Abstract
Background
Exposure to food additives is widespread but up-to-date and accurate intake estimates are rarely available. The safety of the food additive aspartame is the subject of recent controversy and intake estimates for this nonnutritive sweetener are typically derived from surrogates such as diet soda consumption.
Objective
We describe an approach for developing nationally representative dietary exposure estimates for food additives that combines intake from dietary recalls and grocery purchasing information.
Methods
We combined NielsenIQ Homescan Consumer Panel purchasing data with the USDA Global Branded Food Products database and the National Health and Nutrition Examination Survey to estimate aspartame intake and prevalence of consumption for the US population. We examined points of departure for aspartame from CompTox Chemicals Dashboard to provide context for exposures and potential effects.
Results
Mean, 90th percentile, and 95th percentile aspartame intake estimates are below the acceptable daily intake (50 mg/kg/day) and are lower than estimates from previous decades. Groups with the highest aspartame intakes are non-Hispanic whites, 60- to 69-year-olds, and individuals on diabetic diets. Aspartame exposure is highly prevalent (62.6%) in the US including sensitive populations such as pregnant women and children.
Impact statement
Exposure to the widely consumed food additive aspartame is not well characterized, and concerns about potential health effects remain despite assurances of safety when consumed under conditions of intended use. This work provides current intake estimates for the US population with important comparisons across demographic groups and individuals on special diets. The approach includes ingredient statement and grocery purchasing data to capture all aspartame-containing products, beyond diet soda, in intake estimates. This framework also has the potential for application to other food ingredients.
Similar content being viewed by others
Introduction
Aspartame is a nonnutritive artificial sweetener approved by the US Food and Drug Administration (FDA) as a direct food additive in 1981 [1]. It is made of two amino acids found in food, aspartic acid, and phenylalanine, but aspartame is not naturally occurring and must be synthesized. Aspartame is used as a tabletop sweetener and in a wide variety of food and beverages including soft drinks, fruit juice, oatmeal, candy, multivitamins, chewing gum, yogurt, and ice cream. Aspartame is listed as an ingredient on over 900 on-market dietary supplement labels and is approved for use in 19 different types of FDA-approved drug products such as chewable tablets, syrups, and lozenges [2, 3].
Since the submission of the first food additive petition by G.D. Searle and Co. (Searle) in 1973, the safety of aspartame has been challenged repeatedly. Initial concerns about the potential for intellectual disability and endocrine disruption in children from phenylalanine and aspartic acid metabolites and the induction of brain tumors were raised in an objection to FDA’s approval of the petition [1]. A Public Board of Inquiry was appointed to review key studies and issue a decision on whether aspartame should be allowed in the food supply. The Board found aspartame consumption would not increase the risk of intellectual disability or endocrine disruption but may be associated with brain tumors and should not be approved without additional testing. FDA disagreed with the Board and issued a final decision approving aspartame in 1981 [1].
On July 14th, 2023, the International Agency for Research on Cancer issued a conclusion classifying aspartame as “possibly carcinogenic to humans” (IARC classification Group 2B), based in part on a review of more recently published studies [4]. After the release of IARC’s conclusion, the FDA issued a statement maintaining aspartame’s safety as a food additive [5]. As of the date of publication, IARC Monograph 134 containing the full aspartame assessment has not yet been released.
Acceptable daily intakes (ADIs) for aspartame have been set at 50 milligrams per kilogram bodyweight per day (mg/kg/day) by the FDA and 40 mg/kg/day by the Joint FAO/WHO Expert Committee on Food Additives (JECFA). An ADI is the maximum amount of a chemical that can be consumed daily for a lifetime without appreciable health risks. FDA first issued a regulation on aspartame use after approval of the first food additive petition in 1974 and derived an ADI of 20 mg/kg/day based on a no observed effect level (NOEL, critical effect not specified) of 2 g/kg/day and a safety factor of 100, but stayed the regulation after objections to the reliability of the toxicity studies were raised [6]. Later in 1983, after Searle submitted another petition to amend the aspartame regulation for use in carbonated beverages, the FDA established an ADI of 50 mg/kg/day [7, 8]. Traditional risk assessment methods for ADI determination were not used in this instance, i.e., selection of critical toxicity studies and effects and application of safety factors. The FDA assessment detailing the decision to increase the ADI is not publicly available. A detailed evaluation of all unpublished and published studies of aspartame intake, exposures to its metabolites phenylalanine, aspartic acid, and methanol, degradation products, β-aspartame and 5-benzyl-3,6-dioxo-2-piperazine acetic acid, and effects including reproductive and developmental toxicity, neurotoxicity, genotoxicity, cancer, and other endpoints is available elsewhere [9].
Before approving food additives, FDA calculates an Estimated Daily Intake (EDI) and compares it to the ADI. If the EDI falls below or is equal to the ADI, the food additive can be approved. The FDA projected a consumption level of 8.3 mg/kg/day if aspartame is substituted for all sucrose in the average diet of 60 kg man, and 25 mg/kg/day if all carbohydrates were replaced with aspartame [1]. Dietary surveys from Market Research Corporation of America (MRCA) for 12,000 individuals were also used to estimate potential consumption through all products in which Searle expected to market aspartame, including carbonated soda. The FDA estimated intake for all ages at the 99th percentile to be 34 mg/kg/day using the MRCA survey [1]. These projected EDIs were purposefully exaggerated for safety evaluation. After FDA approved aspartame, several studies estimated consumption in the US population (Table 1). All intake estimates fell below the ADI. Studies on aspartame intake in international populations prior to 2007 have been summarized elsewhere [10, 11]. Dietary intakes for Irish adults and pre-school children were estimated to be below the ADI using nationally representative surveys from 2011 [12, 13]. Exposure to aspartame and other non-nutritive sweeteners was also estimated for the Portuguese general population using national dietary surveys from 2015–2016 and was found to be below the ADI [14].
Methods for estimating aspartame consumption in the US have up to this point not included retail purchasing data. Strategies for estimating population-level intake of nonnutritive sweeteners have relied on government or industry sponsored dietary surveys alone or combined with sweetener concentrations [10, 15, 16]. The third National Health and Nutrition Examination Survey (NHANES III, 1988–1994) included aspartame as a variable in the total nutrient file based on concentrations in the University of Minnesota Nutrition Coordinating Center (NCC) nutrient database [17]. The University of Minnesota NCC nutrient database has been continuously updated and is available for licensing [18]. Text in NHANES food code descriptions or other dietary surveys can be searched using terms such as “diet” or “low sugar” to identify foods containing at least one nonnutritive sweetener, or to target product groups such as beverages [10, 19,20,21]. Amounts consumed of selected food codes can then be multiplied by a predetermined aspartame concentration to estimate intake over all foods reported in the dietary recall. These methods are generally worst-case scenarios designed for safety assessment and compare consumption estimates with the ADI using the highest reported use levels. No biomarker of exposure is available for aspartame.
