Abstract
Background
Although the benefits of physical activity (PA) at an early age are well established, there is no robust evidence of the role of PA as well as its intensities in attenuating the association between weight status and metabolic risk among adolescents. In this investigation, we analyzed the association between weight status, intensities of PA, and metabolic risk among adolescents.
Methods
Data from six cross-sectional studies in the International Children’s Accelerometry Database were used (N = 5216 adolescents; boys 14.6 ± 2.1 years and girls 14.7 ± 2.0 years). Weight status was assessed and classified according to body mass index. Fasting glucose, triglycerides, inverse high-density lipoprotein cholesterol, and blood pressure composed the metabolic risk indicator (z-score). PA was measured by accelerometers. The estimated age of peak height velocity was used as a covariate for somatic maturation.
Results
We observed that increase in weight status showed a strong positive relationship with metabolic risk. However, adolescents with overweight or obesity in the highest tertile of PA (moderate-to-vigorous and vigorous intensity) showed a similar metabolic risk score as the normal weight groups. Moderate intensity PA seemed related to metabolic risk even within some categories of vigorous PA.
Conclusions
We conclude that PA attenuates the metabolic risk of adolescents with overweight or obesity. Although this attenuation is largely explained by vigorous PA, moderate intensity seems also important for better metabolic profile.
Similar content being viewed by others
Introduction
The childhood obesity pandemic has changed the profile of chronic disease among children and adolescents [1]. There is increasing attention on the assessment of metabolic risk factors such as homeostatic model assessment of insulin resistance (HOMA-IR), blood pressure, fasting glucose, triglycerides, and high-density lipoprotein cholesterol (HDL-C) during early ages [2, 3]. In a comprehensive sample of 4581 participants of the International Children’s Accelerometry Database (ICAD), Kuzik et al. [2] found that 45% of children and adolescents presented with at least one of these metabolic risk factors. Thus, both the prevention and treatment of early risk factors in children and adolescents should be considered as priorities.
Physical activity (PA) is well established as a key determinant in the prevention and treatment of childhood obesity and early metabolic risk factors [2, 4]. However, unlike in adults [5, 6], the protective effect of PA in attenuating the association between weight status and metabolic risk indicators in youth is unclear. Although PA seems to have a positive influence on inflammatory markers associated with overweight and obesity during adolescence [7], little robust evidence is available in this context. Among the gaps, few studies adopted objective measures of PA, limiting their ability to assess the potential effects of different intensities of PA on metabolic health [2]. Also, maturation plays an important role on metabolic profile especially during adolescence and not accounting for its influence on metabolic markers can confound data interpretation [8, 9].
Identifying the role of PA and its specific intensities on metabolic risk indicators for normal weight, but especially for children and adolescents with overweight or obesity, can provide information for future international recommendations and for clinical practice regarding the prevention and treatment of early metabolic risk factors in early life. Thus, we analyzed the association between weight status, intensities of PA, and metabolic risk among adolescents, with special consideration to the role of PA in attenuating the positive association between obesity and metabolic risk among adolescents.
Methods
Design
The International Children’s Accelerometry Database (ICAD) (http://www.mrc-epid.cam.ac.uk/research/studies/icad) has the aim of pooling data on both cross-sectional and longitudinal PA studies conducted among children and adolescents worldwide. More information about the study process has been previously described elsewhere [10]. Briefly, the dataset has pooled objectively measured ActiGraph accelerometer data (ActiGraph, LLC, Pensacola, Florida). This data set used standardized data reduction techniques on 46,131 raw ActiGraph data files between 2008 and 2010 [10]. Moreover, data of sociodemographic, anthropometric, and cardiometabolic factors were also pooled when available. Participants’ age ranged from 3 to 18 years. For the present study, we used data from six cross-sectional studies from five countries: the ALSPAC (England), the EYHS Denmark (Denmark), the EYHS Estonia (Estonia), the EYHS Portugal (Portugal), the NHANES 2003–04 (United States of America), and the NHANES 2005–06 (United States of America).
