Abstract
Background/objectives
Hypothalamic obesity (HO) occurs in 50% of patients with the pituitary tumor craniopharyngioma (CP). Attempts have been made to predict the risk of HO based on hypothalamic (HT) damage on magnetic resonance imaging (MRI), but none have included volumetry. We performed qualitative and quantitative volumetric analyses of HT damage. The results were explored in relation to feeding related peptides and body fat.
Subjects/methods
A cross-sectional study of childhood onset CPs involving 3 Tesla MRI, was performed at median 22 years after first operation; 41 CPs, median age 35 (range: 17–56), of whom 23 had HT damage, were compared to 32 controls. After exclusions, 35 patients and 31 controls remained in the MRI study. Main outcome measures were the relation of metabolic parameters to HT volume and qualitative analyses of HT damage.
Results
Metabolic parameters scored persistently very high in vascular risk particularly among HT damaged patients. Patients had smaller HT volumes compared to controls 769 (35–1168) mm3 vs. 879 (775–1086) mm3; P < 0.001. HT volume correlated negatively with fat mass and leptin among CP patients (rs = −0.67; P < .001; rs = −0.53; P = 0.001), and explained 39% of the variation in fat mass. For every 100 mm3 increase in HT volume fat mass decreased by 2.7 kg (95% CI: 1.5–3.9; P < 0.001). Qualitative assessments revealed HT damage in three out of six patients with normal volumetry, but HT damage according to operation records.
Conclusions
A decrease in HT volume was associated with an increase in fat mass and leptin. We present a method with a high inter-rater reliability (0.94) that can be applied by nonradiologists for the assessment of HT damage. The method may be valuable in the risk assessment of diseases involving the HT.
Similar content being viewed by others
Introduction
Control of body weight involves several brain regions as well as central and peripheral signaling pathways. The hypothalamus (HT) has a central role in this complex network, serving as a processor and integrator of different signals. The main HT areas involved in energy regulation are ventromedial hypothalamus (VMH), paraventricular nucleus (PVN), arcuate nucleus, dorsomedial HT (DMH), and the lateral HT (LH). Destruction of its distinctive nuclei induces hyperphagia, hyperinsulinemia and weight gain [1] and these structures express high levels of insulin, leptin, and ghrelin receptors [2, 3].
A craniopharyngioma (CP) is a benign pituitary tumor known for its aggressive behavior causing high vascular mortality [4] and morbidity including cognitive deficits [5,6,7]. It is a rare tumor with an incidence is 0.5–2.0 cases/million/year [8], women and men are equally affected and about 40% of cases are seen in children [8]. Total or subtotal excision of CPs followed by cranial radiotherapy (CRT), in about 50% of cases, represents the therapeutic standard of care which in the majority (80%) of cases results in panhypopituitarism [9], a condition which is manageable with hormonal substitution. On the other hand the devastating morbid obesity due to HT damage is very difficult to treat. Thus the primary goal in the treatment of CP is not to avoid hypopituitarism, but to preserve HT function [9, 10].
In up to 50% of CP tumor growth and/or its treatment involves the HT [5, 11, 12], and it is estimated that half of all cases of hypothalamic obesity (HO) occurs in patients treated for CP [5, 10, 13]. The concept of HO dates back to two case reports involving pituitary tumors causing HT damage resulting in hyperphagia and weight gain [14, 15]. The definition of HO has expanded in recent years [13, 16], and knowledge pertaining to the function of the HT calls for a more refined definition. Autonomic imbalance [17] including vagally mediated hyperinsulinemia [18,19,20,21] has been shown and CP patients are leptin resistant [5, 6, 21], and as demonstrated in obese humans [22] fasting ghrelin levels are decreased [6, 23].
Anatomical HT damage is important in this context [16, 24], and with evolving technology in magnetic resonance imaging (MRI) it is now possible to describe HT damage in more detail. There is a general agreement that breaching of the floor of the third ventricle is a definite sign of HT damage [13], but other than that a clear definition of HT damage is currently missing. Several studies have tried to predict the risk of HO among CP based on preoperative and postoperative HT damage on MRIs [7, 13, 25,26,27,28,29,30,31], but none included volume measurements. To our knowledge, there are only two studies that have investigated HT volume in relation to metabolic risk, i.e., among healthy adolescent obese women [32] and among former cranially irradiated acute lymphoblastic leukemia patients [33].
