Abstract
High normal blood pressure (HNBP) is associated with an increased risk of incident high blood pressure (HBP) and of cardiovascular diseases (CVD). To estimate the prevalence of HNBP and related cardiovascular risk factors, a representative sample of 1970 Romanian adults was enrolled in SEPHAR III survey (Study for the Evaluation of Prevalence of Hypertension and Cardiovascular Risk in Romania). All were evaluated for blood pressure values and by a 71-item questionnaire, anthropometric measurements, together with extensive evaluation for target organ damage, blood, and urine sample collection. Prevalence of HNBP was 11% [45.1% had HBP, 43.9% normal BP (NBP)]. HNBP individuals were older (51.14 ± 17.13 years) than subjects with NBP (40.5 ± 15.96 years) but younger than those with HBP (55.79 ± 15.68 years), p < 0.0001 (95% CI 18–85, respectively 18–91). Values of weight, waist circumference, body mass index, total and LDL cholesterol, triglycerides, fasting blood glucose, glycosylated hemoglobin (HbA1c), uric acid, serum creatinine, glomerular filtration rate estimate by CKD-EPI equation, urinary albumin to creatinine ratio, intimae–media thickness, arterial stiffness measurements and diastolic dysfunction, indexed left ventricular mass, interventricular septum and posterior left ventricle wall thickness, left atrial volume, and LA dilatation were significantly higher in HNBP subjects than in NBP. Our study showed that individuals with HNBP represent ~11% and most of them had an elevated total cardiovascular risk. It is essential to educate the public and health care providers to be aware of these individuals and of steps that should be taken to treat modifiable cardiovascular risk factors.
Similar content being viewed by others
Introduction
In 2017, the US guidelines classify the 130–139 mmHg/80–89 mmHg range as stage 1 high blood pressure (HBP) [1]. For the same blood pressure (BP) values, the former 2013, and the new 2018 European Society of Cardiology (ESC) Guidelines for the management of arterial hypertension consider those with 120–129 mmHg and/or 80–84 mmHg to have normal blood pressure (NBP) and those with 130–139 mmHg and/or 85–89 mmHg to have high normal blood pressure (HNBP), with the intent to alert patients and physicians to provide lifestyle education and sometimes medications [1,2,3]. There is heterogeneity within this category, who include 25–50% of adults worldwide, associated with an increased risk of incident HBP at a rate of 8–20% reported in studies lasting 2–4 years, and also associated with increased risk of cardiovascular diseases (CVD). Among these individuals, for those who are middle-aged and without diabetes (DM), the 10-year absolute risk of CVD is about 10% and rises to 40% in the presence of DM and/or established CVD. ESC guidelines use the term HNBP instead of “prehypertension” because the risk of progressing to HBP and developing CVD is higher in individuals with BP values of 130–139/85–89 mmHg than in those with BP values of 120–129/80–84 mmHg [3, 4]. The use of antihypertensive therapy in patients with HNBP who are at high risk (calculated Risk SCORE ≥5% and <10%) and very high risk (calculated Risk SCORE ≥10%) decreases the risk of CVD and death by about 15% [4, 5].
Romania, as previously shown in the three-national-representative surveys [SEPHAR I (2005), SEPHAR II (2012)], and SEPHAR III (2016)—Study for the Evaluation of Prevalence of Hypertension and Cardiovascular Risk in Romania], is a high cardiovascular risk East European country with a high prevalence of HBP of 45.1% [6,7,8,9].
This study aims to evaluate the prevalence of HNBP and to find if adults with HNBP associate more often other cardiovascular risk factors than normotensives, and also to provide a basis for future preventives strategies for HBP and CVD.
Methods
Detailed SEPHAR III methodology has been previously published; therefore, we present below only those aspects regarding the collection of SEPHAR III data that are the object of this study [8, 9].
SEPHAR III: sample selection and data collection
The SEPHAR III survey was conducted between November 2015 and April 2016, in two stages. For an adult Romanian population of 16,269,839 adult citizens, of which 40.41% were estimated to be hypertensive patients (based on SEPHAR I and II survey results), with a maximum error of 2.18%, at a confidence level of 95%, the minimum required sample size for representativity was 1379 study participants [6, 7].
