Abstract
Background and objective
The effect of exercise training on whole-body insulin sensitivity has not been systematically summarized. We aimed to summarize the data from randomized controlled trials evaluating the effect of exercise training on insulin action, in adults.
Subjects
MEDLINE, EMBASE, and CENTRAL databases were searched until January 2021. Randomized controlled trials lasting ≥4 weeks, including adults, and evaluating the effect of exercise on insulin-stimulated glucose disposal measured using the hyperinsulinemic euglycemic clamp, were included.
Methods
Three reviewers extracted summary data from published trials. The primary outcome was insulin-stimulated glucose disposal. Standardized weighted mean differences (SMD) in glucose disposal between intervention and control were compared. The PEDro scale was used to assess risk of bias.
Results
We included 25 trials (36 interventions, N = 851). Exercise increased insulin-stimulated glucose disposal relative to control, SMD = 0.52 (95% confidence interval [CI]: 0.39, 0.65; p < 0.001; I2 = 47%) without significantly suppressing hepatic glucose production. In trials without isotopic tracers, exercise increased glucose disposal (SMD = 0.63; 95% CI: 0.48, 0.77; p < 0.001, I2 = 55%). In trials with isotopic tracers, exercise increased glucose disposal only when tracers were added to the exogenous glucose used for clamping (SMD = 0.34; 95% CI: 0.03, 0.66, p = 0.034. I2 = 0%). In a meta-regression model including aerobic exercise, weight change, and tracer technique, only percent weight change explained between trial heterogeneity (β = 0.069; 95% CI: 0.005, 0.013). The PEDro rating indicated relatively low risk of bias (5.8 ± 0.22).
Conclusions
Exercise training for at least four weeks significantly increases insulin-stimulated glucose disposal. Weight loss maximizes the effect and may be needed to improve hepatic insulin sensitivity. Differences in tracer methodology contribute to divergent outcomes and should be considered when assessing conclusions from research examining the effect of exercise on insulin action.
Registration
PROSPERO (CRD42019124381).
Similar content being viewed by others
Introduction
Skeletal muscle is the principal tissue for insulin-dependent glucose disposal from the blood, and a primary driver of whole-body glycemic control [1]. Under basal conditions, plasma free fatty acids are the primary substrate for skeletal muscle. Glucose or meal-stimulated insulin release suppresses adipose tissue lipolysis and plasma free fatty acids, which stimulates muscle glucose uptake and a switch to glucose as the primary energy source for muscle. People with obesity and type 2 diabetes typically have impaired insulin-stimulated muscle glucose oxidation and glycogen synthesis as a result of downregulation or degradation of insulin receptors and defects in post-receptor insulin signaling [2, 3]. Furthermore, insulin delivered into the portal vein is a major regulator of hepatic glucose production (HGP) and people with obesity, prediabetes, and type 2 diabetes have increased gluconeogenesis and an impairment in the capacity of insulin to suppress HGP [4, 5].
Skeletal muscle is the primary site for insulin resistance in people with type 2 diabetes and improving insulin sensitivity in this tissue improves whole-body glucose homeostasis [6]. Skeletal muscle responds to contraction or exercise with a rapid insulin-independent increase in glucose uptake during exercise and a transient insulin-stimulated pathway following exercise [7,8,9,10,11]. Exercise training leads to sustained increases in insulin sensitivity in healthy subjects and in people with type 2 diabetes [12,13,14]. Therefore, exercise training is part of the standard recommendations for treating and preventing obesity, type 2 diabetes, and related diseases that may be linked to insulin resistance [15].
The β-cell response to glucose and the sensitivity of body tissues to insulin are the key variables determining glucose homeostasis. Neither variable is held constant because of a feedback loop. The hyperinsulinemic euglycemic clamp test (hereinafter referred to as clamp) accounts for the simultaneous rather than sequential responses to glucose and insulin. The clamp offers a highly reproducible physiologic method for quantifying β-cell sensitivity to glucose and tissue sensitivity to insulin [16]. Investigators who administer the clamp with isotopic tracer dilution techniques using the hot infusion protocol, which incorporates tracers into the exogenous glucose used for clamping [17], can measure the suppression of HGP.
The main tissues involved in insulin-stimulated glucose disposal (hereinafter referred to as glucose disposal) are skeletal muscle, liver, and adipose tissue, and the impact of exercise training on adipose tissue insulin sensitivity has been summarized [18]. Randomized controlled trials have examined the impact of exercise training on glucose disposal and suppression of HGP, but these randomized trials have not been systematically summarized. Furthermore, it is unknown whether factors such as exercise mode, duration, intensity, and weight loss influence glucose disposal in response to exercise. This systematic review and meta-analysis aimed to summarize the body of evidence from randomized controlled trials investigating the effect of exercise training on insulin action measured using the clamp. We hypothesized that exercise training would increase whole-body glucose disposal, which would be modified by program variables and clamp methodology.
