Abstract
An organic diet may reduce dietary exposure to pesticides but findings based on observational data are scant. We aimed to compare urinary pesticide concentrations between “organic” and “conventional” consumers from the NutriNet-Santé study. Organic food consumption was determined using a self-reported food frequency questionnaire. Individuals with a proportion of organic food in the whole diet (in g/d) below 10% were defined as low organic food consumers and those whose proportion was above 50% as high organic food consumers. A propensity score matching procedure was then used to obtain two similar subsets of 150 participants, differing mostly by the organic valence of their diet. Urinary pesticide and metabolite concentrations (organophosphorus, pyrethroid, and azole compounds) were determined by UPLC-MS/MS, standardized with respect to creatinine. The molar sums of total diethylphosphates, dimethylphosphates, and dialkylphosphates were also computed. Differences in distributions across groups were tested using Wilcoxon signed-rank test for matched data. Mean age was 58.5 years and 70% of participants were women. Significantly lower urinary levels of diethylthiophosphate, dimethylthiophosphate, dialkylphosphates, and free 3-phenoxybenzoic acid were observed among organic consumers compared to conventional consumers. Our findings confirm that exposure to certain organophosphate and pyrethroïd pesticides in adults may be lowered by switching from conventional to organic foods. This is particularly of high interest among conventional fruit and vegetable consumers, as their exposure may be the highest.
Similar content being viewed by others
Introduction
Pesticides are widely used to protect crops against harmful organisms and diseases, but are also utilized as biocides for non-agricultural purposes. Despite these beneficial aspects, there has been an increasing concern during the last decade that these compounds represent a risk to the general population through residues in food commodities. The toxicological outcomes that have been associated with pesticide exposure include neurological, respiratory, dermatological, digestive, carcinogenic, reproductive, and developmental effects, as recently reviewed by several authors [1,2,3]. Five pesticides (malathion, glyphosate, parathion, diazinon, and tetrachlorvinphos) were classified as probably carcinogenic to humans by the International Agency for Research on Cancer [4]. In addition, a high number of agrochemicals have been documented to affect the endocrine system [5, 6], causing reproductive and developmental adverse effects, but also resulting in metabolic disorders [7, 8]. Knowledge on the consequences of exposure levels observed in the general population remains scarce and difficult to interpret [9]. France is one of the largest users in tons of agricultural pesticides in the European Union [10]. Routes of exposure to pesticides are multiple (oral, dermal, and respiratory) but diet is the main source of pesticide exposure in the general population [11]. It is therefore essential to estimate how and to what extent, different dietary consumption patterns—from more or less contaminated food sources (e.g. organically vs. conventionally grown products) may affect exposure.
Organic production and labelling of organic products are held under legal framework of the Council Regulation (EC) No 834/2007, which limits the use of pesticides to a small number (i.e., 35) of natural substances while 488 active substances are approved by (EC) No 1107/2009 as pesticides in conventional agriculture in the European Union [12]. This exclusion of synthetic pesticides results in a significantly lower frequency of (or no) contamination in organic foods when compared to conventional foods, as consistently described in food residue analyses [13,14,15,16]. Some experimental studies, using mostly crossover design, have been carried out among children [17,18,19] and adults [20, 21], and all report that the adoption of a diet mainly based on organic foods leads to a significant reduction in pesticide levels in urine (including organophosphate pesticides and herbicide 2,4-D [17,18,19,20,21]). Furthermore, the considerable recent growth of organic food market [22] is largely due to consumers’ concerns for food safety. Indeed, organic products are perceived healthier by consumers than conventional ones mainly because of their absence of pesticide residues [23,24,25,26,27]. Yet the extent to which day-life high organic food consumption is related to reduced urinary pesticide concentrations, more specifically in European adults, is not well documented.
In this context, the objective of the current study was to test for differences in pesticide exposure, reflected by urinary biomarkers, among adults with low and high self-reported organic food consumption.
Methods
Study population
The NutriNet-Santé study is an ongoing web-based observational prospective study launched in France in May 2009 on a large sample of adult volunteers. Its general aim is to investigate the relationships between dietary patterns, nutrition, and health issues [28]. Participants over age of 18 years are recruited among the general population by means of vast multimedia campaigns. All questionnaires are completed online using a dedicated website.
The NutriNet-Santé study is conducted according to the Declaration of Helsinki guidelines and was approved by the Institutional Review Board of the French Institute for Health and Medical Research (IRB Inserm n°0000388FWA00005831) and the “Commission Nationale de l’Informatique et des Libertés” (CNIL n°908450/n°909216). Clinicaltrials.gov number: NCT03335644.