Like previous SEPHAR surveys, following a multistratified sampling procedure, a representative sample of Romanian adults (aged between 18 and 80 years), has been randomly selected from the database of population records, following the principle of equality of chances of being enrolled in the study, regardless of the size of the place of residency. The stratification criteria were: Romania’s territorial regions (eight regions), type of residence (rural and urban), gender and age groups (18–24, 25–34, 35–44, 45–54, 55–64, and 65–80 years). During the two study visits, scheduled in the morning at a 4-day interval, all enrolled individuals were evaluated by the following: 71-item survey questionnaire [of which 11 items regarding sociodemographic data; 12 items regarding medical history (including past medical and family history) and risk factors (smoking, leisure time, physical activity); 3 items assessing dietary habits (salt, fats and alcohol intake); 11 items about the knowledge of methods of CVD prevention and complications; 2 items regarding medication, 13 items for evaluation of state of depression], anthropometric and BP measurements, together with evaluation for target organ damage, blood, and urine sample collection after proper fasting time (8–10 h prior). The study protocol and its implementation procedures were supervised by the project reviewers and approved by the Local Ethics Committee.
Blood pressure measurement
BP measurement technique and definitions of hypertensions were in line with both 2013 and 2018 ESH/ESC Guidelines [2, 3]. At each study visit, three BP measurements were performed at 1 min interval using an automatic certified BP measuring device, with an adjustable cuff for arm circumferences from 24 to 42 cm. BP values were calculated using the arithmetic mean of the second and third BP measurement of each study visit.
Diagnostic criteria
The classification of optimal, NBP, HNBP, and HBP were done in accordance with the 2013 ESH/ESC Guidelines, unchanged by the new 2018 guidelines [2, 3]. The BP category is defined by the highest level of BP, whether systolic (SBP) or diastolic (DBP): Ex—HNBP, defined as not being on antihypertensive medication and having an SBP of 130–139 mmHg and/or DBP of 85–89 mmHg (136/70 mmHg was classified as HNBP, but 136/90 mmHg as HBP, 126/70 mmHg was classified as NBP, but 126/85 mmHg as HNBP).
HBP was defined as SBP at least 140 mmHg and/or DBP at least 90 mmHg at both visits and previously diagnosed hypertension with antihypertensive treatment during the previous 2 weeks, regardless of BP values. Controlled BP values were defined by SBP less than 140 mmHg and DBP <90 mmHg at both study visits, in hypertensive patients who were receiving antihypertensive treatment.
Risk factors and diagnostic categories
Detailed SEPHAR III data collection for risk factors and diagnostic categories has been previously published [8, 9].
The SEPHAR III’s questionnaire included items dedicated to HBP-related comorbidities: coronary heart disease (CHD), heart failure (HF), atrial fibrillation (AF), transient ischemic attack (TIA), stroke, peripheral artery disease (PAD), and renal failure (RF), including the need for dialysis.
The use of the special medical caravan—SEPHAR BUS, fully equipped with medical and laboratory equipment required for the clinical, paraclinical, and laboratory testing-allowed to perform a complete evaluation of target organ damage in a large number of subjects covering all of the 84 survey sites from the entire country.
Cardiovascular risk classification
We use charts for high risk countries, as recommended for Romania in the 2016 edition of ESC cardiovascular disease prevention guidelines. Total CV risk estimation was done using SCORE risk estimation system recommended for adults > 40 years of age, unless they are automatically categorized as being at high or very high risk [5].
Statistical analyses
Statistical analysis was performed with IBM SPSS Statistics 20.0 software at a significance level of p ≤ 0.05. A descriptive analysis (means, medians, standard deviation, and range for continuous data and frequency analysis for categorical data) was performed for all the target variables. Kolmogorov–Smirnov test was used to analyse continuous data distribution, according to which appropriate tests were further used in analysis: independent samples t test or Mann–Whitney U test for differences between means of two independent groups, and ANOVA or Kruskal–Wallis test for differences between means of three independent groups. Chi-square test was used to analyse differences between categorical data. Binary multiple logistic regression using a stepwise likelihood ratio method including multicollinearity testing (tolerance <0.1 and VIF value >10) was used for validation of predictors of HNBP and HBP (as dependent variable). Variables for which statistically significant differences between the three study subgroups were highlighted, were used as independent variables (predictors) in regression analysis. Data were weighted for region, locality type, age groups, and gender.