In the attempt to standardize the assessment of HT damage the aim of the study was to perform a novel, detailed qualitative and quantitative analyses of the postsurgical HT volume based on 3 Tesla MR volumetric measurements. Comparison was made with a grading system of HT damage based on retrospective analyses of each patient’s operation records, a method used in our previous work [6]. In addition the volumetric results were put into the context of HO by exploring its relation to feeding related peptides and body fat.
Materials and methods
Patients
Forty-one (24 women) patients, aged ≥ 17 years, were recruited from 64 eligible subjects from the South and Southeast regions of Sweden (population 2.5 million). The patients were surgically treated for a childhood-onset CP between 1958 and 2010. Excluded subjects (n = 23) were either assessed too ill (meningioma, n = 1, neuromuscular disease, n = 1, living in a home for disabled, n = 2), too busy (n = 6), investigations to stressful according to patients (n = 2), had aneurysm clip (n = 1), no reason given (n = 7), missing medical journals (n = 1), no reply (n = 2).
Five patients had to withdraw from MRI due to presence of a shunt causing severe MR artifacts (n = 1), pacemaker (n = 1), claustrophobia (n = 2), or weight restrictions (n = 1). These five patients were nevertheless included in the investigation of metabolic risk factors (n = 41). A total of 36 patients completed MRI, but one was excluded from the HT volume analyses (n = 35) due to the presence of silver clips causing poor MRI quality. Three patients were too heavy to perform a dual-energy x-ray absorptiometry (DXA) (n = 38).
Patient baseline characteristics and tumor treatment modalities are shown in Table 1. Sixteen patients had received CRT, median dose 50 Gy (35–55 Gy). Median age at first operation was 12 years in women (3–29) and 9 years in men (3–22), and median age at investigation was 35 (18–56) years in women, and 36 (20–49) years in men. Median time since first operation was 21 (6–49) years in women, and 23 (11–42) years in men. At the time of this study the same neurosurgeon graded the tumor location retrospectively based on each patient’s operation records: intrasellar growth (grade I), suprasellar growth (grade II), suprasellar growth into the third ventricle (grade III, also referred to as tumor growth into the third ventricle, TGTV). Grades I and II are also referred to as non-TGTV. Twenty-three patients had TGTV reflecting HT damage. At the time of the study 76% of patients received growth hormone (GH) therapy. Median daily GH dose was 0.6 (0.4–1.2) mg in women and 0.5 (0.2–0.8) mg in men resulting in a normalization of serum insulin-like growth factor-I (IGF-I) in all patients.
Totally, 71% of the women were on oral sex steroid treatment and 1 woman had androgen supplementation. The remaining women had normal gonadal function according to blood tests. Among men, 82% needed testosterone replacement; 83% of the women and 88% of men received levothyroxine in doses of 150 (50–250) μg and 150 (100–200) μg, respectively, with s-free T4 values in women of 18 (11–29) pmol/L and men 17 (13–21) pmol/L (reference range: 12–22 pmol/L). Five patients had normal adrenocorticotropic-cortisol axes and all others needed hydrocortisone in doses of 25 (20–40) mg in women and 20 (10–30) mg in men. None of the patients were smokers.
A control group consisting of 32 subjects (18 women) with similar age, gender, and smoking habit distributions was established. Twenty controls were recruited from a pool of healthy subjects who were randomly selected and participated in our previous studies [34, 35]. Twelve new controls were randomly selected from a computerized population register as previously described [36]. One control terminated MRI due to claustrophobia and was excluded from all MRI analyses. Thus, 31 controls were included in the MRI analyses. That same subject was included in the investigations of metabolic risk factors. One control subject was too heavy to perform DXA (n = 31).
Study design
The present investigations were performed during a single day in each patient. The responsible ethics committee approved the protocol (DNR 2011/769). All participants gave written informed consent.
Anthropometric measurements
Body mass index (BMI, kg/m2) and body composition using DXA were assessed as previously described [36]. Data for body composition are expressed as estimated fat mass (kg and %) and fat free mass (kg).
