Abstract
Background/Objectives
Fat mass development in infancy contributes to later adiposity, but its relation to ectopic fat depots is unknown. We examined the associations of infant subcutaneous fat with childhood general and organ-specific fat.
Subjects/Methods
Among 593 children from a population-based prospective cohort study, we obtained total subcutaneous fat mass (as sum of biceps, triceps, suprailiacal, and subscapular skinfolds thickness), central-to-total subcutaneous fat ratio (sum of suprailiacal and subscapular skinfold thickness/total subcutaneous fat) at 1.5, 6 and 24 months of age. At 10 years, we assessed BMI, fat mass index (FMI) based on total body fat by dual-energy X-ray absorptiometry, and abdominal subcutaneous, visceral and pericardial fat mass indices, and liver fat fraction by Magnetic Resonance Imaging.
Results
A higher central-to-total subcutaneous fat ratio at 1.5 months only and higher total subcutaneous fat at 6 and 24 months were associated with higher BMI, FMI and subcutaneous fat mass index at 10 years. The observed associations were the strongest between total subcutaneous fat at 24 months and these childhood outcomes (difference per 1-SDS increase in total subcutaneous fat: 0.15 SDS (95% Confidence Interval (CI) 0.08, 0.23), 0.17 SDS (95% CI 0.10, 0.24), 0.16 SDS (95% CI 0.08, 0.23) for BMI, FMI and childhood subcutaneous fat mass index, respectively). Infant subcutaneous fat measures at any time point were not associated with visceral and pericardial fat mass indices, and liver fat fraction at 10 years.
Conclusions
Our results suggest that infant subcutaneous fat is associated with later childhood abdominal subcutaneous fat and general adiposity, but not with other organ-specific fat depots.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The risk of obesity is partly established already in early life [1, 2]. Changes of adipose tissue during infancy in terms of number of adipocytes, as well as fat depot location contribute to fat mass in adulthood [3,4,5]. Rapid weight gain in infants, which correlates with increased fat deposition, is associated with increased risk of later obesity and an adverse cardiovascular profile [6,7,8]. Also, increased body mass index (BMI) tracks from early childhood into adulthood [1, 2]. However, BMI is a suboptimal indicator of fat mass and does not provide information about its distribution [9]. As different fat depots contribute differently to metabolism, body fat distribution seems to be critical for later risk of obesity related diseases [10, 11]. Available studies that assessed childhood and adulthood fat deposition at specific compartments, in particular ectopic fat, focused on its relation to early growth rather than adiposity [12]. These studies often applied imprecise measures of fat as an outcome parameter, or did not take into account adjustment for body size in these fat measures [12]. We have previously shown that infant subcutaneous fat was associated with total body fat, abdominal subcutaneous fat and preperitoneal fat assessed by ultrasound, but poorly associated with cardiovascular risk factors in children at the age of 6 years [13, 14]. It is not known whether subcutaneous fat and the tempo of its increase in particular periods within infancy relates to more specific, directly measured visceral fat, pericardial fat, and liver steatosis. These adipose tissue depots, acting systemically or locally, have been suggested to play a key role for cardiovascular disease risk, independently and additionally to overall obesity [11, 15].
Therefore, we examined in a prospective population-based cohort study among 593 children, the association of subcutaneous fat mass assessed at different time points (1.5, 6 and 24 months) during infancy with BMI, total fat, abdominal subcutaneous and visceral fat mass, pericardial fat mass and liver fat fraction, assessed by Magnetic Resonance Imaging (MRI) at the age of 10 years.