While schemas for integrating nutrition and consumption datasets have been proposed previously and for different purposes [22,23,24], we found no published studies with food additive exposure estimates using merged purchasing, ingredient, and dietary recall datasets. Here, we present a method for combining aspartame concentrations from published literature, NielsenIQ grocery purchasing data, product ingredient statements from the USDA Global Branded Food Products database, and 24-hour dietary recalls from the 2017–2020 National Health and Nutrition Examination Survey (NHANES). This method applies lower aspartame use levels when other sweeteners are present in ingredient lists for more realistic intake estimates, and enables a better understanding of exposure prevalence which is underestimated when exposure assessment is focused on a narrow spectrum of products such as diet soda. Updated intake estimates are presented for aspartame in a nationally representative, non-institutionalized sample of the US population.
Methods
Data Sources
National Health and Nutrition Examination Survey
The National Health and Nutrition Examination Survey (NHANES) is a national health and nutrition survey of the civilian, non-institutionalized US population conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention [25]. NHANES uses a complex multistage cluster sampling design to collect a nationally representative sample of about 5,000 people in 15 different locations per year and includes an in-person interview and an examination at mobile examination centers. The most recent pre-pandemic cycle from 2017–2020 was selected for up-to-date consumption estimates. This cycle combined one full 2-year cycle from 2017–2018 with an incomplete pre-pandemic sample. NHANES operations were suspended in March 2020 due to the COVID-19 pandemic and as a result the nationally-representative sample could not be collected. This sample was combined with the previous cycle to make a nationally representative sample from 2017–2020. The dietary interview component of NHANES, also called “What We Eat in America”, included two 24-hour dietary recalls. The first recall is collected in-person in mobile examination centers along with other laboratory and examination data, and the second 24-hour recall is collected by phone three to ten days later. Reported foods are assigned a USDA food code from the Food and Nutrient Database for Dietary Studies (FNDDS) which contains descriptions, nutrient data, and standard recipes. The FNDDS is updated every two years to reflect changes in dietary patterns and the food supply.
NielsenIQ Homescan Consumer Panel
The NielsenIQ Homescan Consumer Panel dataset (NielsenIQ Homescan) is a longitudinal survey of 40,000 to 60,000 US households. Panelists use Universal Product Code (UPC) scanners or a mobile app to report all food and non-food purchases from any US retail outlet. Demographic information is provided for each household and sampling weights are assigned for nationally representative purchasing estimates. Datasets from 2017 through March 2020 were combined aligning with NHANES pre-pandemic cycles also used in this analysis.
USDA Global Branded Food Products Database
The U.S. Department of Agriculture (USDA) Global Branded Food Products Database is a public-private partnership that provides UPCs, ingredients, nutrients, and other brand information for over 400,000 food products from US, Canada, and New Zealand markets [26]. The database became publicly available online in 2019 and is updated monthly. The October 2022 file release was used in this analysis.
Approach for combining NielsenIQ Homescan, Branded Foods, and NHANES datasets
Approach for assigning food codes and linking datasets
Food and beverage products in US markets with aspartame in the ingredient statement were identified in the BFD. Products containing other non-nutritive sweeteners including sucralose, acesulfame potassium, saccharin, stevia, neotame, cyclamate, monk fruit, thaumatin, luo han guo, sugar alcohols (e.g., xylitol), and caloric sweeteners were also identified to enable categorization and mapping of products to NHANES. All products were categorized as either containing (0) or not containing [1] each of the sweeteners.
To merge NHANES dietary recalls with the products in the BFD (Fig. 1), aspartame-containing (n = 4071) products were assigned a USDA food code. Food code names, additional descriptions, and if applicable, calorie content (kcal/100 g) [27] were used alongside brand, sweetener categories, and ingredients to assign food codes. Next, for each product UPC/food code combination, all similar items fitting the food code profile were assigned the same food code. For instance, all nonfat fruit yogurts were assigned the food code for “yogurt, nonfat milk, fruit” regardless of aspartame content.
Products in the USDA Global Branded Food Products database (BFD) were tagged to indicate the presence of aspartame in ingredient lists. USDA food codes were assigned to products in the BFD to enable linkage to the NHANES dietary recall. The BFD was then merged with the NielsenIQ Homescan dataset by UPC. The market share of products by USDA food code and aspartame category was estimated. Market share estimates were derived from BFD UPC matches only, and with additional imputed matches. Market share percentages of aspartame-containing products were transferred to NHANES dietary data by merging USDA food codes. The reported amount consumed of the food code was then multiplied by an aspartame concentration for the food type and the percent market share.
USDA food codes were transferred to the NielsenIQ Homescan dataset by merging UPCs with the BFD (Fig. 1). UPC standards (synonymous with Global Trade Item Number (GTIN)) include UPC-A, UPC-E, EAN-8, EAN-13, GTIN-14 and can be converted to a single standard for merging purposes. UPCs in the BFD were in several different formats and were cleaned and standardized to match the UPC standard used in the NielsenIQ Homescan dataset. Following analysis of purchasing data and calculation of market share, the BFD and NielsenIQ Homescan combined dataset was merged with NHANES dietary recall by food code.
Missing data approach and market share estimation
Since the BFD is not a complete database of all UPCs for retail items in the US, approximately 52% of UPCs (or 42% of the share of sales by price) in relevant food and beverage categories in the NielsenIQ dataset were not matched to BFD UPCs and did not have ingredient information. We used an imputation approach to address the unmatched UPCs with no ingredient information based on the percent of aspartame-containing products within a category and random assignment of unmatched UPCs to present or absent categories. In NielsenIQ Homescan datasets, we first created subcategories using grocery category (e.g., low calorie carbonated beverages), and existing brand categories combined with brand information derived from UPCs for private label products since the brand names were masked. Some brands make many different types of foods and beverages, necessitating subdivision by grocery category. If all UPCs matched within a food type and brand category contained aspartame, we assumed the rest of the UPCs in the brand category also contained aspartame. For categories containing a variety of products with aspartame or sweetened with other sweeteners, we assigned a probability equal to the percentage of matched products containing aspartame. For example, if 30% of UPCs in a subcategory of low-calorie fruit drinks were unmatched and missing ingredient data, and 25% of the matched products in the subcategory contained aspartame, a probability of 0.25 was assigned to each of the unmatched UPCs with missing ingredient information. Unmatched UPCs with missing ingredient information were then randomly allocated to the group of products containing aspartame using the assigned probability.
Food codes were also missing when UPCs were unmatched. To account for as much purchased product as possible we assigned food codes to unmatched UPCs. We assigned the most frequent food code in the product category to the rest of the unmatched UPCs. For example, if the majority of matched UPCs in a product category were assigned the food code for diet root beer, then all remaining unmatched UPCs were assigned as diet root beer.