Sample
The initial sample was composed of 21,667 adolescents with complete accelerometer data: the ALSPAC (n = 12,746), the EYHS Denmark (n = 2045), the EYHS Estonia (n = 660), the EYHS Portugal (n = 1356), the NHANES 2003–04 (n = 2372), and the NHANES 2005–06 (n = 2488). Due to missing data on sociodemographic, anthropometry, metabolic variables, as well as invalid accelerometer data, the final (included) sample was composed of 5216 adolescents (2730 girls): the ALSPAC (n = 1588), the EYHS Denmark (n = 1452), the EYHS Estonia (n = 421), the EYHS Portugal (n = 596), the NHANES 2003–04 (n = 602), and the NHANES 2005–06 (n = 557), with age ranging from 10 to 17 years of age. Ethical approval for all studies was obtained from the local ethics committees, including the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees.
Metabolic risk score
Fasting blood glucose, triglycerides, and high-density lipoprotein cholesterol (HDL-C) were measured following a fasting period of at least 12 h using standard clinical procedures previously described, with limited between-study variation [10, 11]. Systolic and diastolic blood pressure were measured using manual and automatic methods. As outcome, we created a continuous metabolic risk score, in which, z-scores from the above-mentioned metabolic variables were created according to sex and chronological age (year) [12]. This indicator is widely accepted as an indicator of cardiovascular risk among adolescents [13]. After this, all indicators were summed to create the indicator of metabolic risk score, given that HDL-C was multiplied per-1 (inverse) and mean of blood pressure z-scores (SBPz-score + DBPz-score/2) was included in the sum as shown in the following equation:
Waist circumference was not included in the overall indicator of metabolic risk due to its close association with BMI, which could confound the results. A detailed description of these procedures has been described previously [10, 11].
Body mass index
Body mass index (BMI) was used to indicate weight status, using values of stature (expressed as m) and body mass (expressed as kg). The values provided by Cole et al. [14] were used to classify the participants as normal weight, overweight or obese, which include specific cutoff points for each age group and sex.
Physical activity (PA)
PA was collected using different Actigraph accelerometers. The ALSPAC study used the Actigraph models 7164, 71256, GT1M; the EYHS Denmark, Estonia, and Portugal study used the Actigraph model 7164; and the NHANES 2003–04 as well as the 2005–06 studies used the Actigraph model GT1M. All studies adopted the placement at the hip protocol. Moreover, the ALSPAC and both NHANES cohorts adopted a protocol of 7 consecutive days of wearing the accelerometer, while the EYHS Denmark, Estonia, and Portugal studies adopted the 4 consecutive days (2 weekdays and 2 weekend days) wearing protocol. Aiming to standardize the procedures, ICAD reanalyzed PA data from accelerometers using 60 s epochs. Non-wear time was considered as 60 min of consecutive zeros with tolerance of 2 min of nonzero epochs. Moreover, aiming to minimize the missing data, we adopted the cutoff point of 500 min per day as a valid day of measurement and a minimum of one valid day per week. To classify sedentary time and intensities of PA (moderate [MPA], vigorous [VPA], and moderate-to-vigorous [MVPA]), we used the cut-points provided by Evenson et al. [15]. A detailed description of how PA measures were pooled has been described previously [10].
Confounders
Chronological age, peak height velocity, cohort, accelerometer wear time, and mothers’ educational status were adopted as confounders. The age of peak height velocity was estimated using a logarithm that included chronological age and stature provided by Moore et al. [16].
Statistical procedures
Characteristics of the sample were described using means and 95% confidence intervals, which were used to compare characteristics between groups [17]. Linear regression models adjusted by accelerometer wear time, sedentary time, study, chronological age, and age at peak height velocity were used to analyze the independent associations between intensities of PA and BMI in predicting metabolic risk. The joint association of weight status and tertiles of PA intensities in predicting metabolic risk score was analyzed by ANCOVA using confidence intervals for differences between groups. All analyzes were conducted in STATA 15.1, adopting statistical significance as p < 0.05.
Results
From the initial sample (n = 21,667), only 5216 adolescents presented blood variables from six different studies: the ALSPAC (n = 1588), the EYHS Denmark (n = 1452), the EYHS Estonia (n = 421), the EYHS Portugal (n = 596), the NHANES 2003–04 (n = 602), and the NHANES 2005–06 (n = 557). Characteristics of the included and excluded sample are presented in Table 1. Included participants were relatively older, presented lower PA levels and higher sedentary time. However, the proportion of sex, weight status, and mother’s education were similar between the included and excluded sample.