Subjects and Methods
Study design
This study was embedded in the Generation R Study, a population-based prospective cohort study from fetal life until adulthood, conducted in Rotterdam, the Netherlands [16]. The Medical Ethical Committee of the Erasmus Medical Center, Rotterdam approved the study (MEC 198.782/2001/31). All parents gave written informed consent for participation in the study [16]. Children enrolled in the study were born between April 2002 and January 2006 [16]. Our present analyses are restricted to a subgroup of Dutch children that underwent additional, more detailed assessment of fetal and postnatal growth. Of the total of 1,232 children in this group, 991 singleton children had skinfold thicknesses measured at the age of 1.5, 6 or 24 months. Finally, organ-specific fat measures assessed by MRI at the age of 10 years were available in 593 children. A flow diagram of study participants is provided in the Supplementary Materials, Figure S1
Measures of adiposity in infancy
As previously described, we measured weight to the nearest gram in naked infants at the age of 1.5 and 6 months using an electronic infant scale and at 24 months using a mechanical personal scale (SECA, Almere, The Netherlands) [13, 14]. Body length at the age of 1.5 and 6 months was measured in supine position to the nearest millimetre using a neonatometer and body height at 24 months was measured in standing position using a Harpenden stadiometer (Holtain Limited, Dyfed, UK). We measured skinfold thicknesses at the ages of 1.5, 6 and 24 months on the left side of the body at the biceps, triceps, suprailiacal, and subscapular area using a skinfold calliper (Slim Guide, Creative Health Products) according to standard procedures [14, 17]. We calculated total subcutaneous fat mass from the sum of all four skinfold thicknesses, and central subcutaneous fat mass from the sum of suprailiacal and subscapular skinfold thicknesses [14, 18, 19]. To create a measure of total subcutaneous fat mass independent of length or height and a measure of central subcutaneous fat mass independent of total subcutaneous fat mass, we estimated the optimal adjustment by log–log regression analyses [13, 20]. Details of these regressions are given in the Supplementary Materials. Total subcutaneous fat mass was only weakly correlated with length or height, and was not adjusted for, whereas a central-to-total subcutaneous fat mass ratio was calculated as central divided by total subcutaneous fat mass. Additionally, we calculated the change in the subcutaneous fat mass measures for each age interval (1.5–6, 6–24, and 1.5–24 months).
Measures of adiposity at 10 years
We measured height and weight without shoes and heavy clothing and calculated BMI (kg/m2). We calculated sex- and age- adjusted standard deviation scores (SDS) of childhood BMI based on Dutch reference growth charts (Growth Analyzer 4.0, Dutch Growth Research Foundation) [21]. We measured total body fat mass with the use of dual-energy X-ray absorptiometry (DXA) scanner (iDXA, GE-Lunar, 2008, Madi-son, WI, USA, ENCORE software v.12.6), according to standard procedures [7, 13]. As previously described, this method has been validated against computed tomography for body fat assessment in other studies [13]. We calculated a fat mass index (FMI) uncorrelated with height as total fat mass divided by height [4], following the same approach as for infant fat measures (details given in Supplementary Methods S1).
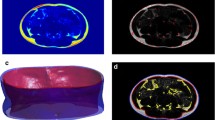
MRI has been described as an accurate and reproducible technique and considered the gold standard for the measurement of intra-abdominal and organ fat deposition [22,23,24,25]. Adiposity measures were obtained from MRI scans as described previously [16]. Briefly, all children were scanned using a 3.0 Tesla MRI (Discovery MR750w, GE Healthcare, Milwaukee, WI, USA) for body fat imaging using standard imaging and positioning protocols. They wore light clothing without metal objects while undergoing the body scan [26]. The scanner was operated by trained research technicians and all imaging data were collected according to standardized protocols. For pericardial, liver, abdominal and pelvic fat measurements four different scans were selected. Scans in the thorax, liver and abdomen were performed instructing the participant to perform breath-hold maneuvers in expiration with a maximum duration of 11 s. For imaging fat around the heart and the coronary arteries, a multi-breath-hold approach was used using an ECG triggered black-blood prepared thin slice single shot fast spin echo acquisition (BB SSFSE). The slice orientation was copied directly from the functional heart scans performed in one of the protocols of the study. In this scan, fat would appear hyper intense around the heart, the coronary arteries and heart chambers. A liver fat scan was subsequently performed using an axial volume and a special 3-point proton density weighted DIXON technique (IDEAL IQ) that could provide not only fat images of the upper abdomen but more importantly was capable of generating a precise fat fraction image demonstrating if liver fat was present [27]. The IDEAL IQ scan is based on a carefully tuned 