On a voluntary basis, participants were also invited to attend health centers for biological sampling and clinical examination (2011–2013). During the visit, volunteers underwent blood and urine sampling as well as a clinical examination including anthropometric measurements. Overall, samples of serum, plasma, buffy-coats, and urine were set up for about 20,000 participants of the cohort [29]. Electronic and paper written informed consents were obtained from all subjects attending the visit. All procedures were approved by the Consultation Committee for the Protection of Participants in Biomedical Research” (C09-42 on May 5th 2010) and the CNIL (n°1460707).
Sociodemographic, anthropometric, and biological data
To be included in the cohort, participants have to fill in a set of questionnaires providing information on sociodemographic (age, sex, educational level, employment status, and place of residence) and lifestyle (smoking status and physical activity) characteristics, and health data (menopausal status for women, medical history, and medication). Every year thereafter, as part of their follow-up, they are also invited to complete this same set of questionnaires to update their information.
During the clinical visits, systolic and diastolic blood pressure, weight, and height were measured using standardized procedures [30]. Fasting blood glucose, total serum cholesterol, HDL-cholesterol, and serum triglycerides were routinely measured as previously described [29].
Assessment of total and organic food consumption
Total and organic food consumptions were assessed using an organic semi-quantitative food frequency questionnaire (Org-FFQ). In June 2014, NutriNet-Santé participants were invited to complete the Org-FFQ through the dedicated secured website. The development of the Org-FFQ has been fully described elsewhere [31]. Briefly, the Org-FFQ consisted of 264 food and beverage items. It was based on a validated FFQ [32], completed by specific questions about organic food consumption frequency. Briefly, for each of the 264 items, subjects were asked to report their frequency of consumption and the quantity consumed over the past year. Additionally, a five-point Likert-type scale ranging from never to always was used to estimate the frequency of organic food consumption of each food item. Organic food intake was obtained for each item by applying a weight of 0, 0.25, 0.5, 0.75, and 1 to the five respective categories of frequency (never, rarely, half of the time, often, and always). Using the Org-FFQ, the proportion of organic food in the whole diet (g/d) was then calculated by dividing the total organic food consumption out of the total food consumption excluding water. Participants were also invited to fill in a questionnaire pertaining to motivations and attitudes towards organic foods.
Selection of the subsample and matching procedure
Of the 33,384 subjects who had completed the Org-FFQ, we selected those with available data regarding total and organic food consumption, with no missing covariates and who had attended the clinical visits (N = 5,746). Among them, we selected subjects who had fasted at least 6 h before the visit and subjects who were no subject to potential metabolic disorders, i.e., subjects with no history of type I diabetes, Crohn’s disease, all types of cancer, neurological diseases, cardiovascular diseases, digestive system diseases (including cirrhosis, hepatitis, celiac disease, and colitis), lupus, spondylolisthesis, and sclerosis (N = 4,598) (Fig. 1). Then low and high organic food consumers were identified as those with a proportion of organic food in the whole diet (g/d) below 10% or above 50%, respectively (N = 2,351). Finally, in order to account for profile differences between low and high organic food consumers, we applied a propensity score matching procedure without replacement. This matching approach, based on a single composite score, enabled to achieve comparability between the two groups in terms of their observed characteristics [33]. We thus obtained two propensity score-matched groups of 150 subjects, differing by the organic valence of their diet. Selection and matching procedures are extensively described in the Supplementary Material.
Urine collection, creatinine, and pesticide analysis
At the clinical visit, urine sample collection was performed using vessels allowing the close-circuit urine transfer from the vessel to the Vacutainer® tube. The Vacutainer® tubes containing the spot urine sample were kept at +4 °C before and during transportation to the central laboratory. After splitting in aliquots, urine samples were stored at −80 °C for further analyses. To account for urine dilution, creatinine concentration (µg/L) was used to adjust analyte concentrations. Urinary creatinine concentration was determined by 1H NMR according to a method adapted from Bouatra et al. [34]. All pesticide assays were performed in the same laboratory. The final list of analyzed pesticides and metabolites has been defined as a compromise between scientific objectives, financial cost and the available measurement methods. The extraction method for Group 1 (dimethylphosphate (DMP), dimethylthiophosphate (DMTP), dimethyldithiophosphate (DMDTP), diethylphosphate (DEP), diethylthiophosphate (DETP), diethyldithiophosphate (DEDTP)) was 96-well µElution Solid Phase Extraction-off line, Oasis Wax well Plate, 30 µm, 2 mg, Waters (Milford, Massachusetts, USA) for purification and concentration purposes. The sample volume used was 200 µl, diluted by 200 µl of water 4% formic acid. The compounds were eluted using a 5% ammonium hydroxide solution in acetonitrile. The extraction for other analytes—Group 2 (chlorpyrifos and metabolites, malathion, dichlorvos, phoxim, diazinon, thiabendazole-5-OH (TBZ-OH), tebuconazole, 2-(diethylamino)-6-methylpyrimidin-4-ol/one (DEAMP) (pirimiphos-methyl metabolite), pyrethroid metabolites (3-phenoxybenzoic acid (3-PBA), 4-fluoro-3-phenoxybenzoic acid (4-FPBA)), and 3,5,6-trichloropyridinol (TCP)) was performed with Solid Phase Extraction-off line, Oasis HLB well Plate, 30 µm, 2 mg, Waters (Milford, Massachusetts, USA). The sample volume used was 250 µl, diluted by 250 µl of water 4% ammonium hydroxide. The elution was done using a methanol–acetonitrile solution acidified with formic acid (0.05%).