Characteristics of the included sample are presented in Table 2. Girls presented lower PA levels, younger age of peak height velocity, smaller waist circumference, and lower fasting glucose and systolic blood pressure compared with boys (p < 0.05). On the other hand, girls had higher BMI, diastolic blood pressure, HDL-C and triglycerides (p < 0.05).
Joint associations between PA intensities (VPA and MVPA) and BMI in predicting metabolic risk score are presented in Table 3. Among the models, both PA and BMI were associated with metabolic risk score. The association between VPA and metabolic risk as well as MVPA and metabolic risk were similar in both sexes. However, the inclusion of PA in the models did not contribute much to the variance in metabolic risk score already explained by BMI.
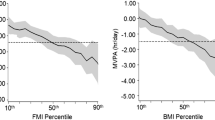
Metabolic risk score according to tertiles of PA (MVPA and VPA) and BMI status are presented in Fig. 1. The group of participants with obesity in the first tertile of both MVPA and VPA presented the most adverse values of metabolic risk. Boys with normal weight in the first tertile of MVPA showed higher metabolic risk compared with their counterparts of the third tertile. The gradual association between mutually exclusive categories of PA/weight status and metabolic risk was clearer for boys, however, the third tertile of PA appeared to be a protective factor in both sexes regardless of weight status. Moreover, girls with overweight in the second tertile of PA showed similar metabolic risk of their counterparts with normal weight.
The association of MPA and body mass index with metabolic risk score according to weight status and tertiles of VPA is presented in Table 4. Body mass index was significantly associated with metabolic risk score among all weight status and tertiles of VPA. MPA was associated with metabolic risk in the tertiles one and three of VPA among boys, as well as in the intermediary tertile among girls.
Discussion
Our main finding was that a lower metabolic risk in adolescents with overweight and obese in the highest tertiles of PA was similar to the metabolic risk of their counterparts with normal weight. We also observed that VPA seemed to be strongly associated with this modeling effect. Moderate PA also explained variations in metabolic risk, reinforcing international guidelines, which suggest 150 min/week of MVPA for health. To our knowledge, this is the first study that analyzed this association and the plausible attenuation effect of PA on metabolic risk using a comprehensive sample with device-based monitoring of PA.
Overweight and obesity are associated with several negative health outcomes even among adolescents, including cardiovascular diseases [2, 5]. Several mechanisms may explain the positive association observed between BMI and metabolic risk. Excess adipose tissue, that often goes along with increased BMI, results in the increased secretion of free fatty acids [18], reduction of adiponectin [19], an increase of inflammatory markers such as IL-6 and CRP [7], and an increase in insulin resistance, all of which contribute to the hyperlipidemia, hypertension, and glucose intolerance components of the metabolic syndrome [20]. PA, on the contrary, is associated with a reduced metabolic risk [11] through the reduction of inflammatory factors [7] and reduction of adiposity [21].
Our results suggest that, overweight/obesity is associated with metabolic risk among adolescents, but PA is capable to attenuate this association. Adolescents with overweight and obesity, in the highest tertile of PA showed reduced metabolic risk compared with counterparts with normal weight. A previous investigation from the ICAD database found that MVPA was only positively associated with metabolic risk (presence of at least one metabolic risk factor) among children and adolescents with normal weight [2], which is somewhat distinct from our findings. This difference may be explained by differences in the analytical procedures used (categorical vs continuous outcomes) and by differences in the age range of participants included (children + adolescents vs adolescents only). In addition, we opted to adjust the analyses for biological maturation, which is an important factor to be considered for both PA and cardiovascular risk in this age group (adolescence) [8, 9]. Thus, it is possible that PA provides more benefit for groups with overweight and obese with advancing age.