6-echo echo planar imaging (EPI) acquisition. Abdominal fat scans followed using an axial volume comprising the lower liver, abdomen and part of the upper pelvis using a proton density weighted 2-point DIXON acquisition (LavaFlex). Finally, a high-resolution free-breathing coronally acquired scan centered at the head of the femurs was performed using a T1-weighted 2-point DIXON technique (LavaFlex). For both IDEAL IQ and LavaFlex measurements, water, fat, in-phase and out-of-phase 3D volumes were reconstructed. The obtained fat scans were subsequently analysed by the Precision Image Analysis company (PIA, Kirkland, Washington, United States). Pericardial, subcutaneous, visceral, and liver fat were quantified using the sliceOmatic (TomoVision, Magog, Canada) software package. All extraneous structures and any image artifacts were removed manually [22]. Total subcutaneous and visceral fat volumes were generated by summing the volumes of the scans. Subcutaneous and visceral fat masses were obtained by multiplying the total volumes by the specific gravity of adipose tissue, 0.9 g/ml. Pericardial fat included both epicardial- and paracardial fat directly attached to the pericardium. Pericardial fat volume was quantified using the summation of discs method and was subsequently multiplied by the specific gravity of adipose tissue, 0.9 g/ml. Liver fat fraction was determined by taking four samples of at least 4 cm2 from the central portion of the hepatic volume. Subsequently, the mean signal intensities were averaged to generate an overall mean liver fat fraction estimation.
To create MRI adiposity measures indices independent of height at 10 years, we estimated the following optimal adjustments by log–log regression analyses: subcutaneous fat mass divided by height4, visceral fat mass by height3 and pericardial fat mass by height3, described in detail in the Supplementary Methods S1 [20].
Covariates
During pregnancy, enrolled women filled out a questionnaire and reported their age, educational level, parity, smoking habits and pre-pregnancy weight. To calculate maternal pre-pregnancy BMI (kg/m2), we used self-reported information about maternal weight and we measured their height at enrolment. We obtained information on child’s sex, birth weight and gestational age at birth from medical records. Information on breastfeeding and timing of introduction of solid foods was obtained by questionnaire in infancy.
Statistical analysis
First, we assessed the associations of infant subcutaneous fat mass measures with childhood BMI, FMI and organ-specific fat mass measures (abdominal subcutaneous and visceral fat mass index, pericardial fat mass index and liver fat fraction) using linear regression models. We calculated the change in the subcutaneous fat mass measures by the differences between subcutaneous fat mass measures in different age intervals (1.5–6, 6–24, and 1.5–24 months). The obtained changes in infant subcutaneous fat measures were then used as determinants in the linear regression model to assess the associations with childhood outcomes. Second, in order to identify specific critical periods of fat mass development in infancy associated with childhood general and organ fat mass measures, we used conditional regression analyses [28, 29]. We obtained the standardized residuals from linear regression models of subcutaneous fat measures at each time point of interest regressed on all prior corresponding subcutaneous fat mass measures (Supplementary Methods S2). This enabled us to include the subcutaneous fat measures at all time points simultaneously in one model, and therefore to estimate the independent and mutually adjusted influence of the change in infant fat mass during each age period on childhood organ-specific fat measures. In our analyses we applied the following models: i) basic model adjusted for child’s sex and child’s age at MRI examination, and ii) confounder model further adjusted for maternal age, educational level, parity, smoking habits during pregnancy and pre-pregnancy BMI, child’s gestational age at birth and birthweight, information on breast-feeding and timing of introduction of solid foods. We selected confounders based on potential causal relationships between these variables, exposures and outcomes based on previous studies. We included these variables in the models when they changed the effect estimates substantially (>10%), or were strongly associated with body fat mass in our or previous studies. Further, we log-transformed the non-normally distributed childhood fat measures indices. We constructed SDS ((observed value -mean)/SD) for all continuous body fat measures at each age that enabled us to compare effect size for different exposure and outcome measures. We tested potential interactions between infant subcutaneous fat mass measures and child´s sex with adiposity measures in childhood. As no consistent interactions were observed, we did not additionally perform stratified analyses. Missing values in covariates (ranging from 0 to 15.7%) were multiple-imputed, using the Markov chain Monte Carlo approach. We performed statistical analyses with the use of SPSS version 21.0 for Windows (SPSS Inc, Chicago, IL, USA).