Methods
Data sources and searches
This systematic review followed the PRISMA reporting guidelines. The protocol was registered with the National Institute for Health Research International Prospective Register of Systematic Reviews (PROSPERO) with registration number, CRD42019124381. A search of three medical databases, including MEDLINE, EMBASE and Cochrane Central Register of Controlled Trials (CENTRAL) was conducted by a research librarian from inception to January 5, 2021 (Supplementary Information Table 1). In addition, reference lists of publications eligible for full text review were searched to identify additional trials. Title, abstract, and full-text screening was conducted in duplicate by independent reviewers (CJR, and ACL or CJE) using Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia). Reasons for exclusion were noted at each stage, and disagreements were resolved by consensus.
Study selection
Eligible trials included randomized controlled trials in the adult population (aged ≥18 years), with an exercise intervention of at least four weeks, and that determined changes in glucose disposal using the clamp procedure. If there were multiple papers from one trial, only the paper that provided clamp-derived glucose disposal measures was chosen. All trials included a control arm where participants did not exercise. Trials that administered a concomitant intervention in the experimental group that was the same in the comparator group to enable isolation of the exercise effect, were also included. For our analyses, trials were excluded if the trial population included children, adolescents (<18 years), pregnant subjects, or did not report glucose disposal. Only trials published in English were included. Unpublished trials were not included.
Data extraction and quality assessment
Data were independently extracted by one reviewer (ACL or CJE) and checked with another (CJR) using a pilot tested form. Disagreements were resolved by consensus or by a third reviewer (JCB). Data extracted from the eligible trials included population characteristics, location, exercise prescription (e.g., frequency, intensity, duration, type), clamp methodology, and overall changes from baseline to the end of the trial.
Exercise intensity was coded as a categorical effect including moderate, moderate to vigorous, or vigorous using the American College of Sports Medicine (ACSM) guidelines [19]. Ratings were based on percent of maximal heart rate or heart rate reserve or maximum oxygen uptake (aerobic) or rate of perceived exertion or number of repetitions or percent of maximum (resistance). When insulin infusion rate was reported as mU/kg/min, it was converted into mU/m2/min by first converting the mean body weight into body surface area (BSA [m2] = 0.1173 * body weight [kg]^0.6466 [20]. The insulin infused reported in mU/kg/min * mean body weight (kg)/BSA (m2) was used to determine the insulin infusion in mU/m2/min.
The primary outcome was mean change in glucose disposal. Secondary outcomes included body weight, BMI, and maximal oxygen uptake (VO2max) and it was determined a priori that these outcomes would be pursued if they were reported by at least 75% of trials. A critical appraisal of all trials was conducted using the Physiotherapy Evidence Database scale (PEDro scale). This checklist was developed to rate the methodological quality of RCTs and is validated for the reliability of a consensus rating from two or more assessors [21]. PEDro scale scores range from 0 to 10 with higher scores indicating greater methodological and reporting quality [22]. Data were independently assessed by one reviewer (ACL or CJE) and checked with another (CJR).
Data synthesis and analysis
To harmonize the various methods used for reporting glucose disposal, we used the standardized mean difference (SMD) to quantify the difference in glucose disposal from baseline to follow-up between the exercise and control groups. The SMD denotes the difference between the mean glucose values of the control and exercise groups, divided by the pooled standard deviation [23]. We present overall weighted mean effect sizes as both fixed- and random effects estimates. Values of 0.3, 0.5, and 0.8 for SMD correspond to small, medium, and large effects, respectively [24]. Potential heterogeneity was calculated as I2 [25]. Begg’s and Egger’s tests were used to examine publication bias [26]. All statistical analyses were conducted using SAS (version 9.4, Cary, NC, USA).
Heterogeneity of changes in glucose disposal, the association between trial-level characteristics, and the magnitude of reduction in glucose disposal was assessed using a modified, weighted least squares regression [27]. Statistically significant regression analyses were integrated into a multiple fixed effects regression to determine which variables explain between-trial variance. Two-sided statistical significance was p < 0.05, and unless specifically stated, values are reported as SMD and 95% confidence intervals (CIs).