We generated market share percentages for each NHANES food code using NielsenIQ Homescan survey weights. The percentage represents the market share of sales by price or volume purchased in the US from 2017 to 2020 of a food or beverage (e.g., fruit flavored soft drink) that contained aspartame. “Market share” is used as a broad term to refer in general to the share of sales. For consistency, share of sales by price is reported in the main text since this metric covers all of the purchased product and is not dependent on the unit of sale. Share by volume was calculated to compare coverage of beverages in the BFD and performance of the imputation method to published per capita purchasing estimates. Product units were converted to ounces and powdered drinks were converted to the diluted amount where applicable. Some products such as chewing gum were sold in “count” units and were not converted to ounces as long as all units were the same for the food code. In some instances, we could not standardize all units, most frequently being “count” when all other units were “ounces”, and the inconsistent units were dropped. In most cases, the share by price for the odd unit was less than 5% (n = 73). In n = 6 cases, the share by price was between 5 and 20%. Even when the share by price for an odd unit was relatively high, share by volume for the aspartame-containing products was similar across units. For instance, light ice cream sold in “count” units captured ~10% of the market share, however, the volume share for aspartame-containing ice cream was 0.04% in products sold by count compared to 0.05% in product sold in ounces. Although volume percentages may not represent all product sold, particularly for non-beverage items, results for volume are available in the Supplemental Information for informational purposes.
Food codes for sushi, breaded shrimp, and borscht were dropped from the analysis because the recipes can vary widely depending on the food source, the purchasing data only represent grocery purchases, and the market share percentages were likely artificially high due to the small number of competing products available in grocery stores. Generally, pickled ginger was the ingredient in sushi that contained aspartame and would therefore not make up a significant portion of the dish. The borscht was a pre-made grocery item with a very low market share compared to other borscht without aspartame as an ingredient, as was a frozen coconut breaded shrimp product.
Aspartame use levels
Aspartame concentrations were selected from published literature, ingredient statements, and patents [10, 21, 28,29,30,31]. In some instances (e.g., frozen strawberries) aspartame concentrations were calculated based on a sucrose to aspartame conversion factor. Aspartame is about 200 times sweeter than sugar [5] and concentrations for products with nonnutritive sweeteners added to replace sugar can be calculated based on added sugars listed in the nutrition fact panel for similar full-calorie products. Since many products contain combinations of sweeteners and not exclusively aspartame, we used low-end and high-end concentrations to account for sweetener blends. One common blend is a mixture of aspartame and acesulfame potassium in a 70:30 proportion [32]. Use levels for aspartame used in this analysis are listed in Table 2. When aspartame was the sole sweetener in the ingredient list, we applied the highest use level. The low-end use level was applied to products listing aspartame and other sweeteners. Concentrations used in this analysis are similar to values collected from Ireland’s National Chemical Food Sampling Program, a study monitoring artificial sweeteners in target retail items from 2008 to 2011 on the Irish market [12]. Beverages contained aspartame at levels ranging from 0.0075% to 0.061% (n = 167), dairy products from 0.004–0.035% (n = 24), edible ices from 0.005–0.013% (n = 4), jams from 0.004–0.029% (n = 12), and sauces from 0.008–0.047% (n = 42) [12]. Likewise, a sample of 68 of the most consumed soft drinks on Portuguese retail markets in 2019 were analyzed for sweetener content and found to contain between 0.002% and 0.023% aspartame [33]. Although these use levels are from international products, we assume sweet taste preferences and product formulations would not differ substantially from US markets. Use levels are also similar to those reported by industry and collected by EFSA for an exposure assessment to aspartame-containing products on European markets [9].
Consumption estimation in NHANES
We identified food codes in NHANES dietary recall food descriptions that may contain a nonnutritive sweetener using search terms including aspartame, sugar-free, artificial sweetener, no calorie sweetener, low calorie sweetener, sugarless, diet, low sugar, no sugar added, no added sugar, and dietetic. We also identified food code ingredients that may contain nonnutritive sweeteners in FNDDS by tagging food descriptions and ingredients using the search terms. Tagged foods and ingredients were reviewed for inclusion against the list of food codes generated from the BFD-NielsenIQ Homescan dataset. Those that were unlikely to contain aspartame were excluded, for example, “diet” frozen meals.
In a few instances, USDA food codes included aspartame in the associated description file, but the food code was not matched with any products in the BFD (e.g., “soft drink, chocolate flavored, diet”). When this was the case, the food code was added (n = 4) and the share and volume percentage were assigned 1 (all products contain aspartame as an ingredient). When a food code had a recipe in FNDDS with an aspartame-containing ingredient, for example, diet cola in a rum and coke, we extracted the percentage of the relevant ingredient. For items such as oatmeal and hot cocoa, NHANES lists multiple food codes by the type of liquid added to reconstitute or cook the food, for instance, water or lowfat milk. We duplicated purchasing percentages for each of the alternate food codes. The final number of food codes with market share percentages was n = 138.
The percent market share for a food code was multiplied by the use rate specific to the product. For each individual (n = 10,830), an intake amount was calculated for both days of dietary recall in NHANES by summing over the aspartame content of all foods reported in the dietary interview using the following equation:
Aspartame intakes (mg/kg/day) were averaged over the first and second 24-hour recalls. Some bodyweight measurements were missing making the final sample size n = 10,723. Reported estimates are for “eaters-only”, meaning the survey respondent reported eating a food containing aspartame at least once over the two days of recall. Participants with only one day of recall (did not participate in the follow up phone interview) are excluded. Averaging over two days of 24-hour recalls is an approximation of the usual intake, a measure of long-term exposure to dietary elements consumed on a regular basis. Results estimated using only the first 24-hour recall [16] represent intake on any “given day” in the US population, but is not a measure of habitual exposure. More complex methods [34] have been developed for estimating usual intake but we use the averaging method, also called the within person mean, to be consistent with methods used in previous exposure studies [10, 15].
Robustness checks
Since exposure estimates may be sensitive to the imputation approach for missing ingredient information, we calculated market share percentages with additional methods. Briefly, we randomly allocated unmatched UPCs using an approach with lower probabilities, and created more specific grocery subcategories based on further parsing of UPCs to decrease the number of assigned USDA food codes. We also set all aspartame use levels for all product categories to either the upper or lower end of the ranges presented in Table 2, and estimated intake for the first 24-hour recall and the second 24-hour recall separately. A more detailed description of the methods and results of the robustness checks can be found in the Supplemental Information.
Points of departure
In order to examine aspartame exposures in the context of existing toxicological information, we compared exposure estimates to the current ADI and other potential points of departure (PODs) using aspartame PODs extracted from the US Environmental Protection Agency CompTox Chemicals Dashboard [35, 36]. PODs from reproductive, neurotoxicity, developmental, “special toxicology”, chronic, subchronic, repeat dose, and clinical studies with administration via the oral route were selected for analysis. We reclassified the three “special toxicology” study types under existing categories (e.g., neurotoxicity, developmental, chronic) based on review of the corresponding publications. We used study type and year to characterize PODs and provide context for potential effects compared to aspartame exposures.