Results
Subject characteristics
Table 1 shows the characteristics of study participants. Of all participants, the mean (SD) birth weight was 3535 (515) g, with the median (95% range) gestational age at birth of 40.3 (36.5, 42.3) weeks. Based on non-response analysis (Supplementary Table S1), as compared to children who did not participate, those who did took part in follow-up studies were born to slightly older and higher educated mothers, were less often prenatally exposed to maternal smoking and were more often breastfed. Participants and non-participants did not differ in terms of all fat measures within infancy. Among assessed childhood outcomes, overall strong correlations were observed interchangeably between BMI, FMI, subcutaneous and visceral fat, whereas moderate and weak correlations (r < 0.6) were present between all other measures as shown in Table S2.
Infant adiposity and childhood general and organ fat
Table 2 shows that, in the basic and confounder model, higher total subcutaneous fat mass at 6 months, but not at 1.5 month, tended to be associated with higher childhood BMI, FMI, and subcutaneous fat mass index. Similarly, but more strongly, in both models higher total subcutaneous fat mass at 24 months was associated with higher childhood BMI, FMI, and subcutaneous fat mass index (differences 0.15 SDS (95% Confidence Interval (CI) 0.08, 0.23), p value < 0.001; 0.17 SDS (95% CI 0.10, 0.24), p value < 0.001; and 0.16 SDS (95% CI 0.08, 0.23), p value < 0.001, respectively, based on the confounder model). Adjusted R-squares of these models were 0.13 for BMI, 0.25 for FMI, and 0.24 for subcutaneous fat mass index. Higher total subcutaneous fat mass at 24 months tended to be associated with higher visceral fat mass index and pericardial fat mass index, but not liver fat fraction at 10 years, in the basic models. However these associations slightly attenuated and were of borderline significance after adjusting for the confounders (differences 0.08 SDS (95% CI 0.00, 0.16), p value = 0.051; and 0.07 SDS (95% CI -0.01, 0.15), p value = 0.090, respectively, based on the confounder model). In both models, no apparent associations were present between infant total subcutaneous fat mass at 1.5 and 6 months with visceral and pericardial fat mass index, and liver fat fraction at the age of 10 years. In the basic and confounder model, central-to-total subcutaneous fat mass ratio at 1.5 months, but not at 6 and 24 months, was associated with a slightly higher childhood BMI, FMI and subcutaneous fat mass index (differences 0.10 SDS (95% CI 0.02, 0.17), p value = 0.012; 0.12 SDS (95% CI 0.05, 0.19), p value < 0.001; and 0.14 SDS (95% CI 0.07, 0.21), p value < 0.001, respectively, based on the confounder model).
Changes in infant subcutaneous fat mass and childhood general and organ fat
As shown in Table 3, based on the basic and confounder model, overall a higher gain in total subcutaneous fat mass, from 1.5 to 24 months only, was associated with a slightly higher childhood BMI, FMI and subcutaneous fat mass index, but not with visceral, pericardial or liver fat.
Critical periods of fat mass development
Figure 1 shows the results from the conditional regression analyses. Infant total subcutaneous fat mass between 6 and 24 months and central-to-total fat mass ratio at 1.5 months were, independently of fat mass in other time intervals, associated with higher childhood BMI, FMI and subcutaneous fat mass index, in the basic and confounder model. Infant fat measures at no time interval were associated with visceral and pericardial fat mass, and liver fat fraction at 10 years based on the basic and confounder model.