Results
Response rate
From the total number of 4226 randomly selected addresses, 2124 adults signed written informed consent and were enrolled in the study. A total number of 154 were lost to follow-up; therefore, only 1970 study participants had eligible data for analysis (complete questionnaires and both study visits). The response rate in SEPHAR III survey was 72.58%.
Prevalence of NBP, HNBP, and HBP
A total of 1970 subjects with complete data were included in the statistical analysis: 1034 were females (52.4%) and 936 males (47.6%), p = 0.03, 95% CI 0.3–9.1, mean age 48.5 ± 17.5 years. Categorized by BP status, 865 (43.9%) subjects had NBP, 216 (11%) subjects had HNBP and 889 (45.1%) subjects had HBP. Individuals with HBP were older (mean age 55.7 ± 15.6 years) than those with HNBP (mean age 51.1 ± 17.1 years) and NBP (mean age 40.5 ± 15.9 years), p < 0.0001 (95% CI 18–85, respectively, 18–91).
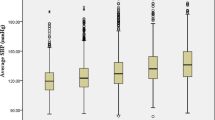
Table 1. and associate Fig. 1 illustrates crude proportions and graphic evolution of NBP, HNBP, and HBP accounted for all participants by age in males and females.
Global rate of HBP awareness accounting for a rate of 80.9% [9].
Characteristics of study groups categorized by gender and blood pressure status
Table 2 shows the characteristics of the three categories of BP groups.
Values of SBP, DBP, weight, waist circumference, BMI, TC, LDL and HDL cholesterol, TG, fasting blood glucose (FBG), glycated hemoglobin HbA1c, uric acid, serum creatinine, eGFR CKD-EPI, and albumin/creatinine ratio were significantly higher in HNBP subjects than in NBP. There were no significant differences for these variables between HNBP and HBP subjects, respectively.
As expected, salt intake was significantly higher in HBP and HNBP subjects compared with NBP ones (13.1 ± 4.1 vs. 12.8 ± 3.6 vs. 11.2 ± 3.6 g/day, <0.0001). There were no significant differences between HBP and HNBP subjects regarding salt intake.
Arterial stiffness and ankle–brachial index (ABI) measurements showed that aortic pulse wave velocity (Ao PWV) was significantly higher in HNBP and HBP, while an ABI < 0.9 was more frequent in HBP group.
The evaluation of carotid arteries showed a higher carotid intimae–media thickness (cIMT) in HNBP and HBP, with more frequent instable plaques in the HBP group.
Transthoracic echocardiography (TTE) measurements showed that values of indexed left ventricular mass (LVMI), interventricular septum (ISV), and posterior left ventricle wall thickness (PV), left atrial (LA) volume, and LA dilatation, were higher in HNBP and HBP groups than in NBP group. The rates of delayed and impaired relaxation, as signs of diastolic dysfunction, defined by E/A and E/e′ ratio were 44% in HBP group vs. 41% in HNBP group (NS) vs. 31% in NBP group (p < 0.0001), being significantly more frequent recorded in HBP and HNBP groups. Left ventricular hypertrophy defined by LVMI and the LV systolic dysfunction (defined by an LVEF ≤ 40%) increases with BP values, being more frequent in the HBP group.
Association of risk factors with HNBP and HBP
The multivariable-adjusted ORs between HNBP and HBP and various risk factors are presented in Table 3. Males are more likely to have HNBP and to develop HBP. Age (beginning from 55 years) and family history of CVD (including HBP) are significantly associated with HNBP and HBP. DM and dyslipidaemia are risk factors for HNBP and significantly increase the risk of HBP. Overweight and obesity were risk factors for both HNBP and HBP. Although the state of depression is a well-recognized risk factor for HBP, in our study the depression state was not associated with HNBP. Compared to subjects with an elementary education status, those with a higher school education were less likely to have HNBP and HBP. Daily alcohol consumption (300 ml wine or 30 ml strong drinks) caused a modest, but non-significant rise in the risk of HNBP and HBP. Cigarette smoking was not associated with HNBP and was also found to have a significantly negative association with HBP. Finally, salt intake is significantly associated with HNBP and HBP, regardless of age or sex.