Statistical analyses
Analyses were performed using Stata v17.0 [37]. Survey weights were applied in all NielsenIQ Homescan purchasing and NHANES dietary analyses to provide nationally representative consumption estimates. Mean, 90th percentile, and 95th percentile intakes were calculated for pregnant women, women of reproductive age (15–49 years), and by sex, race, age, income level, and for those reporting special diets (weight loss, low sugar, or diabetic diet). The arithmetic mean is reported to be consistent with previous exposure studies, however, the geometric mean or median would better characterize the central tendency of the data because the distribution of aspartame intake is right skewed. These summary statistics were also calculated and can be found in the Supplemental Information. Income categories were defined as low if the poverty income ratio (PIR) was less than or equal to 130%, middle if the PIR was 130 to 350%, and high if the PIR was above 350% [38]. Age is categorized as 6 months to 5 years, 6–11, 12–19, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and over 80 years old. Since the American Academy of Pediatrics and the Dietary Guidelines for Americans recommend exclusively feeding infants breast milk or formula for the first six months of life, infants under six months were excluded from the analysis [39, 40]. Prevalence of aspartame exposure (% eaters) was calculated for each demographic subgroup.
The influence of demographic variables on aspartame consumption was examined with multiple linear regression analyses using natural log-transformed intakes from the whole population (eaters and non-eaters). Age and PIR were assessed as continuous variables. Age, sex, race, and PIR were each modeled as independent variables adjusting for the others as covariates. Each race (Mexican American, Hispanic/Other, non-Hispanic Whites, non-Hispanic Black, non-Hispanic Asian, Other) was modeled as a binary variable in separate regressions, adjusting for age, sex, and PIR. Regression models for special diets were adjusted for age, sex, race, and PIR. Associations or differences in mean intakes were considered statistically significant at the p < 0.05 level. As a sensitivity analysis, regressions were repeated excluding intakes above the 99th percentile.
Results
Estimates of aspartame intake
Approximately 95.8% of aspartame intake in the US came from beverages (Table 3). Soft drinks comprised 77.5% and other beverages including tea, energy drinks, hot chocolate, fruit juice drinks, carbonated water, nutritional shakes, coffee, chocolate milk, and horchata beverages made up 18.3% of the beverage category. Sweetener packets contributed 2.8% of aspartame consumption and all other products including candy, chewing gum, mints, frozen fruit, ice cream, yogurt, gelatin and pudding desserts, jams and syrups, cakes and cookies, and vegetable chips and other savory items contributed 1.4% of aspartame intake.
Mean, 90th percentile, and 95th percentile aspartame intakes for the NHANES survey years 2017–2020 presented by sex, age categories, race, income level, for pregnant women, and those on special diets are presented in Table 4. Estimates are for eaters-only, meaning survey participants that reported consuming a product containing aspartame on either of the two days of 24-hour recalls. Eaters-only population sizes and the percent eaters are reported for data derived directly from the BFD and for the imputed sales volumes. Highest intakes among consumers of aspartame are in the 60- to 69-years-old age group, non-Hispanic Whites, individuals on diabetic diets, and at higher income levels. Older individuals are more likely to consume aspartame with an increase in 10 years of age being associated with a 22.9% (β: 0.023, p < 0.001) increase in aspartame intake. Differences in aspartame intake by PIR were not significant across the whole population (both eaters-only and non-eaters) (β: 0.068, p = 0.14). Adjusted aspartame intakes were 91.6% higher in non-Hispanic Whites (β: 0.65, p = 0.00), and 74.8% lower in non-Hispanic Asian (β: −1.38, p = 0.001) and 50.3% lower in non-Hispanic Black (β: −0.70, p < 0.001) populations compared to other racial groups. Aspartame intake is slightly higher in females than males though the difference is not statistically significant (β: 0.056, p = 0.74). There were no significant differences in intake comparing pregnant women to all reproductive age women, nor was there a difference between intake in reproductive age women and the rest of the population. Finally, individuals reporting adherence to weight loss (β: 0.54, p = 0.042) and diabetic diets (β: 1.95, p = 0.005) had significantly higher intakes (corresponding to 71.6% and 603% increases, respectively) than the rest of the population whereas individuals on sugar free/low sugar diets (β: 1.04, p = 0.46) did not consume substantially higher amounts of aspartame than those not on a low sugar diet. Excluding intake values above the 99th percentile did not substantively change results. Regression analyses utilized the imputed dataset and did not differ in a meaningful way from regression coefficients or p-values estimated with the BFD values.
Aspartame consumption was most common among individuals 30- to 39-years old (69.5%), Mexican Americans (66.3%), people adhering to a special diabetic diet (71.7%), and at the middle income levels (64.2%). Trends are consistent across the data generated from the BFD and imputed values. Imputed intake values are slightly lower than those computed only from the BFD while prevalences are slightly higher. The higher prevalence from the imputed matches is due to the capture of UPCs with some market share in the same food code category as BFD UPCs that had zero sales for the time period. Individuals who reported eating the food would therefore move into the eaters-only category under the imputed method. All values are below established ADIs (whole population mean: 0.40 mg/kg/day, 95th percentile: 2.2 mg/kg/day) and are lower than all previous estimates (Table 1). However, compared to previous estimates, it appears aspartame exposure prevalence is higher, with pregnant women reporting consumption in proportions (61.6%) similar to the rest of the population (62.6%) albeit in smaller quantities (mean: 0.25 mg/kg/day, 95th percentile: 1.01 mg/kg/day).
Market coverage of the BFD and imputed UPC matches
We compared volume per capita per day purchasing estimates for aspartame-containing beverages using UPCs matched solely from the BFD, for the BFD plus imputed matches, and for a previously published study examining purchases of products with nonnutritive sweeteners. We focused on evaluating the coverage of the BFD and imputed matches only for beverages since the majority of aspartame intake comes from beverages. The comparison serves as an indicator of the performance of the imputation method and for the coverage of aspartame containing UPCs provided by the BFD compared to more complete commercial nutrition databases. Dunford et al. (2020) combined NielsenIQ Homescan data for the year 2018 with commercial nutrition databases such as Gladson, Label Insight, and Mintel, which are assumed to provide complete nutrition and ingredient information for all UPCs in the Homescan dataset [41]. We use the Dunford et al. (2020) volume per capita purchasing estimate for beverages containing aspartame as a benchmark for market coverage of the BFD and our imputation approach, assuming the estimate includes all or the majority of aspartame containing beverages on the market in 2018. We found the BFD to provide ingredient information for approximately 70% of volume purchased, or 55.3 ml per capita per day in 2018 for beverages containing aspartame, based on an estimated 78.8 ml per capita per day reported by Dunford et al. (2020). With additional volume from UPCs captured using the imputation method, market coverage increased to 98% (77.2 ml per capita per day). A similar amount of market coverage is likely provided by the BFD and the imputation method for all years included in this analysis since the overall share of sales by price for beverages (2017–2020) with matched UPCs from the BFD was 65%, and increased to 94% with the addition of the imputed product. The BFD was reported to cover approximately 85% of the total sales volume of food and beverages in NielsenIQ retail sales databases as of 2022 (NielsenIQ dataset is not specified) [42].