Subcutaneous fat mass in specific periods within infancy and general and organ fat measures at the age of 10 years (n = 383) [1, 2] a Childhood body mass index. b Childhood fat mass index. c Childhood subcutaneous fat mass index. d Childhood visceral fat mass index. e Childhood pericardial fat mass index. f Childhood liver fat fraction. 1Values are regression coefficients (95% Confidence Intervals) from conditional regression models and represent differences in childhood outcomes in SDS per standardised residual change of infant subcutaneous fat in each time interval. 2Basic model is adjusted for child´s sex and child’s age at outcome measurements (except for sex- and age-adjusted BMI SDS). The confounder model additionally includes maternal age, educational level, parity, smoking habits during pregnancy, pre-pregnancy BMI, gestational age, birth weight, breastfeeding, and timing of introduction of solid foods. SDS, standard deviation scores
Discussion
In this population-based prospective cohort study, higher total subcutaneous fat at 6 and 24 months, and a higher central-to-total subcutaneous fat ratio at 1.5 months only, were associated with higher BMI, higher total body fat and higher abdominal subcutaneous fat mass at 10 years. Infant subcutaneous fat measures, as well changes in these measures, were not associated with ectopic fat depots, namely visceral, pericardial and liver fat at 10 years.
Interpretation of main findings
Adipose tissue is heterogeneous, with distinct metabolic activity of different fat compartments, that develop at specific time pre- and postnatally [5, 15]. Existing evidence on precisely measured specific fat compartments, in particular ectopic fat, in relation to early life adiposity is scarce [12]. We have shown previously among 393 children that abdominal subcutaneous fat and preperitoneal fat mass measures by ultrasound, considered as a proxy for visceral fat, track from the age of 2 to 6 years [30]. We have also shown that subcutaneous fat at 24 months of age, was positively associated with total fat mass and preperitoneal fat mass area at the age of 6 years, though poorly indicated cardiovascular risk profile of these children [13, 14].
On the basis of skinfolds thickness measurements in infancy and childhood MRI-assessed organ-specific fat, this study demonstrates that infant subcutaneous fat is positively associated with childhood BMI, total fat and abdominal subcutaneous fat. Excessive abdominal subcutaneous fat storage has important clinical implications. Accumulation of fat in this location was previously reported by us to be even more strongly associated with cardiovascular risk factors in children, than preperitoneal fat mass [31]. Also, among US adults, both visceral and subcutaneous fat were associated with metabolic risk factors independently of overall adiposity [32]. Unlike the previous report assessing preperitoneal fat mass area by ultrasound at 6 years [13], we did not observe infant subcutaneous fat to be clearly associated with visceral fat at 10 years. Yet, different fat imaging techniques, and age at outcome assessment might explain this discrepancy. Available longitudinal studies on liver fat investigated its relation to early growth only in the context of non-alcoholic fatty liver disease [33,34,35,36], whereas studies on pericardial fat are lacking. Based on Australian cohort study, skinfolds thickness measures at 1 year were not associated with the risk of non-alcoholic fatty liver disease in adolescents [36]. We also did not observe infant subcutaneous fat to be associated with liver fat, as well as pericardial fat, at the age of 10 years. Thus, taking into account previous and our study, it seems that fatness in infancy, within the meaning of subcutaneous fat, does not determine later visceral, liver or pericardial fat. As considerable increase of visceral fat starts after the age of 2 years, it is likely that periods other than infancy, play substantial role in the development of this, and other ectopic fat depots [37].
It has been underlined that the tempo of early growth rather than the levels of different anthropometric characteristics per se, plays a role in the programming of body composition. In the longitudinal study conducted in the US, rapid weight gain in infancy was associated with increased abdominal subcutaneous and visceral fat assessed by MRI among 233 adults. Though this effect did not persist when visceral fat was adjusted for total body fat [38]. Also, we previously showed that infant weight gain was associated with abdominal subcutaneous and preperitoneal fat assessed by ultrasound at the age of 6 years [7]. In contrast, several observational studies that assessed adult waist-to-hip ratio, as a proxy for visceral fat, found no apparent associations with early growth [39,40,41]. In our study, gain in total subcutaneous fat between 1.5 and 24 months, was associated with only slightly higher childhood BM, total fat and increased subcutaneous fat, while we did not observe similar association for visceral fat. Previous studies demonstrated that rates of change in adiposity between birth and 1 year of age were not associated with liver outcomes, including liver fat assessed by ultrasound, in adolescence [35, 36]. In line, changes in subcutaneous fat during infancy were not associated with later liver fat, and pericardial fat in our study. Summarizing, our findings suggest that changes in infant subcutaneous fat are unlikely to substantially influence childhood fat accumulation in other than under skin depots. As our sample mainly consisted of children born appropriate for gestational age, we can only speculate if these findings would differ in those prenatally growth restricted with later catch-up growth.