A second analysis was performed for the deconjugated compounds before the same protocol of extraction: an enzymatic hydrolysis was performed, on 250 µl sample, for total (free and conjugated) TBZ-OH, tebuconazole, DEAMP, pyrethroid metabolites (3-PBA and 4-FPBA), and TCP, using β-glucuronidase/arylsulfatase (Helix pomatia) from Roche (Mannheim, Germany), after stabilization with sodium acetate buffer solution, pH 4.5–5.5, as described by the supplier (Sigma-Aldrich, Seelze, Germany), during 16 h at 37 °C.
Analyses of pesticides and metabolites (for correspondence between metabolites and parent compounds, see Supplementary Table 1) were performed using UPLC H-Class system coupled with a tandem mass spectrometry Xevo TQ-S (UPLC-MSMS) (Waters, Milford, USA).
For the pesticides from Group 1, a volume of 15 µl was injected in a column BEH amide 1.7 µm 2.1 × 100 mm (Waters, Milford, USA) in an oven at 35 °C. The eluents used were water 50 mM ammonium acetate/acetonitrile with a gradient from 90% to 20% acetonitrile, with a flow rate of 0.4 ml/min. The analysis was performed in ES-mode. For the pesticides from Group 2, a volume of 10 µl was injected in a column BEH C18 1.7 µm 2.1 × 100 mm (Waters, Milford, USA). The eluents used were water/acetonitrile + 0.05% formic acid with a gradient from. 85.5 to 2% water, in an oven at 40 °C temperature with a flow rate of 0.4 ml/min. The analysis was performed in ES+ and ES− mode.
Quality control/quality assurance
All standards used were certified standards provided by Sigma-Aldrich (Seelze, Germany), A2S (St Jean d’Illac, France) and Dr Ehrenstorfer (Augsburg, Germany).
A set of five calibrations in the range 0.01–10 µg/L for samples of human urine free of the target biomarkers was analyzed each day for 5 days for inter-assay precision and accuracy. For intra-assay precision and accuracy, three levels of concentrations (near limit of quantification, average, and high level) were analyzed in different human urine free of the target biomarkers ten times. A weighted linear regression model (1/×) was used for the construction of calibration curve. Throughout the study, a quality control was analyzed every ten samples and a blank every twenty samples. Dichlorvos-D6, chlorpyrifos-methyl-D6, chlorpyrifos-D10, malathion-D6, diazinon-D10, DETP-D10, and DMTP-D6 served as internal standards.
The limit of detections (LODs) ranged from 0.003 to 0.6 µg/L. The limit of quantification (LOQs) ranged from 0.01 to 2 µg/L with a coefficient of variation ranging from 10 to 25%. LODs were threefold higher than the intensity of the background noise and LOQs were, overall, threefold higher than LODs.
Statistical analysis
For comparative purposes and as previously done in other publications [18, 21], we calculated the following molar sums (µmol/g), based on concentration of individual organophosphorus metabolites (µg/g creatinine) and molecular weights (g/mol):
where DMP denotes dimethylphosphate; DMTP dimethylthiophosphate; DMDTP dimethyldithiophosphate; DEP diethylphosphate; DETP diethylthiophosphate; DEDTP diethyldithiophosphate; DAPs dialkylphosphates; MPs dimethylphosphates; and EPs diethylphosphates.
Samples containing concentrations below the LOD were assumed to have concentrations equal to ½ LOD. Samples containing concentrations below the LOQ were assumed to have a concentration equal to the midpoint between the LOD and the LOQ [35].