Points of departure
The PODs available for aspartame in CompTox Chemicals Dashboard lacked entries for critical effects, did not include studies conducted after 2006, and the information presented reflects FDA grading criteria for toxicity studies which measures adherence to the Toxicological Principles for the Safety Assessment of Food Ingredients (Redbook) standards. The source database COSMOS, which further sources data from the FDA Center for Food Science and Nutrition Priority-based Assessment of Food Additive database, supplied additional information but was generally not specific enough to further categorize PODs based on critical effects. For instance, for three entries labeled “special toxicology study”, a reference to the original published paper was listed [43,44,45] and further details on effects were provided for only one of the study entries [43]. The majority of PODs (n = 54 out of 72) were from studies submitted directly to FDA as part of the food additive petition process and are not publicly available; n = 50 of those PODs are from studies conducted before 1978. References to published studies were listed for 18 PODs. All available PODs for aspartame in CompTox are from studies conducted through 2006. The latest study in the database is a carcinogenicity study from Soffritti et al. (2006) [46], which was given a “C” grade meaning it does not meet FDA’s core standards for oral toxicology studies [47]. The PODs recorded for Soffritti et al. (2006) were 2500 mg/kg/day (highest no effect level) and 5000 mg/kg/day (lowest no effect level) with decreased feed consumption and bodyweight noted as effects. In contrast, Soffriti et al (2006) reports carcinogenic effects at 20 mg/kg/day [46].
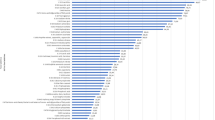
Since information on effects associated with the PODs was limited, Fig. 2 shows only PODs from study types labeled “neurotoxicity”, “neurotoxicity subchronic”, “neurotoxicity chronic”, “reproduction”, “developmental”, and “reproduction developmental” conducted in animal models (rat (n = 12), rabbit (n = 12), mouse (n = 4), monkey (n = 1), guinea pig (n = 1)). These studies are coded by year, either before the FDA increased the ADI from 20 to 50 mg/kg/day in 1983 or after 1983. Of note are three neurotoxicity studies conducted after 1983 with PODs at 13 (NOEL) [48], 71 (lowest observed effect level (LOEL)) [43], 133 (LOEL) [48], and 500 (NOEL) mg/kg/day [49]. More detail is provided on these studies in the discussion.
PODs are no observed effect levels (NOELs) or lowest observed effect levels (LOELs) from animal studies. Reference lines mark the mean, 95th percentile (p95), and the acceptable daily intake (FDA ADI). PODs are coded to highlight whether the study was conducted before or after 1983, the year the FDA increased the ADI from 20 to 50 mg/kg/day.
Discussion
We describe an approach for integrating nationally representative retail purchasing and consumption datasets with a publicly available ingredient database and concentrations of the artificial sweetener aspartame. To our knowledge, this is the first attempt to use these data sources together to estimate exposure to a food additive.
Consumption of aspartame is widespread with almost two-thirds of the of the US population reporting intake of a product containing aspartame between 2017–2020. Research on aspartame and other nonnutritive sweeteners (NNS) indicates this high prevalence follows a trend of increasing use over time. A 1988 consumption analysis using the USDA Continuing Survey of Food Intakes by Individuals, a nutrition survey that preceded NHANES, reported 25% of surveyed women aged 19 to 50 years old consumed aspartame (Table 1). Trend analyses of NNS consumption in the US from 1999 through 2012 found drastic increases in the percentage of adults and children consuming a food, beverage, or packets with NNS. Among children, the prevalence of NNS consumption increased from 6.1% in NHANES cycle 1999–2000 to 25.1% in cycles 2009–2012, and in adults from 18.7% to 41.4% during the same time period [20, 50]. Respondents from NHANES cycles 2009–2012 who were female, obese, non-Hispanic white, or those with higher socioeconomic status were more likely to report consumption of NNS [20]. Our prevalence estimate of 62.6% for the entire population follows this trend of increasing use over time although there were no major differences between adults and children or across demographic subgroups except in six months to five year olds (50.5%) and in non-Hispanic Asians (47.0%).
The higher prevalence for aspartame exposure is attributed to inclusion of products that are not labeled with terms such as “diet” or “low sugar.” Previous analyses likely underestimated the prevalence of nonnutritive sweetener consumption by selecting foods and beverages from NHANES food codes and FNDDS descriptions with specific adjectives [10, 20]. This analysis used the same method to ensure all food codes with descriptions indicating presence of aspartame were included, but also added food codes that did not contain these terms based on ingredient statements for purchased products. Soft drink manufacturers have been rebranding beverages as “zero sugar” instead of “diet” due to shifts in perceptions of dieting [51], and companies may add NNS to “reduced sugar” foods and beverages to maintain palatability. This may lead to unintentional purchase and consumption of products with NNS that are perceived as healthier choices due to labeling claims [52].
Aspartame consumption estimates in this study are lower than intakes reported previously. As compared to earlier estimates of aspartame exposure (e.g., mean: 4.9 mg/kg/day, 95th percentile: 13.3 mg/kg/day [10], Table 1), our lower intakes (mean: 0.4 mg/kg/day, 95th percentile: 2.2 mg/kg/day) may be explained by differences in estimation methods or the influence of ingredient trends and changing health perceptions related to NNS. For instance, the major yogurt manufacturer Yoplait replaced aspartame with sucralose in their reduced calorie yogurts in 2014 due to declining sales and customer concerns about aspartame [53]. Examining food code descriptions in NHANES and assigning aspartame concentration by food type is a method used to estimate intake and was replicated here to ensure consistency with previous exposure studies. However, adding an additional market-based exposure factor based on percentage of sales for products with aspartame will result in lower intakes unless all products in a category contain aspartame as the sole sweetener. Only 13.8% of product with aspartame in BFD ingredient lists had aspartame as the sole sweetener, and nine out of 138 USDA food codes had a market share percentage of 100% (e.g., all sweetener packets sold contained aspartame). The Supplemental Information contains a full list of food codes, volume and price share and associated aspartame concentrations used in this analysis.