To determine the effect of program variables, we conducted sub-group analyses of trials by: demonstration of significant weight loss (p < 0.05), older adults (age > 60), use of tracers, exercise mode (Aerobic/Resistance/both), provision of standardized meals at least 2–3 days before the clamp, inclusion of patients with type 2 diabetes, and completion of the clamp at least 72 h after the last exercise session (to determine the chronic effect of exercise). The criterion for sub-group analysis was that at least eight trials reported these data. If the analysis showed differing results within a subgroup, the variables were examined as covariates. In addition, percent weight change (% baseline body weight), insulin infusion rate, drop-out rate, age, baseline fasting glucose and insulin, and exercise training program variables such as number of minutes per session, frequency (days/week), duration of the intervention, overall intensity, and type were explored as covariates. Trials eligible for each synthesis were determined by tabulating the trial intervention characteristics and comparing against planned groups for each synthesis.
Exploratory sub-analyses
Studies that use tracers report glucose disposal as glucose infusion rate + HGP, whereas studies that do not use tracers report glucose disposal as glucose infusion rate alone. Given the unexpected difference in the effect size between the studies that used tracers and those that did not use tracers, we conducted a sub-analysis to determine the effect of exercise on suppression of HGP during the clamp. If studies did not specifically report % suppression of HGP, it was calculated as the % difference between basal and clamp measures of HGP. As these analyses were unplanned, data should be interpreted as hypothesis-generating.
Results
Description of studies
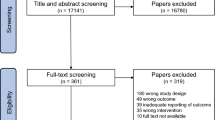
Our search yielded 292 citations (Fig. 1), of which 25 RCTs including 36 comparisons of exercise versus control (N = 851) were eligible for inclusion in our analysis (Table 1). A list of excluded trials at full-text stage and the reasons for exclusion are provided in the Supplementary Information Table 2. Trials were conducted in North America (N = 16) or Europe (N = 9) and ranged in duration from four to 43 weeks.
Fifteen trials evaluated one intervention [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. Nine trials evaluated two interventions which included intensity (high and moderate) [43,44,45], exercise type (aerobic and resistance) [46, 47] and with or without food compensation for energy expended through exercise [48,49,50,51]. One trial evaluated three interventions (aerobic, resistance, and the combination) [52]. Five trials used the two-step clamp protocol: at the first step insulin was infused at a low dose (10–20 mU/min/m2) and at the second step insulin was infused at a higher dose (40–100 mU/m2/min) [32, 42, 44, 48], except in one trial where the first step insulin infusion was 40 mU/m2/min and the second step was 200 mU/m2/min [28] (Table 1). Eight trials including 10 interventions used intent-to-treat analytic strategies [28, 32, 33, 38, 43, 47, 52], and one trial had a sample size exceeding 30 in each group [39].
The baseline characteristics of the subjects are presented in Table 2. Eight trials specifically enrolled subjects with type 2 diabetes [30, 35, 36, 38, 40, 43, 47, 48] and the other trials included subjects with fasting or postprandial blood glucose concentrations in the normal to prediabetes range. Five trials [31, 32, 44, 45, 51] specifically included subjects who were in the overweight category (BMI 25–29.9 kg/m2), and two trials [34, 46] included subjects with BMI < 30 kg/m2. The remaining trials included subjects with overweight, or obesity (BMI > 30 kg/m2).
Quality (risk of bias) and publication bias assessment
The mean PEDro score of the exercise interventions was 5.8 ± 1.08 (mean ± SD, Supplementary Information Table 3). The PEDro score was equal to or greater than 6.0 in 64% of trials indicating relatively high quality [21]. Similar PEDro scores were observed for trials that provided data on body weight, BMI, and VO2max. Few trials reported adherence to the intervention; however, five trials [37, 39, 46, 49, 50] reported more than 20% drop out rates. Trials did not specifically report adverse events but the reasons for withdrawal due to adverse events were reported in seven trials (Supplementary Information Table 4). Five trials including seven interventions [32, 38, 41, 43, 48], used the hot infusion protocol to correct for perturbations in plasma glucose specific activity, by adding tracers to exogenous glucose used for clamping. Four trials including five interventions used the cold infusion protocol without adding tracers to exogenous glucose used for clamping [29, 35, 40, 44]. Seven trials reported the effect of exercise training on suppression of HGP [32, 35, 38, 40, 41, 44, 48], and three of these trials used the cold infusion protocol [35, 40, 44]. In four studies the exercise was unsupervised [31, 33, 45, 51], three studies used a combination of supervised and unsupervised exercise [36, 38, 40], and in the remaining 18 studies exercise was supervised. All testing in the intervention and control groups was conducted in a controlled research setting. Begg’s test (p = 0.21), and Egger’s test (p = 0.19) identified no asymmetries in the effect size distribution suggestive of publication bias.