We performed a balance diagnostic of the matching procedure to obtain standardized differences for variables included in the propensity score model as recommended [33], using the SAS macro %pmdiag [33]. Characteristics of the participants are presented by group of consumers and were compared using Wilcoxon signed-rank test for matched samples for continuous variables and McNemar test (binary variables) or conditional logistic regression for categorical variables (> 2-class variables). We computed the modified Programme National Nutrition Santé Guideline Score (mPNNS-GS), an a priori nutritional index reflecting the adherence to the French food-based nutritional guidelines [36]. A higher score (max = 13.5) reflects a higher nutritional quality of the diet [37]. Distribution indicators, frequency of detection and of quantification are provided.
Additionally, in a sensitivity analysis, in order to increase the discriminating power of our analyses, we only considered subjects with a proportion of organic food in the diet below 5% for the conventional group and their matching organic pairs (n = 218). All analyses were performed using 9.4 version of the SAS software (SAS Institute Inc., Cary, NC, USA).
Results
The balance diagnostic of the matching procedure is presented in Supplementary Table 2. The vast majority of the variables, including health, sociodemographic, and diet, displayed similar distributions across organic and conventional groups, except for consumption of mixed dishes (p-value < 0.05). Participants’ characteristics are shown in Table 1. The average proportions of organic food in the diet were 3% (± 3) and 67% (± 13) in the conventional and organic groups, respectively. Diet quality, assessed using the mPNNS-GS, was relatively high. Mean age of the participants was 58 years, about 30% of the participants were men and more than 60% were highly educated.
Levels of exposure to pesticides through urinary parent moieties and metabolites are presented in Tables 2 and 3. Parent pesticides were detectable in a limited number of samples in the two groups (max = 9% for malathion in the conventional group). The mean and median molecule concentrations were mostly below the LOD (Table 2). In contrast, for some metabolites such as total DEAMP, total TBZ-OH, total tebuconazole, most EPs, and MPs (except DEDTP and DMDTP) and total 3-PBA, the frequency of quantification was high in both groups (>10), and generally lower in the organic group (Table 3). The mean concentrations of DETP, DMTP, and free 3-PBA were significantly higher in the conventional group compared to the organic one while for the rest of metabolites, no significant difference between groups was detected (Table 3).
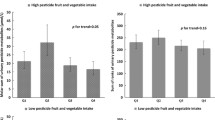
The molar sums of EPs, MPs, and DAPs are presented in Fig. 2 and Table 4. While no significant difference in urinary concentrations across groups was observed for total MPs (p-value = 0.47) and total EPs (p-value = 0.09), the total DAPs concentration was lower in the organic group compared to the conventional group, with means of 0.29 and 0.41 µmol/g creatinine respectively (p-value = 0.03). Some consumers had high exposure levels in both groups as shown by rather different orders of magnitude of means and medians.
When the population sample was restricted to pairs using a different criteria, namely <5% of organic food in the diet for conventional consumers (instead of 10% as previously stated) (n = 218), the mean difference for total EPs was accentuated and reached statistical significance (Table 5), indicating a possible dose-response relationship.
Discussion
In this observational study, when comparing urine pesticide metabolites in consumers with discriminant consumption of organic food, significantly higher urinary levels of DETP, DMTP, total DAPs (organophosphorus metabolites) and free 3-PBA (a pyrethroid metabolite) were found among conventional consumers compared to organic consumers, while, overall, low detection rates were found for parent compounds in both groups. In a sensitivity analysis, with conventional consumers with a maximum of 5% of organic food in their diet, differences between groups were more salient, in particular for EPs. This suggests that organophosphorus and pyrethroid pesticide exposure in adults may be noticeably lowered by introducing organic foods in the diet.
During the last decade, several studies comparing the levels of urine pesticides between “organic” and “conventional” consumers have been conducted in children [17,18,19, 38] and in adults [20, 21]. Beyond disparities in age range and dietary patterns of the populations as well as the periods when they have been conducted, these studies largely differed in their methodology. Most of them were indeed interventional crossover studies [17, 19, 21, 38]; two were observational [18, 20]. Exposure to a wide range of metabolites was assessed, including organophosphorus pesticide metabolites but also, less frequently, pyrethroids and some herbicides. Studies conducted among children reported reduction in total DAPs [17, 18] or metabolites of malathion and chlorpyrifos [19, 38] after switching to an organic controlled diet or when comparing organic and conventional participants. A drastic and immediate reduction was reported in crossover studies. For instance, among 40 Mexican–American children aged 3–6 years, the total DAP reduction was 40% after 7 days of organic diet [17]. Similarly, in a study conducted among preschool children, mean DMP urinary concentration was 9 times lower among 18 children following an organic diet compared to 21 children eating a conventional one [18]. Overall, these studies argue for a central role of the mode of production of food in organophosphorus pesticide exposure among children. These results were observed even in the study with an observational design reflecting actual levels of organic food consumption [18].