Exposures to aspartame estimated in this analysis (mean: 0.4 mg/kg/day, 95th percentile: 2.2 mg/kg/day) were well below the ADI of 50 mg/kg/day established by the FDA. Maximum doses were approximately half of the ADI (23.4 mg/kg/day). We reviewed existing PODs available in CompTox and identified three studies with four PODs ranging from 13 to 500 mg/kg/day that all indicate aspartame exposure may result in reductions of important neurotransmitters (Fig. 2) [43, 48, 49]. The first, which was not included in an evidence review that determined aspartame is safe [10], is a rodent study on the effects of chronic ingestion of aspartame. After rats were fed ad libitum a solution containing 0.1% aspartame (equivalent to use levels in chewing gum or syrup; POD recorded by FDA as 71 mg/kg/day) over a 14-week period beginning at weaning, neuropeptide Y levels decreased by 23% at the site of its synthesis in the arcuate nucleus of the hypothalamus [43]. Neuropeptide Y is a signaling molecule linked to physiological functions including appetite control, anxiety, and a variety of important metabolic pathways in the body [54]. The European Food Safety Authority (EFSA) reviewed Beck et al. (2002) in their 2013 re-evaluation of aspartame as a food additive, agreeing with the author’s conclusion that more investigation is warranted [9]. Also not included in evidence reviews or the EFSA re-evaluation are results from Sharma and Coulombe (1987), which found reduced levels of serotonin and its metabolite, 5-hydroxyindoleacetic acid, in the hypothalamus of mice after administration of 130 mg/kg/day aspartame for 30 days [48]. The third study demonstrated that prenatal exposure to aspartame decreased levels of excitatory neurotransmitters, glutamic and aspartic acid, in the brains of rats [49]. The changes were reversible when exposure ceased for three weeks. A POD of 500 mg/kg/day was recorded as a NOEL is CompTox likely because the main effect under investigation was changes in the kinetics of glutamatergic receptor binding [49]; the study was initiated because of previous findings on reductions of glutamic and aspartic acid in rat brains [55], which were duplicated in this follow-up study [49]. We acknowledge the CompTox POD database for aspartame is not complete and represents a wide range of studies of varying quality conducted over four decades and that regulatory agencies have drawn conclusions on the safety of aspartame based on extensive review of select toxicity studies. Nonetheless, consumption of aspartame by pregnant women and effects on the developing fetus remains an important understudied population and at least one epidemiologic study has suggested an association between maternal consumption of diet soda and poorer performance on cognitive functioning tests in offspring [56].
The FDA, EFSA, Health Canada, and industry associations maintain the safety of aspartame when used under approved conditions and when consumed in amounts within the ADI. The ADI is not applicable to individuals with the genetic disease phenylketonuria (PKU) [9], and products with aspartame are require to bear a warning, “Phenylketonutrics: contains phenylalanine.” PKU causes phenylalanine to build up in the body, and aspartame consumption is a concern because it metabolizes to phenylalanine, aspartic acid, and methanol. Increased levels of phenylalanine can cause brain damage and intellectual disabilities in individuals with PKU, infants born to mothers with PKU, and individuals with advanced liver disease. Regulatory bodies have acknowledged the possibility of developmental toxicity in animals due to phenylalanine, but concluded that at exposure levels below the ADI, plasma phenylalanine concentrations would not lead to adverse effects in normal fetuses [1, 9]. FDA set a plasma phenylalanine threshold level for pregnant women at 50 µmole/dL to protect the fetus from intellectual disabilities, and in clinical studies it took a dose of aspartame at approximately 200 mg/kg/day to create plasma phenylalanine levels of 50 µmole/dL [1]. Likewise, aspartic acid, alone or in combination with its conversion product glutamate (i.e., found in MSG and naturally in many foods), can also cause brain lesions and endocrine disorders at high doses. FDA and EFSA also found that at aspartame doses below the ADI, plasma levels of these amino acids stay within normal ranges are not a cause for concern [1, 9].
Epidemiologic studies have highlighted the potential for adverse effects from aspartame or diet soda consumption. IARC reviewed three studies with four prospective cohorts conducted in the US and EU all reporting positive associations between cancer and consumption of diet beverages but concluded the evidence supplied limited evidence for liver cancer and inadequate support for other cancers [57,58,59]. Another large cohort study conducted in France of over 100,000 adults found an association between exposure to aspartame in food and beverages and increased cancer risk [60]. Current summaries published in 2022–23 of the epidemiological, toxicological, and mechanistic evidence for aspartame consumption and cancer are available. The two reviews were funded by industry trade associations and both concluded aspartame is not carcinogenic [61, 62]. Although epidemiologic studies using diet soda consumption as a proxy for aspartame intake would include any blend of sweeteners present in the beverage, we found no studies that measured a mixture of aspartame and other sweeteners with the intention to compare independent effects to combined effects or to assess interactions among sweeteners.
Food chemicals with problematic hazard profiles are consumed daily in the US. Over 10,000 food additives are allowed in the food supply, and more than 300 chemicals with evidence of adverse effects on neurological development are permitted in food and beverages [63]. Like aspartame, safety assessments for these compounds are typically conducted in isolation, without consideration for concurrent exposures from multiple chemicals that may have cumulative effects on the same health outcome. Although aspartame doses required to increase plasma levels above the threshold for phenylalanine are beyond levels that would normally be consumed, considering the multitude of chemicals that may impact the same endpoint, it may be prudent to reduce the intake of aspartame if it can be avoided. Furthermore, toxicity studies suggest aspartame consumption may impact production of neurotransmitters, which may have consequences for fetal brain development and cognitive function [64]. The WHO recently issued guidelines on NNS with a recommendation to avoid the use of NNS for weight loss since undesirable consequences may outweigh any short-term benefits [65].
This study had several limitations. The FNDDS is updated for every 2-year NHANES cycle and USDA food codes will require mapping to the BFD on a regular basis to track trends in consumption. Manufacturers also frequently reformulate products based on trends and consumer preference. Repeated matching of the BFD to NHANES is also needed to update product ingredient lists. In some cases, beverages are cross-over products that could fit definitions for multiple food codes. For instance, a kombucha beverage with added vitamins and minerals may fit the energy drink or kombucha categories, or a beverage advertised as sparkling water may better fit the definition of a diet soda. Classification decisions for these types of products can be based strictly on predefined variables (e.g., calorie content, primary ingredient), however, NHANES dietary recalls are dependent on the memory of the respondent and how the respondent reports the product. So, a kombucha energy beverage could be classified as an energy drink based on the ingredient statement but may be recalled by the respondent as kombucha. NHANES dietary survey is administered using the USDA Automated Multiple Pass Method (AMPM) instrument where participants answer data retrieval questions designed to improve accuracy in recalling foods and beverages. The AMPM instrument was found in one study to accurately elicit 83% of foods truly eaten by participants [66]. Aspartame intake may therefore be underestimated due to recall errors or when products are mismatched to food codes relative to a respondent’s memory. This analysis did not account for aspartame consumed in dietary supplements and pharmaceuticals. Underestimation may also have occurred since the BFD does not contain ingredient information for all possible UPCs sold during 2017–2020. However, the method used to assign unmatched UPCs likely mitigated the effects of this source of error.
In summary, we found aspartame intake to be lower than estimates from past decades but the consumption of aspartame-containing foods and beverages to be more common in the population that previously estimated. We also highlight toxicity studies reporting changes in neurotransmitters levels following aspartame exposure, which may warrant further investigation. The methods presented here can be applied to monitor trends and consumption of other nonnutritive sweeteners and food additives.
Data availability
The NHANES dataset analyzed is publicly available and can be found at https://www.cdc.gov/nchs/nhanes/index.htm (accessed April 12, 2024). The USDA Global Branded Food Products Database dataset analyzed is publicly available and can be found at https://fdc.nal.usda.gov/ (accessed April 12, 2024). While NielsenIQ Datasets are not publicly available, the dataset generated using NielsenIQ Homescan datasets is available in the Supplemental Information.
References
U.S. Food and Drug Administration. Aspartame: Commissioner’s Final Decision. Fed Register. 1981;46:38283.