Association of intervention with end points
Exercise training was related to greater glucose disposal relative to control in both the fixed effects (SMD = 0.52; 95% CI: 0.39, 0.65; p < 0.001; I2 = 47%) and random effects models (SMD = 0.55; 95% CI: 0.37, 0.73; p < 0.001, Fig. 2). Furthermore, exercise training reduced body weight, (SMD = −0.40; 95% CI: −0‧54, −0.26; p < 0.001; I2 = 46%), and BMI (SMD = −0.48; 95% CI: −0.62, −0.35; p < 0.001; I2 = 41%), and increased VO2max (SMD = 0.78; 95% CI: 0.64, 0.92; p < 0.001; I2 = 74%).
In the sub-group analysis (Fig. 3), trials that administered aerobic exercise (interventions: N = 26 aerobic alone, N = 5 resistance, and N = 5 aerobic and resistance) increased glucose disposal compared to other modes (aerobic, SMD = 0.68; 95% CI: 0.53, 0.84; p < 0.001; I2 = 45%; other modes SMD = 0.21; 95% CI: −0.008, 0.42; p = 0.06; I2 = 2.5%). Although exercise increased glucose disposal regardless of significant (p < 0.05) weight loss compared to control, reduction in body weight enhanced the effect (significant weight loss: SMD = 0.89; 95% CI: 0.69, 1.10; p < 0.001; I2 = 58%; non-significant weight loss: SMD = 0.32; 95% CI: 0.16, 0.48; p < 0.001; I2 = 0.5%). In trials that did not use tracers exercise increased glucose disposal (SMD = 0.63; 95% CI: 0.48, 0‧77; p < 0.001; I2 = 55%), whereas in trials that used tracers exercise did not increase glucose disposal (SMD = 0.22; 95% CI: −0.02, 0.47; p = 0.078; I2 = 0%). The difference in glucose disposal between the trials that used and did not use tracers was moderate in magnitude (SMD = −0.46; p = 0.007, Fig. 2).
When trials with tracers used the recommended hot infusion protocol, exercise increased glucose disposal versus control (SMD = 0.34; 95% CI: 0.03, 0.66; p = 0.034; I2 = 0%). However, this effect size was smaller than trials that did not use tracers. In trials that used the cold infusion protocol, exercise did not increase glucose disposal (SMD = 0.04; 95% CI: −0.05, 0.43; p = 0.84; I2 = 0%). A hypothetical example showing differences in the equation used to calculate glucose disposal in studies that used and did not use tracers is presented in Table 3.
Sub-group analysis of trials (N = 17) that conducted the clamp at least 72 h after the last exercise session showed that exercise increased glucose disposal, and the conduct of the clamp less than 72 h after the last exercise session [31, 34,35,36, 38, 41, 51] (one study did not provide the timing of the clamp after the last exercise session [39]) did not affect the outcome. Similarly, no differing results were obtained for the sub-group analysis by age, standardized meals, and inclusion of patients with type 2 diabetes. In the exploratory analysis of studies that reported suppression of HGP, exercise training did not influence HGP (SMD = −0.29; 95% CI: −0.64, 0.06; p = 0.1; I2 = 94%). Excluding trials that reported HGP but used the cold infusion protocol, did not improve the outcome.
When used as a covariate in the model, aerobic exercise influenced glucose disposal (β: −0.46, 95% CI: −0.81, −0.12). Furthermore, the use of tracers as a covariate in the model was a predictor of glucose disposal (β: 0.44; 95% CI: 0.07, 0.80). Except for percent weight change (β: 0.08; 95% CI: 0.02, 0.14), other covariates used in the analyses did not change the exercise-induced effect on glucose disposal. (Supplementary Information Table 5). In the multiple meta-regression model that included aerobic exercise, percent weight change, and use of tracers, only percent weight change explained the between-trial heterogeneity in the effect of exercise on glucose disposal (β: 0.069; 95% CI: 0.005, 0.013). Thus, each 5% loss of body weight was associated with SMD = 0.345.
Discussion
This systematic review and meta-analysis demonstrated that in adults structured exercise training improves glucose disposal compared to control, and this gain extends beyond the effect of a single acute bout of exercise. The improvement in insulin action occurs regardless of weight loss but is enhanced by reductions in body weight. There was no improvement in hepatic insulin sensitivity. Similarly, the improvement in insulin action occurred regardless of whether or not the analysis included studies with subjects greater than 60 years of age or those with type 2 diabetes. Exercise frequency, intensity, duration, or type did not influence insulin action, but modality of exercise, specifically aerobic exercise, was an important predictor of the improvement in glucose disposal. The implications of these data are that exercise can favorably modulate a critical factor that drives metabolic disease in obesity, and weight loss enhances the effect, possibly through induction of mechanisms related to hepatic and adipose tissue insulin sensitivity.