Timing is important for white adipose tissue development and setting the number of fat cells, as well as it plays role in nutritional transition during infancy [4]. Previous epidemiological studies do not provide evidence that allows to identify periods within infancy, critical for long term adiposity outcomes. Our findings suggest that fatness at 1.5 months, likely reflecting fetal period, and from 6–24 months might be of greatest importance for childhood BMI, total fat and abdominal subcutaneous fat.
Strength and limitations
Our study has several major strengths, including its prospective population-based design, and availability of detailed fat measurements, both in infancy and later childhood. To our knowledge this is one of the first large scale studies with the use of MRI technique in children to assess distribution and content of body fat. This enabled us to obtain precise and accurate measures of fat, in particular ectopic fat deposition in various regions. Due to repeated fat measurements in infancy we were able to study different time intervals in infancy rather than single time points. Although we obtained fat measures in infancy, obviously we were lacking data on other than subcutaneous fat depots in this age period. Out of 991 infants with skinfolds thickness measurements available, 398 (40%) were lost to follow-up. However, we consider selection bias as unlikely since children participating in the follow-up study did not differ from those lost to follow-up in terms of fat measures in infancy. Our sample consisted solely of Dutch children which may limit generalizability of the findings, as ethnic differences regarding body fat distribution have been previously observed [42]. We are aware that skinfold thickness is considered as a valid measure of subcutaneous fat, though more prone to measurement error than other anthropometric measures. Finally, in our analyses we took into account a number of potential confounders, still residual confounding cannot be excluded due to observational design of this study.
Conclusions
Our findings suggest that subcutaneous fat in infancy is associated with childhood general adiposity and abdominal subcutaneous fat, but does not determine accumulation of ectopic fat, namely visceral, pericardial and liver fat. Future studies on organ-specific fat development as well as on interplay of different fat compartments across childhood are warranted along with research progress on their role for cardio metabolic and vascular health.
References
Guo SS, Chumlea WC. Tracking of body mass index in children in relation to overweight in adulthood. Am J Clin Nutr. 1999;70:145S–8.
Rolland-Cachera MF, Deheeger M, Guilloud-Bataille M, Avons P, Patois E, Sempe M. Tracking the development of adiposity from one month of age to adulthood. Ann Hum Biol. 1987;14:219–29.
Kuzawa CW. Adipose tissue in human infancy and childhood: an evolutionary perspective. Am J Phys Anthropol. 1998;Suppl 27:177–209.
Budge H, Sebert S, Sharkey D, Symonds ME. Session on ‘obesity’. adipose tissue development, nutrition in early life and its impact on later obesity. Proc Nutr Soc. 2009;68:321–6.
Berry DC, Stenesen D, Zeve D, Graff JM. The developmental origins of adipose tissue. Development. 2013;140:3939–49.
Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331:929.
Gishti O, Gaillard R, Manniesing R, Abrahamse-Berkeveld M, van der Beek EM, Heppe DH, et al. Fetal and infant growth patterns associated with total and abdominal fat distribution in school-age children. J Clin Endocrinol Metab. 2014;99:2557–66.
Perng W, Rifas-Shiman SL, Kramer MS, Haugaard LK, Oken E, Gillman MW, et al. Early weight gain, linear growth, and mid-childhood blood pressure: a prospective study in project viva. Hypertension. 2016;67:301–8.
Demerath EW, Schubert CM, Maynard LM, Sun SS, Chumlea WC, Pickoff A, et al. Do changes in body mass index percentile reflect changes in body composition in children? Data from the Fels Longitudinal Study. Pediatrics. 2006;117:e487–95.
Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–13.
Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:e837–41.
Wells JC, Chomtho S, Fewtrell MS. Programming of body composition by early growth and nutrition. Proc Nutr Soc. 2007;66:423–34.
Santos S, Gaillard R, Oliveira A, Barros H, Abrahamse-Berkeveld M, van der Beek EM, et al. Associations of infant subcutaneous fat mass with total and abdominal fat mass at school-age: The Generation R Study. Paediatr Perinat Epidemiol. 2016;30:511–20.
Santos S, Gaillard R, Oliveira A, Barros H, Hofman A, Franco OH, et al. Subcutaneous fat mass in infancy and cardiovascular risk factors at school-age: The generation R study. Obes (Silver Spring). 2016;24:424–9.
Lim S, Meigs JB. Ectopic fat and cardiometabolic and vascular risk. Int J Cardiol. 2013;169:166–76.
Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH, van IMH, et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31:1243–64.
Ay L, Hokken-Koelega AC, Mook-Kanamori DO, Hofman A, Moll HA, Mackenbach JP, et al. Tracking and determinants of subcutaneous fat mass in early childhood: the Generation R Study. Int J Obes (Lond). 2008;32:1050–9.
Ketel IJ, Volman MN, Seidell JC, Stehouwer CD, Twisk JW, Lambalk CB. Superiority of skinfold measurements and waist over waist-to-hip ratio for determination of body fat distribution in a population-based cohort of Caucasian Dutch adults. Eur J Endocrinol. 2007;156:655–61.
Freedman DS, Wang J, Ogden CL, Thornton JC, Mei Z, Pierson RN, et al. The prediction of body fatness by BMI and skinfold thicknesses among children and adolescents. Ann Hum Biol. 2007;34:183–94.
Wells JC, Cole TJ. steam As. Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int J Obes Relat Metab Disord. 2002;26:947–52.
Fredriks AM, van Buuren S, Wit JM, Verloove-Vanhorick SP. Body index measurements in 1996-7 compared with 1980. Arch Dis Child. 2000;82:107–12.
Hu H, Nayak K, Goran M. Assessment of abdominal adipose tissue and organ fat content by magnetic resonance imaging. Obes Rev. 2011;12:e504–15.
Shuster A, Patlas M, Pinthus J, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85:1–10.
Thomas E, Fitzpatrick J, Malik S, Taylor-Robinson S, Bell J. Whole body fat: content and distribution. Progress Nucl Magn Reson Spectrosc. 2013;73:56–80.
Mitra S, Fernandez-Del-Valle M, Hill J. The role of MRI in understanding the underlying mechanisms in obesity associated diseases. Biochim Biophys Acta. 2016;1863:1115–31.
Langeslag S, Schmidt M, Ghassabian A, Jaddoe V, Hofman A, van der Lugt A, et al. Functional connectivity between parietal and frontal brain regions and intelligence in young children: The Generation R Study. Hum Brain Mapp. 2013;34:3299–307.
Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729–49.
Keijzer-Veen MG, Euser AM, van Montfoort N, Dekker FW, Vandenbroucke JP, Van Houwelingen HC. A regression model with unexplained residuals was preferred in the analysis of the fetal origins of adult diseases hypothesis. J Clin Epidemiol. 2005;58:1320–4.
Jones A, Charakida M, Falaschetti E, Hingorani AD, Finer N, Masi S, et al. Adipose and height growth through childhood and blood pressure status in a large prospective cohort study. Hypertension. 2012;59:919–25.
Vogelezang S, Gishti O, Felix JF, van der Beek EM, Abrahamse-Berkeveld M, Hofman A, et al. Tracking of abdominal subcutaneous and preperitoneal fat mass during childhood. The Generation R Study. Int J Obes (Lond). 2016;40:595–600.
Gishti O, Gaillard R, Durmus B, Abrahamse M, van der Beek EM, Hofman A, et al. BMI, total and abdominal fat distribution, and cardiovascular risk factors in school-age children. Pediatr Res. 2015;77:710–8.
Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132:1639–47.