U.S. Food and Drug Administration. Inactive Ingredient Search for Approved Drug Products. 2023. Available from: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm?event=BasicSearch.page.
National Intitutes of Health, Office of Dietary Supplements,. Dietary Supplement Label Database (DSLD). 2023. Available from: https://dsld.od.nih.gov/search/aspartame/bWFya2V0X3N0YXR1cz1vbl9tYXJrZXQvZW50cnlfZGF0ZT0yMDExLDIwMjMvc29ydD1tYXRjaC9wYWdlX3NpemU9MjAv.
Riboli E, Beland FA, Lachenmeier DW, Marques MM, Phillips DH, Schernhammer E, et al. Carcinogenicity of aspartame, methyleugenol, and isoeugenol. Lancet Oncol. 2023;24:848–50.
U.S. Food and Drug Administration. Aspartame and Other Sweeteners in Food. 2023. Available from: https://www.fda.gov/food/food-additives-petitions/aspartame-and-other-sweeteners-food.
U.S. Food and Drug Administration. FDA establishes conditions for safe use of aspartame as a sweetener. Fed Register. 1974;39:27317.
U.S. Food and Drug Administration. Food additives permitted for direct addition to food for human consumption; Aspartame. Fed Register. 1983;48:31376.
U.S. Food and Drug Administration. Food additives permitted for direct addition to food for human consumption; Aspartame. Fed Register. 1984;49:6672.
European Food Safety Authority, Panel on Food Additives and Nutrient Sources Added to Food. Scientific opinion on the re-evaluation of aspartame (E 951) as a food additive. EFSA J. 2013;11:3496.
Magnuson BA, Burdock GA, Doull J, Kroes RM, Marsh GM, Pariza MW, et al. Aspartame: A safety evaluation based on current use levels, regulations, and toxicological and epidemiological studies. Crit Rev Toxicol. 2007;37:629–727.
Renwick AG. The intake of intense sweeteners–an update review. Food Addit Contam. 2006;23:327–38.
Martyn DM, Nugent AP, McNulty BA, O’Reilly E, Tlustos C, Walton J, et al. Dietary intake of four artificial sweeteners by Irish pre-school children. Food Addit Contam: Part A. 2016;33:592–602.
Buffini M, Goscinny S, Van Loco J, Nugent AP, Walton J, Flynn A, et al. Dietary intakes of six intense sweeteners by Irish adults. Food Addit Contam: Part A 2018;35:425–38.
Carvalho C, Correia D, Severo M, Magalhães V, Casal S, Ramos E, et al. Dietary exposure to artificial sweeteners and associated factors in the Portuguese population. Food Addit Contam: Part A. 2022;39:1206–21.
Kuk JL, Brown RE. Aspartame intake is associated with greater glucose intolerance in individuals with obesity. Appl Physiol, Nutr, Metab. 2016;41:795–8.
Heybach J, Smith J, editors. Intake of aspartame in 19–50 year old women from the USDA Continuing Survey of Food Intakes by Individuals (CSFII-85). Federation of American Societies for Experimental Biology; 1988: FASEB J.
Centers for Disease Control and Prevention, National Center for Health Statistics,. NHANES III Total Nutrient Intakes File Documentation, 1998 Series 11, No. 2A.
University of Minnesota Nutrition Coordinating Center. Database Licensing. 2023. Available from: http://www.ncc.umn.edu/food-and-nutrient-database/.
Butchko HH, Kotsonis FN. Acceptable daily intake vs actual intake: the aspartame example. J Am Coll Nutr. 1991;10:258–66.
Sylvetsky AC, Jin YC, Clark EJ, Welsh JA, Rother KI, Talegawkar SA. Consumption of low-calorie sweeteners among children and adults in the United States. J Acad Nutr Diet. 2017;117:441–8.
Tran NL, Barraj LM, Hearty AP, Jack MM. Tiered intake assessment for low- and no-calorie sweeteners in beverages. Food Addit Contam: Part A. 2021;38:208–22.
U.S. Department of Agriculture, Economic Research Service. Purchase to Plate Suite. 2023. Available from: https://www.ers.usda.gov/data-products/purchase-to-plate/.
Ng SW, Popkin BM. Monitoring foods and nutrients sold and consumed in the United States: dynamics and challenges. J Acad Nutr Diet. 2012;112:41–5.e4.
Carlson A, Lino M, Juan W, Marcoe K, Bente L, Hiza HAB, et al. Development of the CNPP Prices Database. US Department of Agriculture, Center for Nutrition Policy and Promotion. 2008. https://ageconsearch.umn.edu/record/45851.
Akinbami LJ, Chen TC, Davy O, Ogden CL, Fink S, Clark J, et al. National Health and Nutrition Examination Survey, 2017-March 2020 prepandemic file: sample design, estimation, and analytic guidelines. Vital Health Stat 1. 2022:1–36.
U.S. Department of Agriculture. FoodData Central: USDA Global Branded Food Products Database. 2023. Available from: https://fdc.nal.usda.gov/.
U.S. Department of Agriculture, Agricultural Research Service,. What We Eat in America Food Categories 2017 - March 2020 Prepandemic 2022. Available from: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/dmr-food-categories/.
Goff DH, Jordan WK. Aspartame and polydextrose in a calorie-reduced frozen dairy dessert. J Food Sci. 1984;49:306–7.
Steensen WL, Weaver RC, inventors; Nabisco Brands, asignee. Aspartame-sweetened gelatin dessert and sweetening composition therefor. US patent 4,574,091. 1986.
Franz M. Diet soft drinks: how safe are they? Diabetes Self Manag. 2010;27:11–13.
Chandan RC, O’Rell K Ingredients for yogurt manufacture. Manufacturing Yogurt and Fermented Milks, 2013. p. 217-37.
Pszczola DE. Sweetener + sweetener enhances the equation. Food Technology Magazine. 2023. https://www.ift.org/news-and-publications/food-technology-magazine/issues/2003/november/columns/products-and-technologies_ingredients.
Silva PD, Cruz R, Casal S. Sugars and artificial sweeteners in soft drinks: A decade of evolution in Portugal. Food Control. 2021;120:107481.
Herrick KA, Rossen LM, Parsons R, Dodd KW. Estimating usual dietary intake from National Health and Nutrition Examination Survey data using the National Cancer Institute Method. Vital- Health Stat 2. 2018;178:1–63.
U.S. Environmental Protection Agency. Comptox Chemicals Dashboard, Aspartame - Chemical Details. 2023. Available from: https://comptox.epa.gov/dashboard/chemical/details/DTXSID0020107.
Williams AJ, Grulke CM, Edwards J, McEachran AD, Mansouri K, Baker NC, et al. The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. J Cheminf. 2017;9:61.
StataCorp. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC; 2021.
Alaimo K, Olson CM, Frongillo Jr EA, Briefel RR. Food insufficiency, family income, and health in US preschool and school-aged children. Am J Public Health. 2001;91:781.
Meek JY, Noble L. Breastfeeding So. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics. 2022;150:e2022057988.