In the present study conducted in French adults, significant reductions in median urinary concentrations—ranging from 17 to 55%—were observed for DETP, DMTP, and free 3-PBA in organic consumers compared to conventional consumers while no significant results were found for the other pesticides investigated.
To the best of our knowledge, only one experimental crossover study has been carried out among adults to test for modification in urinary pesticide metabolites following a period of organic diet [21]. In that crossover study conducted among 13 adults in Australia, participants alternated two 7-day periods during which diets were either composed of at least 80% of conventional foods or organic foods. All DAPs were less frequently detected during the organic period than during the conventional one. After applying the same calculation as in our study for left-censored data, a reduction of 89% of total DAPs was observed. Indeed, during the organic phase, total DAPs mean concentration was 0.032 ± 0.038 µmol/g creatinine while total DAPs mean concentration among “organic consumers” was 0.29 ± 0.42 in our study. During the conventional diet period, total DAPs mean concentration was 0.294 ± 0.435 µmol/g creatinine while we found total DAPs mean concentration of 0.41 ± 0.64 among conventional consumers. These differences in the level of exposure may be explained by different designs (experimental vs. observational) as well as by different methodological approaches. Indeed, our study is based on observational data, i.e., based on actual self-reported data with participants that did not consume 100% organic or conventional food. In other words, given the cutoff used herein (i.e., minimum 50% of the diet coming from organic sources) conventional foods could also make up a quite important part of the diet in the “organic group”, leading to a certain and variable dietary pesticide exposure. In the same line, our sensitivity analysis clearly showed that reducing the proportion of organic foods (from maximum 10% to 5%) in the conventional consumer diet exacerbated the differences between groups in some metabolite concentrations. In addition, in our study, the definition of organic consumers was based on data collected using a self-administered food frequency questionnaire covering the past year. Use of a self-administered food frequency questionnaire, prone to some measurement error may have led to misclassification. Moreover, as organophosphorus metabolites exhibit a short half-live [39], it is possible that some participants identified as organic consumers have consumed highly contaminated (conventional) food just before the urine sampling. As underlined in another study [20], the very short half live of these compounds may strongly limit the relevance of this type of measure to assess the overall exposure to organophosphorus.
In the only other observational study carried out in adults, urinary DAPs and self-reported organic food consumption habits (using a three-categories question) were assessed in 480 US participants [20]. In that study, total DAPs concentrations significantly decreased while increasing consumption of organic food, with a difference of 65% between never organic food consumers and often/always organic food consumers. The magnitudes of the effects of switching from conventional to organic diets were comparable to those observed in our study, i.e., lower than in controlled experimental trials.
Herein, total DEAMP, total TBZ-OH, total tebuconazole, most EPs, and MPs, and free 3-PBA were frequently detected with rather high levels, even among organic consumers. As already mentioned, this may be explained by the fact that, in so-called high organic food consumers, organic food made up, on average, “only” 63% of the diet, meaning that, on average, almost 40% of the food was conventional. These individuals may have also been big consumers of conventional fruit and vegetables, leading to an overall quite high dietary pesticide exposure.
Compared with pesticide exposures estimated in 2006 in a random representative French survey (ENNS), participants of the conventional group in our study exhibited comparable urinary pesticide levels for DETP, DMTP and lower for DMDTP [40]. In contrast, mean urinary concentrations of DEP and DMP were markedly higher in our study [40]. For instance, mean and median of DEP urinary concentrations among our conventional group were 31.68 and 0.97 µg/g creatinine while in the ENNS study the respective values were 3.89 and 3.66. These findings reveal extremely different distributions of pesticide exposure levels between the ENNS survey and our study in which some participants had very high exposure levels. These differences can also partly be explained by the different approach used for left-censored data. Concerning pyrethroid metabolites, the median urinary concentration of free 4-F-3-PBA was very low in our study, as in the ENNS study . For 3-PBA, the urinary concentration was higher in the ENNS study with a geometric mean of 0.72 μg/g creatinine which compares to a mean of 0.13 μg/g creatinine in the conventional group of our study. As we mentioned above, it can be hypothesized that since subjects in our sample were very high consumers of fruit, vegetables and whole grains compared to the French population [41], they must have been particularly exposed to pesticides from plant-based products, which are indeed largely sprayed by synthetic chemicals [16]. This hypothesis is reinforced by the observation that some urinary organophosphorus pesticide metabolite concentrations (namely DETP and DMTP) and 3-PBA are linearly associated with conventional fruit and vegetable consumption (data not shown) in our study. For instance, a factor 4 was observed for DETP urinary concentration between 1st and 3rd tertiles of consumption of conventional fruit and vegetables.