During exercise, muscle glucose uptake increases but the process induced by muscle contractions is not dependent on insulin [53]. Muscle contractions do not activate the steps proximal to the receptor in the canonical insulin signaling cascade [54]. Following a single exercise bout, the mechanisms underlying improved insulin action involve a coordinated effect of insulin action on vasodilation, augmented capillary perfusion, and increased glucose transporter 4 (GLUT4) translocation to the muscle surface membrane [55, 56]. The molecular adaptations that regulate insulin sensitivity after one exercise bout lead to sustained elevations in insulin action with regular physical training [57,58,59,60].
Our analysis including 36 interventions showed that in adults structured exercise training increases glucose disposal compared to control. The analysis predominantly included subjects with overweight and obesity, and we found that the cumulative effect of exercise training was not intensity- or length of exercise session-dependent. Despite large differences in training intensity and exercise time, moderate-intensity continuous training and high-intensity interval training show similar improvements in glucose disposal in adults with obesity after 12 weeks [61]. Additionally, four weeks of moderate-intensity continuous training and high-intensity interval training were both effective in reducing intrahepatic lipids independent of changes in abdominal adiposity or body mass [62]. Our analysis supports the findings from these studies [61, 62], and from a meta-analysis showing that adipose tissue insulin resistance index reduces with aerobic exercise training but is not affected by exercise intensity [18].
One interesting finding was that glucose disposal was enhanced when studies were limited to exercise interventions reporting significant weight loss. Furthermore, meta regression showed that every 5% weight loss was associated with SMD = 0.345. Weight loss clearly improves insulin sensitivity but the effects in different organ systems have individual variability depending upon the duration and extent of the metabolic dysregulation. In general, insulin-mediated suppression of lipolysis in adipose tissue and suppression of HGP occur maximally at 5% to 8% weight loss [63, 64]. Insulin-mediated glucose disposal in skeletal muscle continues to increase with additional weight loss [63]. The effects of exercise on glucose disposal are primarily driven by skeletal muscle [6]. We did not find a significant effect of exercise training on suppression of hepatic glucose production. Engin et al. did not find an effect of exercise training on suppression of free fatty acids during the clamp except when accompanied by significant weight loss, in their meta-analysis summarizing the findings from intervention studies measuring the impact of exercise on adipose tissue insulin sensitivity [18]. Sufficient weight loss is perhaps needed before the effect of exercise training on insulin sensitivity extends to the liver and adipose tissue.
The cold infusion protocol results in a marked underestimation of glucose disposal, generating biologically implausible negative values of HGP [17]. Our subgroup analyses of trials using tracers showed that exercise increased glucose disposal compared to control, only when trials that followed the cold infusion protocol were excluded. However, the effect size was not as large as the trials that did not use tracers, which may in part be attributed to the methodology for evaluating glucose disposal. Glucose infusion accounts for the suppression of HGP from basal levels, that could arise from the intervention. For example, when insulin is infused at 40 mU/m2/min during the clamp it is unlikely to completely suppress HGP in adults with insulin resistance [65, 66]. If basal HGP is 2 mg/kg/min and the insulin infusion results in suppression of HGP to 1 mg/kg/min without an increase in glucose disposal, glucose infusion at 1 mg/kg/min would be needed to maintain euglycemia. If glucose disposal also increased by 1 mg/kg/min then glucose infusion would need to be 2 mg/kg/min. Therefore, glucose infusion = ∆glucose disposal + ∆HGP. The trials that do not use tracers report glucose infusion which accounts for changes in hepatic insulin action without adding HGP which is expected to reduce at the end of an intervention (Table 3). Since glucose infusion = ∆glucose disposal + ∆HGP, our analysis raises the question as to whether adding HGP to glucose infusion is necessary to calculate the overall effect of the intervention on insulin action. This anomaly in the gold standard for measuring insulin action where a less comprehensive method provides a more intuitive result, warrants resolution.
This review is the first to summarize findings relating to the effect of exercise training on glucose disposal. We were also able to synthesize the findings on hepatic insulin sensitivity assessed as insulin-mediated suppression of HGP. A key strength of this review is that we evaluated studies that used the gold standard technique for assessing insulin action. Given the controlled settings for conduct of the trials, the evidence supporting the effect of exercise training on insulin action, has high certainty. However, some considerations are noteworthy. In studies included in our meta-analysis, disparate methods were adopted in the administration of the clamp particularly in the use of tracers. Trials included in our analysis used an array of measures to report glucose disposal, such as per unit of body weight or per unit of fat free mass/skeletal muscle, or normalized to blood insulin concentrations, which made comparison between trials difficult. Therefore, we chose to focus on the SMD, and the statistical interpretation of the association between exercise and insulin action. Furthermore, variables such as the timing of the test following the last exercise bout and insulin infusion rate were not standardized. Lastly, we noted some consistent methodological weaknesses throughout the literature, such as small sample sizes, poor reporting of both adverse events and compliance with the intervention, and failure to follow intent-to-treat analytic strategies. However, there was no evidence of publication bias and overall, the trials were of high quality which facilitated a fair interpretation of our results.