U.S. Department of Agriculture, U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 2020, 9th Edition.
Dunford EK, Miles DR, Ng SW, Popkin B. Types and Amounts of Nonnutritive Sweeteners Purchased by US Households: A Comparison of 2002 and 2018 Nielsen Homescan Purchases. J Acad Nutr Diet. 2020;120:1662–71.e10.
Larrick B, Kretser A, McKillop K. Update on “A Partnership for Public Health: USDA Global Branded Food Products Database”. J Food Compos Anal. 2022;105:104250.
Beck B, Burlet A, Max J-P, Stricker-Krongrad A. Effects of long-term ingestion of aspartame on hypothalamic neuropeptide Y, plasma leptin and body weight gain and composition. Physiol Behav. 2002;75:41–7.
McAnulty PA, Collier MJ, Enticott J, Tesh JM, Mayhew DA, Comer CP, et al. Absence of developmental effects in CF-1 mice exposed to aspartame in utero. Fundam Appl Toxicol. 1989;13:296–302.
Trocho C, Pardo R, Rafecas I, Virgili J, Remesar X, Fernández-López JA, et al. Formaldehyde derived from dietary aspartame binds to tissue components in vivo. Life Sci. 1998;63:337–49.
Soffritti M, Belpoggi F, Degli Esposti D, Lambertini L, Tibaldi E, Rigano A. First experimental demonstration of the multipotential carcinogenic effects of aspartame administered in the feed to Sprague-Dawley rats. Environ Health Perspect. 2006;114:379–85.
Neltner TG, Alger HM, Leonard JE, Maffini MV. Data gaps in toxicity testing of chemicals allowed in food in the United States. Reprod Toxicol. 2013;42:85–94.
Sharma RP, Coulombe RA Jr. Effects of repeated doses of aspartame on serotonin and its metabolite in various regions of the mouse brain. Food Chem Toxicol. 1987;25:565–8.
Reilly MA, Lajtha A. Glutamatergic receptor kinetics are not altered by perinatal exposure to aspartame. Neurochem Int. 1995;26:217–22.
Sylvetsky AC, Welsh JA, Brown RJ, Vos MB. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr. 2012;96:640–6.
Enten H A die-hard diet soda drinker investigates the zero sugar trend. CNN, 2022. Available from: https://www.cnn.com/2022/06/05/health/diet-soda-vs-zero-sugar-wellness/index.html.
Sylvetsky AC, Dietz WH. Nutrient-content claims — guidance or cause for confusion? N. Engl J Med. 2014;371:195–8.
Choi C Yoplait Light to Remove Aspartame From Yogurt: Manufacturing. net; June, 2014. Available from: https://www.manufacturing.net/home/news/13179432/yoplait-light-to-remove-aspartame-from-yogurt.
Zhang L, Hernandez-Sanchez D, Herzog H. Regulation of feeding-related behaviors by arcuate neuropeptide Y neurons. Endocrinology. 2019;160:1411–20.
Reilly M, Debler E, Lajtha A. Perinatal exposure to aspartame does not alter aminergic neurotransmitter systems in weanling rat brain. Res Commun Psychol Psychiatry Behav. 1990;15:141–59.
Cohen JFW, Rifas-Shiman SL, Young J, Oken E. Associations of prenatal and child sugar intake with child cognition. Am J Prev Med. 2018;54:727–35.
Jones GS, Graubard BI, Ramirez Y, Liao LM, Huang WY, Alvarez CS, et al. Sweetened beverage consumption and risk of liver cancer by diabetes status: A pooled analysis. Cancer Epidemiol. 2022;79:102201.
McCullough ML, Hodge RA, Campbell PT, Guinter MA, Patel AV. Sugar- and artificially-sweetened beverages and cancer mortality in a Large U.S. Prospective Cohort. Cancer Epidemiol Biomark Prev. 2022;31:1907–18.
Stepien M, Duarte-Salles T, Fedirko V, Trichopoulou A, Lagiou P, Bamia C, et al. Consumption of soft drinks and juices and risk of liver and biliary tract cancers in a European cohort. Eur J Nutr. 2016;55:7–20.
Debras C, Chazelas E, Srour B, Druesne-Pecollo N, Esseddik Y, Szabo de Edelenyi F, et al. Artificial sweeteners and cancer risk: Results from the NutriNet-Santé population-based cohort study. PLOS Med. 2022;19:e1003950.
Pavanello S, Moretto A, La Vecchia C, Alicandro G. Non-sugar sweeteners and cancer: Toxicological and epidemiological evidence. Regul Toxicol Pharm. 2023;139:105369.
Borghoff SJ, Cohen SS, Jiang X, Lea IA, Klaren WD, Chappell GA, et al. Updated systematic assessment of human, animal and mechanistic evidence demonstrates lack of human carcinogenicity with consumption of aspartame. Food Chem Toxicol. 2023;172:113549.
Maffini MV, Neltner TG. Brain drain: the cost of neglected responsibilities in evaluating cumulative effects of environmental chemicals. J Epidemiol Community Health. 2015;69:496–9.
Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13:330–8.
World Health Organization. Use of non-sugar sweeteners: WHO guideline. In: Guidelines Review Committee NaFS, editor. 2023.
Kirkpatrick SI, Subar AF, Douglass D, Zimmerman TP, Thompson FE, Kahle LL, et al. Performance of the Automated Self-Administered 24-hour Recall relative to a measure of true intakes and to an interviewer-administered 24-h recall123. Am J Clin Nutr. 2014;100:233–40.
Butchko HH, Stargel WW. Aspartame: Scientific evaluation in the postmarketing period. Regul Toxicol Pharmacol. 2001;34:221–33.
U.S. Government Accountability Office. Food and Drug Administration: food additive approval process followed for aspartame. HRD-87-46. 1987. https://www.gao.gov/products/hrd-87-46.
Acknowledgements
Researcher(s)’ own analyses calculated (or derived) based in part on data from NielsenIQ Consumer LLC and marketing databases provided through the NielsenIQ Datasets at the Kilts Center for Marketing Data Center at The University of Chicago Booth School of Business. The conclusions drawn from the NielsenIQ data are those of the researcher(s) and do not reflect the views of NielsenIQ. NielsenIQ is not responsible for, had no role in, and was not involved in analyzing and preparing the results reported herein.
Author information
Authors and Affiliations
Contributions
LR designed and conducted analyses, wrote the first draft, and revised the manuscript. BH advised on statistical analyses, and KN advised on point-of-departure analyses. BH and KN reviewed, provided comments, and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Riess, L.E., Huynh, B.Q. & Nachman, K.E. Aspartame exposures in the US population: Demonstration of a novel approach for exposure estimates to food additives using NHANES data. J Expo Sci Environ Epidemiol (2024). https://doi.org/10.1038/s41370-024-00678-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41370-024-00678-7
- Springer Nature America, Inc.
Keywords
This article is cited by
-
Aspartame, as an artificial sweetener, does not affect renal function and antioxidative states in mice
BMC Research Notes (2024)