 4th versus 1st–3rd quartile of other four tested parameters. Low sRAGE (1st quartile) was associated with ≈60% higher risk of accelerated aortic stiffening, defined as ∆PWV/year ≥ 0.2 m/s, but predictive potential disappeared if ∆PWV/year ≥ 0.1 m/s was used as a dependent variable. High skin AGEs/sRAGE ratio (4th quartile) was associated with increased risk of accelerated arterial stiffening, defined either as ∆PWV/year ≥ 0.2 m/s or as ≥0.1 m/s.
As a final step, we repeated all the above-mentioned regression models, but after excluding 56 subjects with overt diabetes at the follow-up visit. The results were confirmatory to analyses performed in a full sample (Table 3).
We also exploratorily repeated all analyses in Table 3, but using HbA1c, instead of AGE–RAGE axis components. No significant relation of this parameter to increased risk of accelerated arterial stiffening was found either in all samples [odds ratio for increase in HbA1c amounting to one quartile was 0.98 (95% CI: 0.80–1.20), while 1.38 (95% CI: 0.84–2.27) for HbA1c ≥ 42 mmol/mol, if ∆PWV/year ≥ 0.2 m/s was used as a dependent variable], or if patients with overt diabetes were excluded.
Discussion
In this study, we compared five different expressions of AGE–RAGE axis activity to find the best match with a natural course of vascular aging in the general population. Two primary estimated parameters, circulating sRAGE and skin AGEs (measured noninvasively using autofluorescence technique), showed significant independent association with individual dynamics of arterial stiffening during ≈8 years, but the closest association was observed for the ratio of these two factors, i.e., skin AGEs/sRAGE ratio. This result seems to be also biologically plausible, because this ratio represents the joint factor of the individual course of deposition of AGEs in solid tissue on one side, and the activity of the protective factor on the other. The key finding of our study is that high skin AGEs/sRAGE ratio (≥3.3), i.e., mutual combination of accelerated individual deposition of AGEs in previous years with failed protection factor against this process, was associated with twofold higher relative risk of ∆PWV/year ≥ 0.2 m/s (=2 m/s per age decade). Taking into account that “natural course” (average age-dependent increase) of arterial stiffening is consistent with the increase in PWV by 1 m/s per age decade [24], we can interpret this finding that individuals with high skin AGEs/sRAGE ratio have twice as fast vascular aging, than subjects with a lower ratio. Indeed, this association remained significant also after adjustment for other potential covariates of arterial stiffening (age, gender, BP, chronic pharmacotherapy… ).
Our findings are in agreement with already-known evidence. Several cross-sectional studies confirmed the role of sRAGE in pathophysiology of arterial stiffness. We have already shown in a cross-sectional population-based study that nondiabetic hypertensive subjects with decreased sRAGE (<918 pg/mL) have significantly higher aortic PWV than those with higher circulation concentration levels [15]. In line with this finding, also Dimitriadis et al. [14] demonstrated an inverse relation between sRAGE and aortic PWV, and another study group showed an association between sRAGE and pulse pressure [9]. Finally, our recent study demonstrated even in a prospective cohort design, that high status of circulating sRAGE at baseline acted protectively against accelerated age-dependent increase in aortic PWV during follow-up [16]. With one exception, only smaller and usually highly selected population studies were available to confirm the association between skin autofluorescence AGEs and arterial stiffness. Watfa et al. [26] found an association between aortic PWV and skin AGEs in 55 nondiabetic individuals, younger than 65 years (but not in older subjects). Similar association was reported in 120 patients with end-stage renal disease plus 110 control subjects [27], and additionally, also in 105 type I diabetic patients in another cross-sectional study [28]. The largest part of the evidence represents the Maastricht study that confirmed the independent relation between aortic PWVV and skin AGEs in 862 participants (including normoglycemic subjects, those with impaired glucose metabolism, as well as type II diabetic patients). However, no study was dealing with the ratio to circulating sRAGE, and no one tested the PWV as a longitudinal prospective variable, until now.
In this study, we did not find any relation between ∆PWV/year (or just PWV at the follow-up visit) and circulating AGEs (serum CML), either as a single factor or in ratio with sRAGE. In contrast, a study by McNulty et al. [12] reported a positive association between CML and arterial stiffness in hypertensive patients, and similar association was found in older subjects in another general population-based cross-sectional analysis [13]. In line with these two observations, we performed an exploratory subanalysis, limited to hypertensive patients, aged ≥65 years (not mentioned in “Results”). A marginally significant association (with p = 0.030) was found between ∆PWV/year and circulating AGEs (as continuous variables and by multivariate linear regression), but the subsample used was rather limited (n = 191). Indeed, an association between CML and PWV was reported in diabetic patients [29], but we were not able to repeat this analysis (due to low sample size). The missing association of CML with arterial stiffness in our study can be explained by biological behavior of this factor. Namely, this substance represents a non-cross-linking member of “AGEs family”, i.e., it is able to bind to only one protein residue [30]. Thus, circulating status of CML probably does not give the best information, in the context of the fact that the pathophysiological target of glycation is solid tissue, and plasma concentrations of AGEs give only indirect information. It can be speculated that circulating CML reflects the increased activity of the AGE–RAGE axis only, and especially in more affected subjects, while in generally healthy persons lacking the necessary sensitivity. That would mean we just chose a less-sensitive biomarker of the circulating status of AGEs, while another can be more appropriate (e.g., high-mobility group box 1, a cross-linking member of “AGEs family” [31]).