In conclusion, exercise for at least four weeks is associated with improved insulin action in adults, compared to control. Since the exercise-stimulated increase in glucose disposal largely occurs in skeletal muscle, sufficient weight loss may be necessary before the effects of exercise extend to the liver and adipose tissue. While aerobic exercise is an important predictor of the improvement in insulin action, the duration and intensity of exercise do not influence the effect of exercise training on glucose disposal. Distinct methods produce distinct results, which is important for the field to recognize when designing and interpreting trials using the clamp. Therefore, our results highlight the need for evaluating and harmonizing methods for the clamp to enable a better understanding of the effects of exercise on insulin action.
Data availability
The protocol for the study (CRD42019124381) is publicly available at: https://www.crd.york.ac.uk/prospero/. The datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP.The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization.Diabetes. 1981;30:1000–7.
Mitrakou A, Kelley D, Veneman T, Jenssen T, Pangburn T, Reilly J, et al. Contribution of abnormal muscle and liver glucose metabolism to postprandial hyperglycemia in NIDDM. Diabetes. 1990;39:1381–90.
Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98:2133–223.
Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA. San Antonio metabolisms. Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia. 2004;47:31–9.
Smith GI, Polidori DC, Yoshino M, Kearney ML, Patterson BW, Mittendorfer B, et al. Influence of adiposity, insulin resistance, and intrahepatic triglyceride content on insulin kinetics. J Clin Invest. 2020;130:3305–14.
Sylow L, Tokarz VL, Richter EA, Klip A. The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab. 2021;33:758–80.
DiMenna FJ, Arad AD. Exercise as ‘precision medicine’ for insulin resistance and its progression to type 2 diabetes: a research review. BMC Sports Sci Med Rehabil. 2018;10:21.
Ross R. Does exercise without weight loss improve insulin sensitivity? Diabetes Care. 2003;26:944–5.
O’Gorman DJ, Karlsson HK, McQuaid S, Yousif O, Rahman Y, Gasparro D, et al. Exercise training increases insulin-stimulated glucose disposal and GLUT4 (SLC2A4) protein content in patients with type 2 diabetes. Diabetologia. 2006;49:2983–92.
Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C. Physical activity/exercise and type 2 diabetes. Diabetes Care. 2004;27:2518–39.
Maarbjerg SJ, Sylow L, Richter EA. Current understanding of increased insulin sensitivity after exercise - emerging candidates. Acta Physiol (Oxf). 2011;202:323–35.
Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of training on the dose-response relationship for insulin action in men. J Appl Physiol (1985). 1989;66:695–703.
Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286:1218–27.
Fatone C, Guescini M, Balducci S, Battistoni S, Settequattrini A, Pippi R, et al. Two weekly sessions of combined aerobic and resistance exercise are sufficient to provide beneficial effects in subjects with Type 2 diabetes mellitus and metabolic syndrome. J Endocrinol Invest. 2010;33:489–95.
Kanaley JA, Colberg SR, Corcoran MH, Malin SK, Rodriguez NR, Crespo CJ, et al. Exercise/physical activity in individuals with type 2 diabetes: a consensus statement from the American College of Sports Medicine. Med Sci Sports Exerc. 2022;54:353–68.
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23.
Finegood DT, Bergman RN, Vranic M. Modeling error and apparent isotope discrimination confound estimation of endogenous glucose production during euglycemic glucose clamps. Diabetes. 1988;37:1025–34.
Engin B, Willis SA, Malaikah S, Sargeant JA, Yates T, Gray LJ, et al. The effect of exercise training on adipose tissue insulin sensitivity: a systematic review and meta-analysis. Obes Rev. 2022;23:e13445.
Medicine ACoS. ACSM’s Guidelines for Exercise Testing and Prescription. Philadelphia: Lippincott Williams & Wilkins; 2021.
Livingston EH, Lee S. Body surface area prediction in normal-weight and obese patients. Am J Physiol Endocrinol Metab. 2001;281:E586–91.
Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–21.
Cashin AG, McAuley JH. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J Physiother. 2020;66:59.
Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7:105–25.
Lipsey MW, Wilson DB. Practical Meta-Analysis vol. 49. Sage Publications: California, 2001.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Hedges LV, Higgins JPT, Rothstein HR, Borenstein M. Introduction to Meta-Analysis, First edn Wiley: New Jersey, 2009.
Marcus RL, Lastayo PC, Dibble LE, Hill L, McClain DA. Increased strength and physical performance with eccentric training in women with impaired glucose tolerance: a pilot study. J Womens Health (Larchmt). 2009;18:253–60.