Some limitations should be highlighted. First, our study design is entirely observational and based on self-reported data. Thus, consumption data are prone to misestimation, and in particular organic food consumption may have been overestimated [31]. While food consumption data are relatively precisely recorded, we did not quantify the extent of other sources of exposure (i.e. occupational, home, or environmental ones). For instance, thiabendazole is not only authorized as a plant protection product, but is also a biocide for wood treatment (see Supplementary Table 1). In addition, it is a preservative authorized for use in foodstuffs intended for human consumption (E233), and an anthelminthic drug used in human and veterinary medicine. In this case, the presence of thiabendazole metabolites in urine can be due to multiple non-dietary sources, although it should be born in mind that the diet remains the main source of pesticide exposure in the general population. The absence of difference in pesticide exposure may also be due to a lack of power, at least for contaminants with very low detection or quantification levels. The use of a middle bound scenario for left-censored data may also have led to the overestimation of some estimates.
Another limit pertains to the efficiency of matching. Although a wide range of confounders were used to estimate the propensity score, we cannot rule out possible residual confounding between organic and conventional consumers. In addition, propensity score matching leads to the exclusion of “particular” subjects unable to be matched, thus avoiding the external validity of findings as previously highlighted [33]. Our study sample, composed of volunteers, was particular with respect to sociodemographic characteristics and dietary intakes and thus is not comparable to the general population. NutriNet-Santé participants have indeed higher intakes of fruit and vegetables than the general French population [41]. Finally, as previously mentioned, concerning urinary DAPs and 3-PBA concentrations, the reliability of such biomarkers to reflect long-term exposure (usual diet, as assessed with the Org-FFQ) is questionable as their half live is short [39, 42] and only one biological sampling has been available. It should also be noted that given most pesticide moieties studied herein were no longer authorized for use in conventional agriculture at the time of urine sampling, the differences in overall pesticide exposure between the two groups of consumers are likely underestimated.
Our study also exhibits major strengths. Detailed data were used to assess organic and conventional consumption in the overall diet. We used an effective method, i.e. propensity score, allowing to match organic and conventional consumers using a wide range of covariates (including sociodemographic, dietary, and health data). Finally, this is the first study conducted in Europe comparing pesticide urinary concentrations from different classes of pesticides, in adults who differed by their organic consumption in real conditions.
Conclusions
Compared to individuals with low organic food consumption, individuals with a high proportion of organic food in their diet had significantly lower levels of various metabolites of pesticides of the organophosphate and pyrethroid families, suggesting that an organic food-based diet may help reduce the dietary pesticide exposure, at least for some agrochemicals as tested herein. Overall, low detection rates were found for parent compounds in both groups. It also should be stressed that urines of participants in our study displayed rather high exposure levels, irrespective of the group considered, compared to other populations. It would be also of high interest to conduct similar comparisons in large and representative samples. Accurate assessment of organic food consumption in representative national surveys is therefore required to better evaluate the clinical effects of such differences at the national level. Additional research is also needed to assess the part attributable to dietary pesticide exposure and non-dietary sources as well as the effects of such differences over the long term on health status.
References
Kim K-H, Kabir E, Jahan SA. Exposure to pesticides and the associated human health effects. Sci Total Environ. 2017;575:525–35.
Mostafalou S, Abdollahi M. Pesticides: an update of human exposure and toxicity. Arch Toxicol. 2017;91:549–99.
Nicolopoulou-Stamati P, Maipas S, Kotampasi C, Stamatis P, Hens L Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front Public Health 2016; 4. https://doi.org/10.3389/fpubh.2016.00148.
Guyton KZ, Loomis D, Grosse Y, El Ghissassi F, Benbrahim-Tallaa L, Guha N, et al. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015;16:490–1.
Mnif W, Hassine AIH, Bouaziz A, Bartegi A, Thomas O, Roig B. Effect of endocrine disruptor pesticides: a review. Int J Environ Res Public Health. 2011;8:2265–303.
Mostafalou S, Abdollahi M. Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol. 2013;268:157–77.
Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135–62.
Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120:779.
Collectif INSERM. Pesticides: Effets sur la santé, une expertise collective de l’Inserm. Salle Presse Inserm. 2013.http://presse.inserm.fr/pesticides-effets-sur-la-sante-une-expertise-collective-de-linserm/8463/ (accessed 21 Aug2016).
Eurostat. Agriculture, forestry and fishery statistics. 2016.
Nougadère A, Sirot V, Kadar A, Fastier A, Truchot E, Vergnet C, et al. Total diet study on pesticide residues in France: levels in food as consumed and chronic dietary risk to consumers. Environ Int. 2012;45:135–50.
Union Européenne. Pesticides database. 2016.ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/.