After adjusting for age, gender, and family history of CVD/HBP, individuals with overweight/obesity and those with a high salt intake showed an increased risk for HNBP: OR 1.62, CI 1.32–1.98, p < 0.001, respectively OR 2.12, CI 1.67–2.68, p < 0.001. Clustering of these two factors was associated with a 3.52 higher OR (CI 2.78–4.76, p < 0.001) of HNBP compared with absence of the association.
Study groups related comorbidities and the risk of CVD
Related comorbidities among NBP, HNBP, and HBP subjects are presented in Table 4.
Although the majority of the HBP subjects (78%) had at least one comorbidity, 50% of subjects from HNBP group had at least one associated comorbidity, while only 30% of subjects from NBP group associated at least one comorbidity.
The rates of CHD, HF, PAD, TIA, and stroke were significantly highest in HNBP and HBP patients compared with NBP, whereas the rates of AF and RF were correlated with the HBP status. Nevertheless, the use of statins and antiplatelet treatment was not frequent (from 3 to 5%) with not significantly differences between the groups.
CV risk estimation using SCORE chart was possible for 1303 (66.1%) subjects: 312 (36.06%) in NBP, 102 (47.2%) in HNBP, and 889 (100%) in HBP subjects. Table 5 shows the characteristics of study groups associated with calculated SCORE risk. In each SCORE category, the proportion of HBP subjects is significantly higher than the proportion of NBP and HNBP subjects, respectively. The percentages of subjects at low and moderate CV risk is similar in NBP and HNBP groups, but the percentages of subjects at high and very high CV risk in higher in HNBP group than in NBP group: 18.05% (39 subjects out of total 216 subjects) vs. 10.6% (92 subjects our of total 865); p = 0.002, 95% CI: 2.3–13.4.
Discussion
This study focuses on a large sample of adults aged between 18 and 80 years, representative for the Romanian population. The prevalence of HNBP was 11% and the prevalence of HBP was 45.1% in all participants, which together means ~56% of population. By extrapolating the results from the SEPHAR III survey to the entire adult population of Romania, we can estimate that in 2016, there were ~7.4 million HBP patients and 1.8–1.9 million HNBP adult subjects.
The prevalence of HNBP in our study was only 11%, which is significantly less than the 25% in Brisighella Heart Study (Italy/Croatia), or the reported range of 30–40% in others European countries, but similar with the one reported in Turkey—14.5% [10,11,12]. The overall pooled prevalence of HNBP is thought to be 36% based on the results of a meta-analysis performed in 2011, on a total sample of 250,741 individuals from 20 cross-sectional studies [13]. The maximum value of HNBP’s prevalence is 58.7% and was reported in a study coming from Nigeria [14]. In a more recent meta-analysis, HNBP’s prevalence ranged from 32.6 to 41.1% for the five US studies, from 25.2 to 46.0% for the five Japanese studies, and from 30.0 to 35.3% for the three Chinese studies [15]. It is well known that studies who excluded individuals with HBP generally reported a higher prevalence of HNBP, but this cannot explain the surprisingly lower prevalence of HBP in our sample [4]. In contrast, the prevalence of 45.1% HBP in our study is on the higher end between European Countries and this could explain the distribution analysis ascertained that most participants have HBP [16].
The gender difference in the prevalence rate of HNBP in Romanian population, 13.8% male’s vs. 8.4% females, was not as drastic as that seen in American adults, where 44.8% of male’s vs. 27.3% of females were reported to have prehypertension [17]. In our study the prevalence for males is highest for those between 25 and 34 years (15.9%), respectively, 35–44 years (11.9%) for females, and increase after the age of 75 years (19.3% males, 14% females). The prevalence of HNBP would be expected to be higher with increasing age, but especially for men, the prevalence decrease slightly in those between 45 and 74 years, in parallel with the net increase (×1.5–5) of HBP prevalence with age—Table 1 and Fig. 1. Although many reports have observed a significant correlation between HNBP and increasing age, in our study the distribution of HNBP is more similar to those from East Asian Countries (Mongolia and China) where the prevalence decreased with age in males [4, 11, 18, 19]. Yet, sexual differences in the distribution of HNBP apparently exist and seem to vary from culture to culture, which implies an interaction between social and biological the prevalence decreased with age in males [4, 11, 18, 14]. Also, prevalence of obesity, which is an important determinant of HNBP was 9.7% (18–24 years), 15% (25–34 years), 30% (35–44 years), 43.5% (45–54 years), 52.9% (55–64 years), and 44.2% (65–80 years), with more 18–44 years overweight and obese HNBP males (36/59) than females (11/29) [4, 18]. However, another possible explanation could be the observations coming from recent studies that autonomic dysfunction is evident in prehypertensive young males aged from 20–40 years, with an enhanced sympathetic activity and decreased parasympathetic activity [20, 21].