The observed risk attenuation by PA was clearer for boys. This result can be explained in part by the lower level of general PA [22] and VPA among girls with overweight or obese compared with boys, potentially due to the effects of obesity on motor competence and difficulty with movement of their higher body mass [23, 24]. Here, for example, girls in the third tertile showed significantly less MVPA (girls: ~67 min/day vs boys: ~94 min/day) and VPA (girls: ~23 min/day vs boys: ~36 min/day) than boys. This also suggests that higher amounts of PA are needed for metabolic risk attenuation among adolescents with overweight and obesity.
Interestingly, we observed similar results when we compared models with MVPA and VPA. Although there is clear evidence that more intense PA provides additional benefits for health [25], our findings suggest that even MPA seems important for a better metabolic profile. This may have a special practical implication, as adolescents, especially those who are overweight and obese, are more likely to take part in and adhere to PA programs of lighter intensity (i.e., MPA instead of VPA) [26].
The potential attenuation effect of PA in the association between weight status and metabolic risk can occur through different mechanisms. Both PA and obesity present convergent mechanisms to metabolic risk factors such as inflammation [7]. PA is associated with a reduction of inflammatory factors, while obesity is associated with increased inflammatory factors. Moreover, PA improves adiponectin levels while obesity decreases them [19, 27]. In this sense, PA should be promoted for the prevention of cardiovascular diseases, even without the reduction of body weight.
Some limitations of the current study should be mentioned. First, we considered a minimum of one day of valid wear time in the present study, which can present bias as one day may not be representative of usual habitual PA. Second, the different models of accelerometers used in each study could represent possible variations between-study outcomes. Third, the large number of missing data on metabolic variables must be highlighted, being a potential selection bias for the included sample. The main difference between the included and excluded sample was on chronological age, PA level, and sedentary time. However, age-adjusted estimates such as BMI and mother educational status (a socioeconomic proxy) were not different between the included and excluded sample. Fourth, the use of a continuous outcome has operational advantages, but it is also relevant to recognize that approaches adopting a categorical diagnosis of metabolic syndrome have its advantages as well. Finally, the lack of control of other potential metabolic risk determinants (e.g., energy intake), and the cross-sectional design preclude evidence of causality from the interpretation of the findings. However, the comprehensive sample with device-based monitoring of PA from four different countries represents the main strength of the study. The understanding of the potential effects of different amounts and intensities of PA on health is only possible through objective/device-based measurements. Moreover, we adjusted the analyses for somatic maturation, which is an important potential confounder because of its association with metabolic risk factors, adiposity, and PA [8, 9].
Conclusions
In conclusion, we found that PA attenuates metabolic risk of adolescents with overweight and obesity. Although VPA seems to explain a great part of this attenuation, MPA also appears important for better metabolic profile. During growth and development, adolescents with overweight and more PA show, in general, similar metabolic risk to their counterparts with normal weight.
References
Han JC, Lawlor DA, Kimm SY. Childhood obesity. The Lancet. 2010;375:1737–48.
Kuzik N, Carson V, Andersen LB, Sardinha LB, Grøntved A, Hansen BH, et al. Physical activity and sedentary time associations with metabolic health across weight statuses in children and adolescents. Obesity. 2017;25:1762–9.
Kuschnir MCC, Bloch KV, Szklo M, Klein CH, Barufaldi LA, De Azevedo Abreu G, et al. ERICA: prevalence of metabolic syndrome in Brazilian adolescents. Revista de Saude Publica. 2016;50:1s–3s.
Moore JB, Beets MW, Brazendale K, Blair SN, Pate RR, Andersen LB, et al. Associations of vigorous-intensity physical activity with biomarkers in youth. Med Sci Sports Exerc. 2017;49:1366–74.
Werneck AO, Oyeyemi AL, Gerage AM, Cyrino ES, Szwarcwald CL, Sardinha LB, et al. Does leisure-time physical activity attenuate or eliminate the positive association between obesity and high blood pressure? J Clin Hypertens. 2018;20:959–66.
Loprinzi P, Smit E, Lee H, Crespo C, Andersen R, Blair SN. The “fit but fat” paradigm addressed using accelerometer-determined physical activity data. N Am J Med Sci. 2014;6:295–301.
Rubin DA, Hackney AC. Inflammatory cytokines and metabolic risk factors during growth and maturation: influence of physical activity. Med Sport Sci. 2010;55:43–55.