Barański M, Średnicka-Tober D, Volakakis N, Seal C, Sanderson R, Stewart GB et al. Higher antioxidant and lower cadmium concentrations and lower incidence of pesticide residues in organically grown crops: a systematic literature review and meta-analyses. Br J Nutr 2014;112(5):794–811.
Lairon D. Nutritional quality and safety of organic food. A review. Agron Sustain Dev. 2010;30:33–41.
Smith-Spangler C, Brandeau ML, Hunter GE, Bavinger JC, Pearson M, Eschbach PJ, et al. Are organic foods safer or healthier than conventional alternatives?A systematic review. Ann Intern Med. 2012;157:348–66.
Authority (EFSA) EFS. Monitoring data on pesticide residues in food: results on organic versus conventionally produced food. EFSA Support Publ. 2018;15:1397E.
Bradman A, Quirós-Alcalá L, Castorina R, Aguilar Schall R, Camacho J, Holland NT et al. Effect of organic diet intervention on pesticide exposures in young children living in low-income urban and agricultural communities. Environ Health Perspect. 2015. https://doi.org/10.1289/ehp.1408660.
Curl CL, Fenske RA, Elgethun K. Organophosphorus pesticide exposure of urban and suburban preschool children with organic and conventional diets. Environ Health Perspect. 2002;111:377–82.
Lu C, Toepel K, Irish R, Fenske RA, Barr DB, Bravo R. Organic diets significantly lower children’s dietary exposure to organophosphorus pesticides. Environ Health Perspect. 2006;114:260–3.
Curl CL, Beresford SAA, Fenske RA, Fitzpatrick AL, Lu C, Nettleton JA, et al. Estimating pesticide exposure from dietary intake and organic food choices: The Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health Perspect. 2015. https://doi.org/10.1289/ehp.1408197
Oates L, Cohen M, Braun L, Schembri A, Taskova R. Reduction in urinary organophosphate pesticide metabolites in adults after a week-long organic diet. Environ Res. 2014;132:105–11.
Agence Bio/CSA. Baromètre de consommation et de perception des produits biologiques en France, 13ème édition. 2016.http://www.agencebio.org/sites/default/files/upload/documents/4_Chiffres/BarometreConso/1400610_agence_bio_rapport_2015_vp.pdf (accessed 11 Aug2016).
Dickson-Spillmann M, Siegrist M, Keller C. Attitudes toward chemicals are associated with preference for natural food. Food Qual Prefer. 2011;22:149–56.
Grunert KG. Food quality and safety: consumer perception and demand. Eur Rev Agric Econ. 2005;32:369–91.
Hoefkens Christine, Verbeke Wim, Aertsens Joris, Mondelaers Koen, Van Camp John. The nutritional and toxicological value of organic vegetables: Consumer perception versus scientific evidence. Br Food J. 2009;111:1062–77.
Hughner RS, McDonagh P, Prothero A, Shultz CJ, Stanton J. Who are organic food consumers? A compilation and review of why people purchase organic food. J Consum Behav. 2007;6:94–110.
Padilla Bravo C, Cordts A, Schulze B, Spiller A. Assessing determinants of organic food consumption using data from the German National Nutrition Survey II. Food Qual Prefer. 2013;28:60–70.
Hercberg S, Castetbon K, Czernichow S, Malon A, Mejean C, Kesse E, et al. The Nutrinet-Santé Study: a web-based prospective study on the relationship between nutrition and health and determinants of dietary patterns and nutritional status. BMC Public Health. 2010;10:242.
Lassale C, Galan P, Julia C, Fezeu L, Hercberg S, Kesse-Guyot E. Association between adherence to nutritional guidelines, the metabolic syndrome and adiposity markers in a french adult general population. PLoS ONE. 2013;8:e76349.
Lassale C, Péneau S, Touvier M, Julia C, Galan P, Hercberg S, et al. Validity of web-based self-reported weight and height: results of the Nutrinet-Santé study. J Med Internet Res. 2013;15:e152.
Baudry J, Méjean C, Allès B, Péneau S, Touvier M, Hercberg S, et al. Contribution of Organic Food to the Diet in a Large Sample of French Adults (the NutriNet-Santé Cohort Study). Nutrients. 2015;7:8615–32.
Kesse-Guyot E, Castetbon K, Touvier M, Hercberg S, Galan P. Relative validity and reproducibility of a food frequency questionnaire designed for French adults. Ann Nutr Metab. 2010;57:153–62.
Ali MS, Groenwold RH, Klungel OH. Best (but oft-forgotten) practices: propensity score methods in clinical nutrition research. Am J Clin Nutr. 2016;104:247–58.
Bouatra S, Aziat F, Mandal R, Guo AC, Wilson MR, Knox C, et al. The Human Urine Metabolome. PLoS ONE. 2013;8:e73076.