Assessments of physical activity and fatigue
The degree of physical exercise during work and spare time was assessed by a self-rating questionnaire in which patients and matched controls classified their physical activity according to a four-grade scale [37].
The multidimensional fatigue inventory is a self-report instrument, which covers the following dimensions: general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity [38].
Biochemical assays
Blood samples were drawn in the morning, after an over-night fasting and before drug intake, except for hydrocortisone. Venous plasma (p)-glucose (mmol/L) was analyzed with a blood glucose analyzer (Hemocue AB, Ängelholm). Details on leptin, ghrelin and IGF-I measurements are shown in Follin et al. [33]. Standard measurements for plasma levels of insulin, estradiol, testosterone, free thyroxine, and free triiodothyronine were used.
Neuroimaging protocol
Imaging sequences were acquired on a 3-Tesla MR scanner (MAGNETOM Skyra, Siemens healthcare, Erlangen, Germany) using a 20 channel head/neck receive coil. For volumetric measurements axial T1-weighted MPRAGE images were acquired (1 mm isotropic resolution, TE 3 ms, TR 1900 ms, flip angle 9).
Measurement of HT volume and a description of the borders of anterior/medial/posterior HT for a qualitative assessment of HT damage
The HT volumetry was performed manually (ANALYZE 10.0 software package, Biomedical Imaging Resource, Mayo foundation, Rochester, MN) using a pen and digitized drawing pad. It was carried out on T1-weighted images preprocessed by acquiring cubic spine interpolation and interpolation of original voxel size to 0.5 × 0.5 × 0.5 mm. The HT delineation was based on anatomical borders established first in postmortem histological sections of the human HT [39] and then on 3T MRI scans [39].
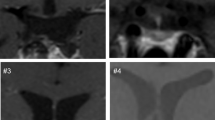
The HT delineation in the present study was performed on a rostral to caudal axis on coronal images as described previously [40, 41]. The CV (standard deviation of the HT volume/mean of the HT volume × 100) was previously estimated for a number of controls resulting in an intraclass correlation (ICC) coefficient of 0.94 [40]. A single person blinded to the identity of the subjects carried out all HT segmentation (Fig. 1).
Delineation of the hypothalamic region in 3 Tesla magnetic resonance images (MRI). A representative overview of the delineation principles of the hypothalamus (HT) in T1-weighted MRI acquired at 3 T according to Gabery et al. [40]. Between 14 and 16 images were used for per case analysis. Images were preprocessed with cubic spine interpolation of original voxel size to 0.5 × 0.5 × 0.5 mm3. The images were taken from slices 1, 4, 8, 12, and 16 with 2 mm spacing. From a rostral to caudal direction, the images in the A1-E1 column are representative 3 T MR images in a coronal plane. Column A2–E2 represents a schematic overview of the corresponding level modified from Mai et al. [41]. Column A3–E3 illustrates an overlap of the schematic overview on the MR image. The light blue lines illustrate how the HT region was delineated. Landmarks such as the HT sulcus (represented by a white star) and the lateral edge of the optical tract (represented by red star) were identified for the delineation and a straight line between these two points was drawn to set the superior/lateral border of the area in a reproducible fashion. The optical tract was excluded in all slides. Abbreviations: d dorsomedial hypothalamic nucleus; F fornix; i infundibular nucleus; l lateral hypothalamus; MB mammillary body; OT optical tract; P paraventricular nucleus; s supra optic nucleus; v ventromedial hypothalamic nucleus
A second rater, blinded to the identity, evaluated the HT volume of 29 subjects, i.e., all TGTV (n = 18) and a subsample of controls (n = 11). In addition the first rater re-evaluated the same 29 subjects. The ICC between the two raters was 0.97 for all subjects, and separately for TGTV ICC = 0.94 and for controls ICC = 0.95. The manual segmentation of the HT for volumetric analysis was performed by two raters with expertize in hypothalamic anatomy [39, 40]. One of the investigators who performed the volumetry also made the detailed qualitative description of the HT, and was again blinded for the neurosurgeons assessment. It is our experience that new raters need around 10 h of manual segmentation practice prior to begin volumetric data acquisition given that they have prior knowledge of the human hypothalamus such as identification of anatomical structures, including the hypothalamic sulcus, optical tract, fornix, and the mammillary body (MB). The anterior boundary of the HT region was defined with the appearance of the preoptic HT. Medial HT region was set once the fornix ascended and positioned below the HT sulcus. The manual segmentation criteria used for volumetric analysis in the current study will only include a small portion of the anterior MB. The posterior boundary was defined once the fornix was merged with the MB and thus, only a small portion of the anterior MB was included in the estimated HT volume. The spectrum of HT involvement is qualitatively described from low to high as follows: indentation, distortion, missing (totally, largely, and partially missing). A comparison was made between the neurosurgeons assessment and the combined qualitative and quantitative measurements from the volumetric analysis. Qualitative assessments are presented in Fig. 2a–c.