Werneck AO, Silva DR, Collings PJ, Fernandes RA, Ronque ERV, Barbosa DS, et al. Biological maturation, central adiposity, and metabolic risk in adolescents: a mediation analysis. Child Obesity. 2016;12:377–83.
Cumming SP, Sherar LB, Pindus DM, Coelho-e-Silva MJ, Malina RM, Jardine PR. A biocultural model of maturity-associated variance in adolescent physical activity. School Health. 2012;5:37–41.
Sherar LB, Griew P, Esliger DW, Cooper AR, Ekelund U, Judge K, et al. International children’s accelerometry database (ICAD): design and methods. BMC Public Health. 2011;11:485.
Ekelund U, Luan J, Sherar LB, Esliger DW, Griew P, Cooper A, et al. Moderate to vigorous physical activity and sedentary time and cardiometabolic risk factors in children and adolescents. JAMA. 2012;307:704–12.
Eisenmann JC. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc Diabetol. 2008;7:17.
Andersen LB, Harro M, Sardinha LB, Froberg K, Ekelund U, Brage S, et al. Physical activity and clustered cardiovascular risk in children: a cross-sectional study (The European Youth Heart Study). Lancet. 2006;368:6.
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ (Clin Res Ed). 2000;320:1240–3.
Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci. 2008;26:1557–65.
Moore SA, McKay HA, Macdonald H, Nettlefold L, Baxter-Jones ADG, Cameron N, et al. Enhancing a somatic maturity prediction model. Med. Sci. Sports Exerc. 2015;47:1755–64.
Gardner MJ, Altman DG. Confidence intervals rather than P values: estimation rather than hypothesis testing. Br Med J (Clin Res Ed). 1986;292:746–50.
Frohnert BI, Jacobs DR, Steinberger J, Moran A, Steffen LM, Sinaiko AR. Relation between serum free fatty acids and adiposity, insulin resistance, and cardiovascular risk factors from adolescence to adulthood. Diabetes. 2013;62:3163–9.
Simpson J, Smith ADAC, Fraser A, Sattar N, Lindsay RS, Ring SM, et al. Programming of adiposity in childhood and adolescence: associations with birth weight and cord blood adipokines. J Clin Endocrinol Metabol. 2017;102:499–506.
Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74.
Tarp J, Bugge A, Andersen LB, Sardinha LB, Ekelund U, Brage S, et al. Does adiposity mediate the relationship between physical activity and biological risk factors in youth? A cross-sectional study from the International Children’s Accelerometry Database (ICAD). Int J Obesity. 2017. [Epub ahead of print]. https://doi.org/10.1038/ijo.2017.241.
Werneck AO, Oyeyemi AL, Fernandes RA, Romanzini M, Ronque ERV, Cyrino ES, et al. Regional socioeconomic inequalities in physical activity and sedentary behavior among brazilian adolescents. J Phys Activity Health. 2018;15:338–44.
Spees CK, Scott JM, Taylor CA. Differences in amounts and types of physical activity by obesity status in US adults. Am J Health Behav. 2012;36:56–65.
Augustijn MJCM, DʼHondt E, Van Acker L, De Guchtenaere A, Lenoir M, Caeyenberghs K, et al. Role of motor competence and executive functioning in weight loss. J Dev Behav Pediatr. 2018;39:642–51.
Howard B, Winkler EAH, Sethi P, Carson V, Ridgers ND, Salmon J, et al. Associations of low- and high-intensity light activity with cardiometabolic biomarkers. Med Sci Sports Exerc. 2015;47:2093–101.
Ekblom-Bak E, Ekblom Ö, Bergström G, Börjesson M. Isotemporal substitution of sedentary time by physical activity of different intensities and bout lengths, and its associations with metabolic risk. Eur J Prev Cardiol. 2016;23:967–74.
Martinez-Gomez D, Eisenmann JC, Gomez-Martinez S, Veses A, Romeo J, Veiga OL, et al. Associations of physical activity and fitness with adipocytokines in adolescents: the AFINOS Study. Nutr Metab Cardiovasc Dis. 2012;22:252–9.