HNBP individuals appear to have a greater prevalence of CVD risk factors, compared to those with NBP. In this study, waist circumference, BMI, TC, LDL, and HDL cholesterol, TG, FBG, HbA1c, uric acid, serum creatinine, eGFR CKD-EPI and albumin/creatinine ratio (ACR) were significantly highest in HNBP subjects. There is no significant difference for these values between HNBP and HBP subjects and the results are generally concordant with the other studies [4, 10,11,12].
The multiple logistic regression analysis showed that male sex, age > 55 years, overweight, obesity, and salt intake, were significantly associated with both HNBP and HBP. In addition, dyslipidaemia, DM, depression state, and a low level of education were significantly associated with HBP. A high education level was shown to be a protective factor, suggesting as in other studies, that those with a higher education were better informed about hypertension and subsequently had a healthier lifestyle [4, 13]. In our study, alcohol use was not a predictor of HNBP or HBP and we also found that smoking appeared to be a protection factor for HBP [OR 0.60, CI 0.48–0.75, p = 0.001]—Table 3. The relationship between smoking and development of HBP is still unclear and controversial, but it was noted that a lower BP in smokers than non-smokers might be ascribed to the effect of smoking reducing weight [22]. Regarding smoking and HNBP, our study did not conclude any association which is in line with other studies. Only one study showed a positive association between smoking and HNBP, but only among men, among non-whites and among nonobese subjects [18, 23]. Like in other studies, an index BMI, which defined overweight [OR 1.18, CI 0.82–1.70, p = 0.06] and obesity [OR 1.33, CI 0.98–1.81, p = 0.04], was a strong modifiable predictor of HNBP and HBP [4, 13, 15, 17]—Table 3.
In SEPHAR III survey, the salt intake was estimates for first time in a representative cohort for the general adult population of Romania, based on the Kawasaki formula [8, 9]. As expected, salt intake is significantly associated with HNBP and HBP regardless of age or sex, being significantly higher than in NBP (Table 3). Like other Central/East European Countries, daily salt intake in Romania is almost double beyond the recommended intake by current guidelines [3, 5, 9, 16].
Arterial stiffness measurements estimated by Ao PWV were significantly higher in HNBP and HBP, while an ABI <0.9 was more frequent in the HBP group. Our results confirm previous findings that claim that arterial functions are impaired even at the prehypertensive stage [24, 25]. Also, the evaluation of carotid arteries showed a higher cIMT in HNBP and HBP, with more frequents instable plaques in HBP group [26].
TTE measurements showed values of LVMI, ISV, PV, LA volume, and LA dilatation, being highest in HNBP and HBP groups than in NBP. The MONICA/KORA Augsburg trial was a study of individuals with HNBP with a follow-up of 10 years, which found a significantly greater age-related increase in LV wall thickness (11.9 vs. 4.7%, p < 0.001) and LV mass (15.7 vs. 8.6%, p = 0.006) and an increased incidence of LV concentric remodeling (hazard ratio- HR 10.7; 95% CI 2.82–40.4) and LVH (HR 5.3; 95% CI 1.58–17.9), compared with individuals with NBP [27]. Few studies have shown an association between the diastolic dysfunction and HNBP status, but in our study, the rates of delayed relaxation, LA volume augmentation and LA dilatation like markers of diastolic dysfunction, appears to be significantly more frequent in HNBP than in NBP individuals, with no differences between HNBP and HBP. Our data confirm the continuous relationship between increasing degree of BP and deterioration of diastolic dysfunction, showing that changes in diastolic function are already present in prehypertensive stages [28,29,30,31].