It holds true that individual dynamics of tissue deposition of AGEs is completely different in patients with overt diabetes, and moreover, antidiabetic treatment can play a role in these terms. Therefore, we repeated the main analysis also after excluding all patients with overt diabetes, i.e., those already treated with any antidiabetic drugs or with fasting glycemia ≥7 mmol/L (see Table 3), or also excluding those with treatment (not mentioned in “Results”); the results were confirmatory to the main findings. We also performed exploratory analysis only in subjects with overt diabetes (not mentioned in “Results”). The predictive power raised for low sRAGE (<918 pg/mL) [with adjusted odds ratio for ∆PWV/year ≥ 0.2 m/s was 5.21 (1.22–22.32), p = 0.026], but surprisingly was no more significant for skin AGEs/sRAGE ratio [3.25 (0.87–12.11), p = 0.08]. However, the subsample of diabetic patients was too small (n = 56) to draw more conclusions.
Potential clinical implications
The mandatory question in any kind of biomarker is its relevance regarding subsequent specific treatment or any other clinical application (stratification of disease, personalization of therapeutic approach, etc.). Currently, no specific AGE- or arterial stiffness-directed treatment exists. The clinical development of alagebrium, a nonenzymatic breaker of AGEs, causing cross-links between collagen fibers, was interrupted several years ago [32]. Exogenous administration of recombinant sRAGE was also tested, but only in animal models [33, 34]. Nearly all types of antihypertensives decrease arterial stiffness, but its potential benefit through this mechanism can be only hardly substantiated from their primary therapeutic effects (decrease in BP). More promising seems to be in this way new classes of antidiabetics, namely SGLT-2 (sodium–glucose cotransporter-2) inhibitors. It has been suggested that evident renoprotective effect of SGLT-2 inhibitors is mediated through a pathway involving HMGB1, another member of AGE family [35]. Treatment with SGLT-2 inhibitor was also followed by a decrease in aortic PWV in diabetic patients [36]. The beneficial effect mediated by the AGE–RAGE axis was reported also in another newer group of antidiabetics, glucagon-like peptide-1 receptor agonists, [37, 38], and also this kind of drugs has potential to decrease arterial stiffness [37]. The crucial advantage of these new classes of antidiabetics is that they can be safely used also in nondiabetic subjects, and one of its potential indications can be supposedly vascular protection against AGEs. We can speculate that a biomarker in the form of proposed skin AGEs/sRAGE ratio may be potentially useful to identify the target population, and to objectify the effect of the treatment.
Study limitations
First of all, as indicators of the activity of AGE–RAGE axis, we used the individual values from the follow-up visit and not from baseline (which is more conventional in prospective studies). The reason is technical: the analysis allows two measurements of PWV, but the autofluorescence quantification of skin AGEs was not available to us in 2008. Moreover, we have not availed serum aliquots to estimate the baseline serum AGEs (after more than 10 years from baseline visit). On the other hand, the age-dependent stiffening and AGE deposition are probably continuous and linear processes, and only its dynamics (steepness) may individually vary. Hence, it may be less important to which part of the continuum the factors relate. We are able to compare the association between ∆PWV/year and either baseline or follow-up concentration of sRAGE, with virtually similar results. We believe that the time factor will not play a major role in other parameters as well.
Second, we did not measure esRAGE, another circulating species of RAGE with presumed similar physiological properties, like sRAGE. Since the physiological levels of sRAGE are about five times higher than esRAGE, the skin AGEs/sRAGE seems to be a more sensitive biomarker than skin AGEs/esRAGE.
Finally, our study cohort is based on a random population sample, but as in other surveys, we strongly depend on the willingness of volunteers to attend the examination program (in our case, twice—at baseline and again after ≈8 years). It is a well-known phenomenon that this kind of survey is attended paradoxically by a relatively healthier part of the population. Thus, our results are not fully generalizable.