Acknowledgements
The authors thank all participants and funders of the original studies that contributed data to the ICAD, all ICAD collaborators, and Prof. Chris Riddoch, Prof. Ken Judge, and Dr Pippa Griew. The ICAD collaborators include Prof. S. Anderssen, Norwegian School for Sport Science, Oslo, Norway (EYHS, Norway); Prof. G. Cardon, Department of Movement and Sports Sciences, Ghent University, Belgium (Belgium Preschool Study); Centers for Disease Control and Prevention, National Center for Health Statistics, Hyattsville, Maryland, USA (NHANES); Prof. A. Cooper, Centre for Exercise, Nutrition and Health Sciences, University of Bristol, Bristol, UK (Personal and Environmental Associations with Children’s Health [PEACH]); Dr R. Davey, Centre for Research and Action in Public Health, University of Canberra, Canberra, Australia (Children’s Health and Activity Monitoring for Schools [CHAMPS]); Prof. U. Ekelund, Norwegian School of Sport Sciences, Oslo, Norway, and MRC Epidemiology Unit, University of Cambridge, Cambridge, UK; Dr D.W. Esliger, School of Sports, Exercise, and Health Sciences, Loughborough University, Loughborough, UK; Dr K. Froberg, University of Southern Denmark, Odense, Denmark (EYHS, Denmark); Dr P. Hallal, Postgraduate Program in Epidemiology, Federal University of Pelotas, Pelotas, Brazil (1993 Pelotas Birth Cohort); Prof. K.F. Janz, Department of Health and Human Physiology, Department of Epidemiology, University of Iowa, Iowa City, Iowa, USA (Iowa Bone Development Study); Dr K. Kordas, School of Social and Community Medicine, University of Bristol, Bristol, UK (Avon Longitudinal Study of Parents and Children [ALSPAC]); Dr S. Kriemler, Institute of Social and Preventive Medicine, University of Z€urich, Z€urich, Switzerland (Kinder-Sportstudie [KISS]); Dr A. Page, Centre for Exercise, Nutrition and Health Sciences, University of Bristol, Bristol, UK; Prof. R. Pate, Department of Exercise Science, University of South Carolina, Columbia, South Carolina, USA (Physical Activity in Preschool Children [CHAMPS-US] and Project Trial of Activity for Adolescent Girls [Project TAAG]); Dr J.J. Puder, Service of Endocrinology, Diabetes and Metabolism, Lausanne University Hospital, University of Lausanne, Lausanne, Switzerland (Ballabeina Study); Prof. J. Reilly, Physical Activity for Health Group, School of Psychological Sciences and Health, University of Strathclyde, Glasgow, UK (Movement and Activity Glasgow Intervention in Children [MAGIC]); Prof. J. Salmon, School of Exercise and Nutrition Sciences, Deakin University, Melbourne, Australia (Children Living in Active Neigbourhoods [CLAN]); Dr L.B. Sherar, School of Sports, Exercise and Health Sciences, Loughborough University, Loughborough, UK; Dr A. Timperio, Centre for Physical Activity and Nutrition Research, Deakin University, Melbourne, Australia (Healthy Eating and Play Study [HEAPS]); Dr E.M.F. van Sluijs, MRC Epidemiology Unit, University of Cambridge, Cambridge, UK (Sport, Physical activity and Eating behavior: Environmental Determinants in Young people [SPEEDY]). The UK Medical Research Council and the Wellcome Trust (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. Moreover, we acknowledge São Paulo Research Foundation (FAPESP) for the master’s degree scholarship of AOW (FAPESP process: 2017/27234–2).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Werneck, A.O., Silva, D.R., Oyeyemi, A.L. et al. Physical activity attenuates metabolic risk of adolescents with overweight or obesity: the ICAD multi-country study. Int J Obes 44, 823–829 (2020). https://doi.org/10.1038/s41366-020-0521-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-020-0521-y
- Springer Nature Limited
This article is cited by
-
Associations of fitness, motor competence, and adiposity with the indicators of physical activity intensity during different physical activities in children
Scientific Reports (2021)
-
Structural equation model of the effect of biological maturation on metabolic syndrome risk and C-reactive protein: effect of trunk fat and sports participation
Scientific Reports (2021)
-
How do short-term associations between diet quality and metabolic risk vary with age?
European Journal of Nutrition (2021)