GEMS/Food-Euro. Second workshop on reliable evaluation of low-level contamination of food. Report on a workshop in the frame of GEMS/Food-Euro, EUR/HFA target 22. 26-27 May 1995. Kulmbach, German. Geneva: WHO. 1995.
Estaquio C, Kesse-Guyot E, Deschamps V, Bertrais S, Dauchet L, Galan P, et al. Adherence to the French Programme National Nutrition Santé Guideline Score is associated with better nutrient intake and nutritional status. J Am Diet Assoc. 2009;109:1031–41.
Baudry J, Allès B, Péneau S, Touvier M, Méjean C, Hercberg S et al. Dietary intakes and diet quality according to levels of organic food consumption by French adults: cross-sectional findings from the NutriNet-Santé Cohort Study. Public Health Nutr 2017;20:638–48.
Lu C, Barr DB, Pearson MA, Waller LA. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environ Health Perspect. 2008;116:537–42.
Griffith W, Curl CL, Fenske RA, Lu CA, Vigoren EM, Faustman EM. Organophosphate pesticide metabolite levels in pre-school children in an agricultural community: Within- and between-child variability in a longitudinal study. Environ Res. 2017;20(4):638–48.
Fréry N, Saoudi A, Garnier R, Zeghnoun A, Falq G, Guldner L. Exposure of the French population to environmental pollutants–Environmental components of the French National Survey on Nutrition and Health–Initial results. Fr Inst Public Health Surveill St.-Maurice. Fra. 2010.
Andreeva VA, Deschamps V, Salanave B, Castetbon K, Verdot C, Kesse-Guyot E, et al. Comparison of Dietary Intakes Between a Large Online Cohort Study (Etude NutriNet-Santé) and a Nationally Representative Cross-Sectional Study (Etude Nationale Nutrition Santé) in France: Addressing the Issue of Generalizability in E-Epidemiology. Am J Epidemiol. 2016;184:660–9.
Sudakin DL, Stone DL. Dialkyl phosphates as biomarkers of organophosphates: The current divide between epidemiology and clinical toxicology. Clin Toxicol. 2011;49:771–81.
Acknowledgements
We thank all the people who helped carry out the NutriNet-Santé study and all the dedicated and conscientious volunteers. We especially thank Younes Esseddik, Paul Flanzy, Véronique Gourlet, Nathalie Arnault, Fabien Szabo, Laurent Bourhis and Cédric Agaesse.
Author contributions
The author contributions were as follows: JPC, SH, EKG, DL and PG conceived and designed the experiments. JB performed the experiments. GD and AD performed the urinary dosages. JB and EKG analyzed the data. JB and EKG wrote the paper. All the authors were involved in interpreting results and editing the manuscript. JB and EKG had primary responsibility for final content. All authors read and approved the final manuscript.
Funding
The BioNutriNet project was supported by the French National Research Agency (Agence Nationale de la Recherche) in the context of the 2013 Programme de Recherche Systèmes Alimentaires Durables (ANR-13-ALID-0001). The NutriNet-Santé cohort study is funded by the following public institutions: Ministère de la Santé, Santé Publique France, Institut National de la Santé et de la Recherche Médicale (INSERM), Institut National de la Recherche Agronomique (INRA), Conservatoire National des Arts et Métiers (CNAM) and Paris 13 University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DL acts since 2018 as a scientific expert, with no honoraria or personal funding, in two non-for-profit foundations recently funded in France («Fondation Bjorg, Bonneterre et citoyens » and « Fond de dotation Institut de l’alimentation bio »).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Baudry, J., Debrauwer, L., Durand, G. et al. Urinary pesticide concentrations in French adults with low and high organic food consumption: results from the general population-based NutriNet-Santé. J Expo Sci Environ Epidemiol 29, 366–378 (2019). https://doi.org/10.1038/s41370-018-0062-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41370-018-0062-9
- Springer Nature America, Inc.
Keywords:
This article is cited by
-
Urinary dialkylphosphate metabolites in the assessment of exposure to organophosphate pesticides: from 2000 to 2022
Environmental Monitoring and Assessment (2024)
-
Environmental pressures and pesticide exposure associated with an increase in the share of plant-based foods in the diet
Scientific Reports (2023)
-
Organic food consumption and the incidence of cancer in the Danish diet, cancer and health cohort
European Journal of Epidemiology (2023)
-
Reported organic food consumption and metabolic syndrome in older adults: cross-sectional and longitudinal analyses
European Journal of Nutrition (2022)
-
Quantifiable urine glyphosate levels detected in 99% of the French population, with higher values in men, in younger people, and in farmers
Environmental Science and Pollution Research (2022)