Like other studies, hypertension related comorbidities are significantly higher in HBP and HNBP groups than in NBP individuals: 78% HBP participants had at least one comorbidity, 50% in the group of HNBP and 30% between those with NBP [32, 33]. A total of 507 participants (25.7% of the total population) had CHD; 19% in NBP, 27% in HNBP and 32.1% in HBP. This is extremely high and unfortunately Romania holds fourth place in the world in terms of mortality due to ischemic heart disease and stroke in men and third place in women [7, 34].
In addition, a marker of subclinical renal disease, ACR is significantly higher in HNBP than in NBP subjects: 31.5 ± 288.3 vs. 8.3 ± 26.4 mg/mmol, p = 0.02. There is no significant difference in these values between HNBP and HBP subjects (31.5 ± 288.3 vs. 29.8 ± 211.2 mg/mmol) but it is evident that increases in Albumin/Creatinine ratio, parallel BP, and antedate development of HBP [35]. Interestingly, and as suggested by 2018 ESC Hypertension Guidelines, in HNBP and HBP groups, we found an increase in serum uric acid to levels lower than those typically associated with gout, but significantly higher than NBP individuals [3]—Table 3.
The present study showed that 47.2% of adults with HNBP had at least one of the following CVD risk factors (dyslipidaemia, DM, overweight/obesity) and 18.05% were at high or very high cardiovascular risk, as estimated by the SCORE system. The percentage of subjects with high or very high CV risk are more in HNBP group compared with NBP group: 18.05% vs. 10.6%; p = 0.002, 95% CI: 2.3–13.4—Table 5. Since HNBP is a stage in the progression to HBP, this might imply that almost half of these individuals, and especially those at high and very high cardiovascular risk (almost 1 for 5), are at risk of CVD progression [4,5,6,7].
Limitations and strengths of SEPHAR III survey
SEPHAR III methodology enables a complete estimation of BP trends and a complete target organ damage evaluation [8, 9]. The strengths of the study include the large sample size associated with the principle of equality of chances of being enrolled in the study and direct measurement of BP, rather than self-reported values. Use of the automated BP measuring device provided a reliable measurement of BP and was beneficial in eliminating biases related to the traditional manual BP measurement [2, 3]. The response rate in SEPHAR III survey was good (72.58%) but even that, the results of this cross-sectional study may not entirely reflect the health status of the general population in Romania, since the study population represented a convenience sample of those who signed written consent to participate.
Another study limit is the lack of ABPM, since this type of evaluation could not be feasible (mainly for logistic reasons) for use in large sample as SEPHAR III surveys. By using ABPM, the reals percentages of NBP, HNBP, and HBP could be more accurate, due to its superiority in identifying the white-coat effect or masked hypertension.
Conclusions
Individuals with HNBP represent ~11% of the Romanian adult population and they associate in a higher proportion other cardiovascular risk factors compared with normotensive Romanian adults. This might imply that they are at increased risk of developing sustained HBP and other CVD. HNBP and HBP combined afflict ~56% of Romanian adults (18–80 years). Possible explanations of this current alarming situation may be the following: unhealthy lifestyle and diet, including increased salt intake and the increase rate of obesity and DM. It is of paramount importance to inform and educate the general public and health care providers not only about HBP but also to be aware of HNBP subjects at risk for CVD and of steps that should be taken to treat modifiable risk factors in these people.
Summary
What is known about topic
-
The oldest and new 2018 European Society of Hypertension’s/ESC Guidelines for the management of arterial hypertension consider BP 130–139/85–89 mmHg as High normal blood pressure (HNBP).
-
HNBP affects ~25–50% of adults worldwide and increases the risk of incident hypertension, with annual rates ranging from 8 to 20% in studies lasting 2–4 years, and 4 to 9% in longer-term studies.
-
Antihypertensive medications reduce cardiovascular events in adults with HNBP and diabetes or cardiovascular disease, whereas data are lacking, and guidelines do not recommend pharmacotherapy for primary prevention without these comorbidities.
What this study adds
-
HNBP is a common condition across age, sex, ethnicity, and geographical boundaries in countries with developed and developing economies worldwide. For the first time we estimate the prevalence of HNBP ~11% in Romania which is a high cardiovascular risk East European country with a high prevalence of general hypertension around 45.1%.