Joseph LJ, Trappe TA, Farrell PA, Campbell WW, Yarasheski KE, Lambert CP, et al. Short-term moderate weight loss and resistance training do not affect insulin-stimulated glucose disposal in postmenopausal women. Diabetes Care. 2001;24:1863–9.
Wagner H, Degerblad M, Thorell A, Nygren J, Stahle A, Kuhl J, et al. Combined treatment with exercise training and acarbose improves metabolic control and cardiovascular risk factor profile in subjects with mild type 2 diabetes. Diabetes Care. 2006;29:1471–7.
Andersson A, Sjodin A, Olsson R, Vessby B. Effects of physical exercise on phospholipid fatty acid composition in skeletal muscle. Am J Physiol. 1998;274:E432–8.
Shojaee-Moradie F, Baynes KC, Pentecost C, Bell JD, Thomas EL, Jackson NC, et al. Exercise training reduces fatty acid availability and improves the insulin sensitivity of glucose metabolism. Diabetologia. 2007;50:404–13.
Stener-Victorin E, Baghaei F, Holm G, Janson PO, Olivecrona G, Lonn M, et al. Effects of acupuncture and exercise on insulin sensitivity, adipose tissue characteristics, and markers of coagulation and fibrinolysis in women with polycystic ovary syndrome: secondary analyses of a randomized controlled trial. Fertil Steril. 2012;97:501–8.
Christensen B, Nellemann B, Larsen MS, Thams L, Sieljacks P, Vestergaard PF, et al. Whole body metabolic effects of prolonged endurance training in combination with erythropoietin treatment in humans: a randomized placebo controlled trial. Am J Physiol Endocrinol Metab. 2013;305:E879–89.
Otten J, Stomby A, Waling M, Isaksson A, Soderstrom I, Ryberg M, et al. A heterogeneous response of liver and skeletal muscle fat to the combination of a Paleolithic diet and exercise in obese individuals with type 2 diabetes: a randomised controlled trial. Diabetologia. 2018;61:1548–59.
Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J Alzheimers Dis. 2010;22:569–79.
Arad AD, DiMenna FJ, Thomas N, Tamis-Holland J, Weil R, Geliebter A, et al. High-intensity interval training without weight loss improves exercise but not basal or insulin-induced metabolism in overweight/obese African American women. J Appl Physiol (1985). 2015;119:352–62.
Snel M, Gastaldelli A, Ouwens DM, Hesselink MK, Schaart G, Buzzigoli E, et al. Effects of adding exercise to a 16-week very low-calorie diet in obese, insulin-dependent type 2 diabetes mellitus patients. J Clin Endocrinol Metab. 2012;97:2512–20.
Brochu M, Malita MF, Messier V, Doucet E, Strychar I, Lavoie JM, et al. Resistance training does not contribute to improving the metabolic profile after a 6-month weight loss program in overweight and obese postmenopausal women. J Clin Endocrinol Metab. 2009;94:3226–33.
Bogardus C, Ravussin E, Robbins DC, Wolfe RR, Horton ES, Sims EA. Effects of physical training and diet therapy on carbohydrate metabolism in patients with glucose intolerance and non-insulin-dependent diabetes mellitus. Diabetes. 1984;33:311–8.
Yarasheski KE, Cade WT, Overton ET, Mondy KE, Hubert S, Laciny E, et al. Exercise training augments the peripheral insulin-sensitizing effects of pioglitazone in HIV-infected adults with insulin resistance and central adiposity. Am J Physiol Endocrinol Metab. 2011;300:E243–51.
Dengel DR, Pratley RE, Hagberg JM, Rogus EM, Goldberg AP. Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J Appl Physiol (1985). 1996;81:318–25.
Coker RH, Hays NP, Williams RH, Brown AD, Freeling SA, Kortebein PM, et al. Exercise-induced changes in insulin action and glycogen metabolism in elderly adults. Med Sci Sports Exerc. 2006;38:433–8.
DiPietro L, Dziura J, Yeckel CW, Neufer PD. Exercise and improved insulin sensitivity in older women: evidence of the enduring benefits of higher intensity training. J Appl Physiol (1985). 2006;100:142–9.
Reichkendler MH, Rosenkilde M, Auerbach PL, Agerschou J, Nielsen MB, Kjaer A, et al. Only minor additional metabolic health benefits of high as opposed to moderate dose physical exercise in young, moderately overweight men. Obesity (Silver Spring). 2014;22:1220–32.
Poehlman ET, Dvorak RV, DeNino WF, Brochu M, Ades PA. Effects of resistance training and endurance training on insulin sensitivity in nonobese, young women: a controlled randomized trial. J Clin Endocrinol Metab. 2000;85:2463–8.