Conclusions
Both low concentration of circulating sRAGE and high status of skin AGEs were associated with individually accelerated age-dependent increase in aortic PWV, in middle-aged general population. However, as the most sensitive biomarker seems to be a ratio of both factors, it gives complex information about target damage (AGE deposition) and activity of the protective mechanism in one joint parameter. In contrast, circulating status of AGEs and its ratio to sRAGE were unrelated to individual dynamics of arterial stiffening. To our knowledge, our analysis demonstrated these facts for the first time in a prospective design.
Study highlights
What is already known
-
Advanced vascular end-product deposition is a pivotal process of vascular aging.
-
This process is naturally balanced by a specific soluble receptor (sRAGE).
What this study adds
-
Individual course of arterial stiffening during ≈8 years was ascertained.
-
Skin AGE to sRAGE ratio showed an independent association with this process.
References
O’Rourke MF, Mancia G. Arterial stiffness. J Hypertens. 1999;17:1–4.
Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605.
Schleicher ED, Wagner E, Nerlich AG. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Investig. 1997;99:457–68.
Prasad K. Low levels of serum soluble receptors for advanced glycation end products, biomarkers for disease state: myth or reality. Int J Angiol. 2014;23:11–6.
Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YCE, et al. Cloning and expression of a cell-surface receptor for advanced glycosylation end-products of proteins. J Biol Chem. 1992;267:14998–5004.
Basta G. Receptor for advanced glycation endproducts and atherosclerosis: from basic mechanisms to clinical implications. Atherosclerosis. 2008;196:9–21.
Schnider SL, Kohn RR. Effects of age and diabetes mellitus on the solubility and nonenzymatic glucosylation of human skin collagen. J Clin Investig. 1981;67:1630–5.
Vasan S, Zhang X, Kapurniotu A, Bernhagen J, Teichberg S, Basgen J, et al. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature. 1996;382:275–8.
Geroldi D, Falcone C, Emanuele E, D’Angelo A, Calcagnino M, Buzzi MP, et al. Decreased plasma levels of soluble receptor for advanced glycation end-products in patients with essential hypertension. J Hypertens. 2005;23:1725–9.
Grauen Larsen H, Marinkovic G, Nilsson PM, Nilsson J, Engstrom G, Melander O, et al. High plasma sRAGE (soluble receptor for advanced glycation end products) is associated with slower carotid intima-media thickness progression and lower risk for first-time coronary events and mortality. Arterioscler Thromb Vasc Biol. 2019;39:925–33.
Jabaudon M, Blondonnet R, Pereira B, Cartin-Ceba R, Lichtenstern C, Mauri T, et al. Plasma sRAGE is independently associated with increased mortality in ARDS: a meta-analysis of individual patient data. Intensive Care Med. 2018;44:1388–99.
McNulty M, Mahmud A, Feely J. Advanced glycation end-products and arterial stiffness in hypertension. Am J Hypertens. 2007;20:242–7.
Semba RD, Najjar SS, Sun K, Lakatta EG, Ferrucci L. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with increased aortic pulse wave velocity in adults. Am J Hypertens. 2009;22:74–9.
Dimitriadis K, Tsioufis C, Kasiakogias A, Miliou A, Poulakis M, Kintis K, et al. Soluble receptor for advanced glycation end-product levels are related to albuminuria and arterial stiffness in essential hypertension. Nutr Metab Cardiovasc Dis. 2013;23:382–8.
Mayer O, Seidlerova J, Filipovsky J, Vagovicova P, Wohlfahrt P, Cifkova R, et al. Soluble receptor for advanced glycation end products and increased aortic stiffness in the general population. Hypertension Res. 2016;39:266–71.
Gelzinsky J, Mayer O Jr., Seidlerova J, Materankova M, Mares S, Kordikova V, et al. Soluble receptor for advanced glycation end-products independently influences individual age-dependent increase of arterial stiffness. Hypertens Res. 2020;43:111–20.
Prasad K. Is there any evidence that AGE/sRAGE is a universal biomarker/risk marker for diseases? Mol Cell Biochem. 2019;451:139–44.
Cifkova R, Skodova Z, Bruthans J, Adamkova V, Jozifova M, Galovcova M, et al. Longitudinal trends in major cardiovascular risk factors in the Czech population between 1985 and 2007/8. Czech MONICA and Czech post-MONICA. Atherosclerosis. 2010;211:676–81.
Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605.
Meerwaldt R, Graaff R, Oomen PHN, Links TP, Jager JJ, Alderson NL, et al. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47:1324–30.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis. 2016;252:207–74.
National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266.
Herbert A, Cruickshank JK, Laurent S, Boutouyrie P. Establishing reference values for central blood pressure and its amplification in a general healthy population and according to cardiovascular risk factors. Eur Heart J. 2014;35:3122–33.
Collaboration TRVfAS. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31:2338–50.