-
The present study showed that almost half of adults with HNBP had at least one of the following CVD risk factors (dyslipidaemia, DM, and overweight/obesity) and 18.05% were at high or very high cardiovascular risk, as estimated by the SCORE system. Since HNBP is a phase in the progression to HBP, this might imply that almost half of individuals with HNBP, and especially those at high and very high cardiovascular risk (almost 1 for 5), are at risk of CVD progression and need healthier lifestyle and prevention medications.
-
It’s of paramount importance to inform and educate the general public and health care providers not only about HBP but also to be aware of HNBP subjects at risk for CVD and of steps that should be taken to treat modifiable risk factors in these people.
-
Our study has a reproducible method that could be easily implemented on any other set of data from a national-representative survey from any other country.
References
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force On Clinical Practice Guidelines. Hypertension. 2018;71:1269–324.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–219.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–104.
Egan BM, Stevens-Fabry S. Prehypertension—prevalence, health risks, and management strategies. Nat Rev Cardiol. 2015;12:289–300.
Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–81.
Dorobanţu M, Darabont R, Ghiorghe S, Arsenescu-Georgescu C, Macarie C, Mitu F, et al. Hypertension prevalence and control in Romania at a seven-year interval. Comparison of SEPHAR I and II surveys. J Hypertens. 2014;32:39–47.
Dorobantu M, Darabont RO, Badila E, Ghiorghe S. Prevalence, awareness, treatment, and control of hypertension in Romania: results of the SEPHAR study. Int J Hypertens. 2010;2010:970694. https://doi.org/10.4061/2010/970694.
Dorobantu M, Darabont RO, Dimulescu D, Sinescu C, Gusbeth PT, Georgescu CA, et al. New national epidemiological survey for the assessment of trend in hypertension’s prevalence, treatment and control among the adult population of Romania: SEPHAR III: design and methodology. J Hypertens Res. 2016;2:143–52.
Dorobantu M, Tautu OF, Dimulescu D, Sinescu C, Gusbeth-Tatomir P, Arsenescu-Georgescu C, et al. Perspectives on hypertension’s prevalence, treatment, and control in a high cardiovascular risk East European country: data from the SEPHAR III survey. J Hypertens. 2018;36:690–700.
Ivkovic V, Parini A, Vrdoljak A, Karanovic S, Baric MA, Bacchelli S, et al. [PP.19.05] Prehypertension in european rural population—data from the Brisghella heart study (Italy) and Enah study (Croatia). Conference paper. J Hypertens. 2016;34:e234.
Costanzo S, Di Castelnuovo A, Zito F, Krogh V, Siani A, Arnout J, et al. Prevalence, awareness, treatment, and control of hypertension in healthy unrelated male-female pairs of European regions: the dietary habit profile in European communities with different risk of myocardial infarction—the impact of migration as a model of gene–environment interaction project. J Hypertens. 2008;26:2303–11.
Erem C, Hacihasanoglu A, Kocak M, Deger O, Topbas M. Prevalence of prehypertension and hypertension and associated risk factors among Turkish adults: Trabzon Hypertension Study. J Public Health. 2009;31:47–58.
Guo X, Zou L, Zhang X, Li J, Zheng L, Sun Z, et al. Prehypertension: a meta-analysis of the epidemiology, risk factors, and predictors of progression. Tex Heart Inst J. 2011;38:643–52.
Isezuo SA, Sabir AA, Ohwovorilole AE, Fasanmade OA. Prevalence, associated factors and relationship between prehypertension and hypertension: a study of two ethnic African populations in Northern Nigeria. J Hum Hypertens. 2011;25:224–30.
Huang Y, Wang S, Cai X, Mai W, Hu Y, Tang H, et al. Prehypertension and incidence of cardiovascular disease: a meta-analysis. BMC Med. 2013;11:177. https://doi.org/10.1186/1741-7015-11-177.
Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, et al. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Pressure). National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet. 2011;377:568–77.
Gupta AK, McGlone M, Greenway FL, Johnson WD. Prehypertension in disease-free adults: a marker for an adverse cardiometabolic risk profile. Hypertens Res. 2010;33:905–10.
Li H, Xu T, Tong W, Liu Y, Zhao L, Zhang Y. Comparison of cardiovascular risk factors between prehypertension and hypertension in a Mongolian population, Inner Mongolia, China. Circ J. 2008;72:1666–73.