Cuff DJ, Meneilly GS, Martin A, Ignaszewski A, Tildesley HD, Frohlich JJ. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care. 2003;26:2977–82.
Coker RH, Williams RH, Yeo SE, Kortebein PM, Bodenner DL, Kern PA, et al. The impact of exercise training compared to caloric restriction on hepatic and peripheral insulin resistance in obesity. J Clin Endocrinol Metab. 2009;94:4258–66.
Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12:789–98.
Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133:92–103.
Nordby P, Auerbach PL, Rosenkilde M, Kristiansen L, Thomasen JR, Rygaard L, et al. Endurance training per se increases metabolic health in young, moderately overweight men. Obesity (Silver Spring). 2012;20:2202–12.
Davidson LE, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med. 2009;169:122–31.
Richter EA, Garetto LP, Goodman MN, Ruderman NB. Enhanced muscle glucose metabolism after exercise: modulation by local factors. Am J Physiol. 1984;246:E476–82.
Wojtaszewski JF, Hansen BF, Gade, Kiens B, Markuns JF, Goodyear LJ, et al. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes. 2000;49:325–31.
Sjoberg KA, Frosig C, Kjobsted R, Sylow L, Kleinert M, Betik AC, et al. Exercise increases human skeletal muscle insulin sensitivity via coordinated increases in microvascular perfusion and molecular signaling. Diabetes. 2017;66:1501–10.
Miinea CP, Sano H, Kane S, Sano E, Fukuda M, Peranen J, et al. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J. 2005;391:87–93.
Richter EA, Sylow L, Hargreaves M. Interactions between insulin and exercise. Biochem J. 2021;478:3827–46.
Prior SJ, Goldberg AP, Ortmeyer HK, Chin ER, Chen D, Blumenthal JB, et al. Increased skeletal muscle capillarization independently enhances insulin sensitivity in older adults after exercise training and detraining. Diabetes. 2015;64:3386–95.
De Glisezinski I, Crampes F, Harant I, Berlan M, Hejnova J, Langin D, et al. Endurance training changes in lipolytic responsiveness of obese adipose tissue. Am J Physiol. 1998;275:E951–6.
Ziogas GG, Thomas TR, Harris WS. Exercise training, postprandial hypertriglyceridemia, and LDL subfraction distribution. Med Sci Sports Exerc. 1997;29:986–91.
Ryan BJ, Schleh MW, Ahn C, Ludzki AC, Gillen JB, Varshney P, et al. Moderate-intensity exercise and high-intensity interval training affect insulin sensitivity similarly in obese adults. J Clin Endocrinol Metab. 2020;105.e2941-59.
Winn NC, Liu Y, Rector RS, Parks EJ, Ibdah JA, Kanaley JA. Energy-matched moderate and high intensity exercise training improves nonalcoholic fatty liver disease risk independent of changes in body mass or abdominal adiposity - a randomized trial. Metabolism. 2018;78:128–40.
Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591–601.
Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–8.
Solomon TP, Haus JM, Kelly KR, Cook MD, Filion J, Rocco M, et al. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr. 2010;92:1359–68.
Solomon TP, Haus JM, Kelly KR, Cook MD, Riccardi M, Rocco M, et al. Randomized trial on the effects of a 7-d low-glycemic diet and exercise intervention on insulin resistance in older obese humans. Am J Clin Nutr. 2009;90:1222–9.
Acknowledgements
This work was supported in part by a grant from the National Institute on Aging (5K99AG065419-02), the National Cancer Institute (R00 CA218603), the National Institute of General Medical Sciences which funds the Louisiana Clinical and Translational Science Center (U54 GM104940), and the National Institute of Diabetes and Digestive and Kidney Diseases (T32 DK064584) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
National Institute on Aging.
Author information
Authors and Affiliations
Contributions
CJR: conceptualization of research question, methodology, screening and extraction of data, interpretation of data, writing—original draft preparation, review and editing; DZ: Analysis and interpretation of data, and writing—review and editing; JPK; interpretation of data, and writing—review and editing ACL: screening and extraction of data, interpretation of data, writing—review and editing CJE: screening and extraction of data, interpretation of data, writing—review and editing; CLK: methodology, writing—review and editing; LCS: methodology, writing—review and editing; FLG: interpretation, writing—review and editing; WDJ: analysis and interpretation of data, writing—review and editing; JCB: conceptualization of research question, methodology, analysis and interpretation of data, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rebello, C.J., Zhang, D., Kirwan, J.P. et al. Effect of exercise training on insulin-stimulated glucose disposal: a systematic review and meta-analysis of randomized controlled trials. Int J Obes 47, 348–357 (2023). https://doi.org/10.1038/s41366-023-01283-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-023-01283-8
- Springer Nature Limited