Watfa G, Soulis G, Tartagni E, Kearney-Schwartz A, Borghi C, Salvi P, et al. Relationship between tissue glycation measured by autofluorescence and pulse wave velocity in young and elderly non-diabetic populations. Diabetes Metab. 2012;38:413–9.
Ueno H, Koyama H, Tanaka S, Fukumoto S, Shinohara K, Shoji T, et al. Skin autofluorescence, a marker for advanced glycation end product accumulation, is associated with arterial stiffness in patients with end-stage renal disease. Metabolism. 2008;57:1452–7.
Osawa S, Katakami N, Kuroda A, Takahara M, Sakamoto F, Kawamori D, et al. Skin autofluorescence is associated with early-stage atherosclerosis in patients with type 1 diabetes. J Atheroscler Thromb. 2017;24:312–26.
Llaurado G, Ceperuelo-Mallafre V, Vilardell C, Simo R, Gil P, Cano A, et al. Advanced glycation end products are associated with arterial stiffness in type 1 diabetes. J Endocrinol. 2014;221:405–13.
Ahmed N. Advanced glycation endproducts–role in pathology of diabetic complications. Diabetes Res Clin Pr. 2005;67:3–21.
Reddy MC, Christensen J, Vasquez KM. Interplay between human high mobility group protein 1 and replication protein A on psoralen-cross-linked DNA. Biochemistry. 2005;44:4188–95.
Bakris GL, Bank AJ, Kass DA, Neutel JM, Preston RA, Oparil S. Advanced glycation end-product cross-link breakers. A novel approach to cardiovascular pathologies related to the aging process. Am J Hypertens. 2004;17:23s–30s.
Sakaguchi T, Yan SF, Yan SD, Belov D, Rong LL, Sousa M, et al. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Investig. 2003;111:959–72.
Tang SC, Wang YC, Li YI, Lin HC, Manzanero S, Hsieh YH, et al. Functional role of soluble receptor for advanced glycation end products in stroke. Arterioscler Thromb Vasc Biol. 2013;33:585–94.
Yao Y, Zebboudj AF, Shao E, Perez M, Bostrom K. Regulation of bone morphogenetic protein-4 by matrix GLA protein in vascular endothelial cells involves activin-like kinase receptor 1. J Biol Chem. 2006;281:33921–30.
Aroor AR, Das NA, Carpenter AJ, Habibi J, Jia G, Ramirez-Perez FI, et al. Glycemic control by the SGLT2 inhibitor empagliflozin decreases aortic stiffness, renal resistivity index and kidney injury. Cardiovasc Diabetol. 2018;17:108.
Batzias K, Antonopoulos AS, Oikonomou E, Siasos G, Bletsa E, Stampouloglou PK, et al. Effects of newer antidiabetic drugs on endothelial function and arterial stiffness: a systematic review and meta-analysis. J Diabetes Res. 2018;2018:1232583.
Chen S, Yin L, Xu Z, An FM, Liu AR, Wang Y, et al. Inhibiting receptor for advanced glycation end product (AGE) and oxidative stress involved in the protective effect mediated by glucagon-like peptide-1 receptor on AGE induced neuronal apoptosis. Neurosci Lett. 2016;612:193–8.
Acknowledgements
The study realization was supported by the Health Development Agency of Czech Ministry of Health (project 15-27109), by Charles University Research Fund (PROGRES, project Q39), and by Ministry of Health, Czech Republic—conceptual development of research organization (FNPl, 00669806). We would like to acknowledge the hard work of all investigators and other coworkers (study nurses, laboratory technicians, medical students, …) who participated in the post-MONICA project. Namely, we are grateful to Mrs Martina Peštová for her excellent collaboration, and last but not least, to all study subjects, who voluntarily responded to the very time-consuming clinical examination program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mayer, O., Gelžinský, J., Seidlerová, J. et al. The role of advanced glycation end products in vascular aging: which parameter is the most suitable as a biomarker?. J Hum Hypertens 35, 240–249 (2021). https://doi.org/10.1038/s41371-020-0327-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-020-0327-3
- Springer Nature Limited
This article is cited by
-
Time-varying parameters of glycemic control and glycation in relation to arterial stiffness in patients with type 1 diabetes
Cardiovascular Diabetology (2022)
-
Advanced glycation end-products, measured as skin autofluorescence, associate with vascular stiffness in diabetic, pre-diabetic and normoglycemic individuals: a cross-sectional study
Cardiovascular Diabetology (2021)
-
Serum biomarkers, skin autofluorescence and other methods. Which parameter better illustrates the relationship between advanced glycation end products and arterial stiffness in the general population?
Hypertension Research (2021)
-
Accumulation of advanced glycation end products (AGEs) is associated with the severity of aortic stenosis in patients with concomitant type 2 diabetes
Cardiovascular Diabetology (2020)