Sandboge S, Perala MM, Salonen MK, Blomstedt PA, Osmond C, Kajantie E, et al. Early growth and non-alcoholic fatty liver disease in adulthood-the NAFLD liver fat score and equation applied on the Helsinki Birth Cohort Study. Ann Med. 2013;45:430–7.
Breij LM, Kerkhof GF, Hokken-Koelega AC. Accelerated infant weight gain and risk for nonalcoholic fatty liver disease in early adulthood. J Clin Endocrinol Metab. 2014;99:1189–95.
Anderson EL, Howe LD, Fraser A, Callaway MP, Sattar N, Day C, et al. Weight trajectories through infancy and childhood and risk of non-alcoholic fatty liver disease in adolescence: the ALSPAC study. J Hepatol. 2014;61:626–32.
Ayonrinde OT, Olynyk JK, Marsh JA, Beilin LJ, Mori TA, Oddy WH, et al. Childhood adiposity trajectories and risk of nonalcoholic fatty liver disease in adolescents. J Gastroenterol Hepatol. 2015;30:163–71.
Kindblom JM, Lorentzon M, Hellqvist A, Lonn L, Brandberg J, Nilsson S, et al. BMI changes during childhood and adolescence as predictors of amount of adult subcutaneous and visceral adipose tissue in men: the GOOD Study. Diabetes. 2009;58:867–74.
Demerath EW, Reed D, Choh AC, Soloway L, Lee M, Czerwinski SA, et al. Rapid postnatal weight gain and visceral adiposity in adulthood: the Fels Longitudinal Study. Obes (Silver Spring). 2009;17:2060–6.
Gonzalez DA, Nazmi A, Victora CG. Growth from birth to adulthood and abdominal obesity in a Brazilian birth cohort. Int J Obes (Lond). 2010;34:195–202.
Kuh D, Hardy R, Chaturvedi N, Wadsworth ME. Birth weight, childhood growth and abdominal obesity in adult life. Int J Obes Relat Metab Disord. 2002;26:40–7.
McCarthy A, Hughes R, Tilling K, Davies D, Smith GD, Ben-Shlomo Y. Birth weight; postnatal, infant, and childhood growth; and obesity in young adulthood: evidence from the Barry Caerphilly Growth Study. Am J Clin Nutr. 2007;86:907–13.
Samara A, Ventura EE, Alfadda AA, Goran MI. Use of MRI and CT for fat imaging in children and youth: what have we learned about obesity, fat distribution and metabolic disease risk? Obes Rev. 2012;13:723–32.
Acknowledgements
We gratefully acknowledge the contribution of general practitioners, hospitals, midwives, and pharmacies in Rotterdam.
Funding
The general design of the Generation R Study is made possible by financial support from the Erasmus MC, University Medical Center, Rotterdam, Erasmus University Rotterdam, Netherlands Organization for Health Research and Development (ZonMw), Netherlands Organisation for Scientific Research (NWO), Ministry of Health, Welfare and Sport and Ministry of Youth and Families. Research that has leaded to these findings received support by a grant from the Netherlands Organization for Health Research and Development (VIDI 016.136.361), a European Research Council Consolidator Grant (ERC-2014-CoG-648916), an unrestricted grant from Nutricia Research and from the European Union’s Horizon 2020 research and innovation programme under grant agreements no 733206 (LifeCycle). Bernadeta Patro Golab received a research training fellowship grant from the Nestle Nutrition Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Patro Golab, B., Voerman, E., van der Lugt, A. et al. Subcutaneous fat mass in infancy and abdominal, pericardial and liver fat assessed by Magnetic Resonance Imaging at the age of 10 years. Int J Obes 43, 392–401 (2019). https://doi.org/10.1038/s41366-018-0287-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0287-7
- Springer Nature Limited
This article is cited by
-
Role of obesity and blood pressure in epicardial adipose tissue thickness in children
Pediatric Research (2022)
-
Exclusivity of breastfeeding and body composition: learnings from the Baby-bod study
International Breastfeeding Journal (2021)
-
The mysterious values of adipose tissue density and fat content in infants: MRI-measured body composition studies
Pediatric Research (2021)