Lin Y, Lai X, Chen G, Xu Y, Huang B, Chen Z, et al. Prevalence and risk factors associated with prehypertension and hypertension in the Chinese She population. Kidney Blood Press Res. 2012;35:305–13.
Amaral JF, Borsato Diana de MA, Freitas-Guedes IM, Toschi-Dias E, Martinez DG, Laterza MC. Autonomic and vascular control in prehypertensive subjects with a family history of arterial hypertension. Arq Bras Cardiol. 2018;110:166–74.
Moinuddin A, Gupta R, Saxena Y. Autonomic function tests in prehypertensive young adult males of Uttarakhand region. Indian J Physiol Pharmacol. 2016;60:45–51.
Primatesta P, Falaschetti E, Gupta S, Marmot MG, Poulter NR. Association between smoking and blood pressure: evidence from the health survey for England. Hypertension. 2001;37:187–93.
Alshaarawy O, Xiao J, Andrew ME, Burchfiel C, Shankar A. Serum cotinine levels and prehypertension in never smokers. Int J Hypertens. 2013;2013:284524. https://doi.org/10.1155/2013/284524.
Gedikli O, Kiris A, Ozturk S, Baltaci D, Karaman K, Durmus I, et al. Effects of prehypertension on arterial stiffness and wave reflections. Clin Exp Hypertens. 2010;32:84–9.
Tomiyama H, Yamashina A. Arterial stiffness in prehypertension: a possible vicious cycle. J Cardiovasc Transl Res. 2012;5:280–6.
Manios E, Tsivgoulis G, Koroboki E, Stamatelopoulos K, Papamichael C, Toumanidis S, et al. Impact of prehypertension on common carotid artery intima-media thickness and left ventricular mass. Stroke. 2009;40:1515–8.
Markus MR, Stritzke J, Lieb W, Mayer B, Luchner A, Döring A, et al. Implications of persistent prehypertension for ageing related changes in left ventricular geometry and function: the MONICA/KORA Augsburg study. J Hypertens. 2008;26:2040–9.
Yeter E, Akçay M, Keleş T, Durmaz T, Bayram NA, Ozdemir L, et al. The association of diastolic dysfunction and circadian variation of blood pressure in prehypertension. J Am Soc Echocardiogr. 2009;22:726–31.
Jang SY, Kim S, Lee CK, Cho EJ, Cho SJ, Lee SC. Prehypertension and left ventricular diastolic dysfunction in middle-aged Koreans. Korean Circ J. 2016;46:536–41.
Ladeiras-Lopes R, Fontes-Carvalho R, Vilela EM, Bettencourt P, Leite-Moreira A, Azevedo A. Diastolic function is impaired in patients with prehypertension: data from the EPI Porto Study. Rev Esp Cardiol (Engl Ed). 2018;71:926–34.
Celik T, Yuksel UC, Fici F, Celik M, Yaman H, Kilic S, et al. Vascular inflammation, and aortic stiffness relate to early left ventricular diastolic dysfunction in prehypertension. Blood Press. 2013;22:94–100.
Hsia J, Margolis KL, Eaton CB, Wenger NK, Allison M, Wu L, et al. Prehypertension and cardiovascular disease risk in the Women’s Health Initiative. Circulation. 2007;115:855–60.
Mainous AG 3rd, Everett CJ, Liszka H, King DE, Egan BM. Prehypertension and mortality in a nationally representative cohort. Am J Cardiol. 2004;94:1496–500.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–245.
Ninomiya T, Kubo M, Doi Y, Yonemoto K, Tanizaki Y, Tsuruya K, et al. Prehypertension increases the risk for renal arteriosclerosis in autopsies: the Hisayama study. J Am Soc Nephrol. 2007;18:2135–42.
Acknowledgements
All authors have equal contribution to this paper.
Funding
Sephar III survey and this study were realized with financial support from the Romanian Society of Hypertension.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pop, C., Fronea, O.F.G., Pop, L. et al. High-normal blood pressure and related cardiovascular risk factors prevalence in the Romanian adult population: insights from the SEPHAR III study. J Hum Hypertens 35, 884–895 (2021). https://doi.org/10.1038/s41371-020-00417-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-020-00417-z
- Springer Nature Limited