Qualitative assessment pertained separately for anterior/medial/posterior hypothalamus (HT). The Fig. 2 provides an example of this assessment with an overview of the medial hypothalamus in T1-weighted magnetic resonance images (MRI) acquired at 3 Tesla from 3 cases. a Nontumor growth to the third ventricle (non-TGTV) with an intact hypothalamus. b and c Tumor growth to the third ventricle (TGTV) with increasing degree of hypothalamic damage. a’–c’ Represent the corresponding region but with higher magnification of the hypothalamic region. a’ An overlap of a schematic overview of the corresponding level modified from Mai et al. [41] depicts the positions of the hypothalamic nuclei (right hemisphere only). The red dashed lines illustrate how the hypothalamic region was delineated in one hemisphere, for analysis bilateral hypothalamus was quantified. b, b’ The medial hypothalamus was missing bilaterally at the level of the infundibular nucleus and median eminence, while the remaining hypothalamic structures were intact in anterior and posterior regions (not depicted). c, c’ The medial hypothalamus was missing bilaterally. The hypothalamus was also missing bilaterally in the anterior portion (not depicted), in posterior sections the right hypothalamus was missing with only left hypothalamic region remaining intact where fornix merges with the mammillary body (not depicted). Abbreviations: d dorsomedial hypothalamic nucleus; F fornix; i infundibular nucleus; l lateral hypothalamus; MB mammillary body; OT optical tract; P paraventricular nucleus; s supra optic nucleus; v ventromedial hypothalamic nucleus
Measurement of the total intracranial volume
The intracranial volume (ICV) was calculated from the volumetric segmentation of the MPRAGE-sequence using the Free surfer image analysis suite (version 5.1), which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/).
Statistics
Data are presented as median (min–max) with the exception of physical activity where the results are presented as mean ± SD. Differences between patients and controls were compared using Mann–Whitney U test. Bivariate correlations were assessed using Spearman rank correlation coefficient (rs). A linear regression was performed to investigate whether our results were confounded by age and body weight. To ensure that model assumptions were reasonably fulfilled we investigated scatter plots as well as residual analyses. P < 0.05 was regarded as statistically significant. We used SPSS version 22.0 for statistical analyses.
Results
Anthropometric, biochemical, and hormone measurements in CP patients (n = 41) compared to controls (n = 32)
Compared to controls, significantly higher weight, BMI, waist, and fat mass were measured in the CP patients (Table 2). In addition, fasting s-ghrelin levels were significantly lower, whereas plasma levels of insulin, leptin, and leptin/kilogram fat mass levels were higher. Moreover, plasma free T4 levels were significantly higher.
Associations between metabolic risk factors and neuropeptides in CP patients (n = 41) and controls (n = 32)
Fasting s-ghrelin levels correlated significantly with fat mass in kg only among patients (rs = −0.48; P = 0.002), whereas s-leptin levels correlated significantly with fat mass in kg and fat mass as a % of total body weight among CP patients (rs = 0.80; P < .001; rs = 0.90; P < .001, respectively) as well as among controls (rs = 0.56; P = 0.001; rs = 0.90; P < 0.001, respectively). P-insulin levels did also correlate with fasting s-ghrelin-levels among both CP patients (rs = −0.64, P < 0.001) and controls (rs = −0.52, P = 0.002).
Volume of HT in CP patients (n = 35) compared to controls (n = 31)
In univariate linear regression models group affiliation was significantly associated with HT volume (patients: 769 (35–1168) mm3; controls: 879 (775–1086) mm3; P < 0.001), whereas there was no significant association between ICV and HT volume (P = 0.43). When group affiliation and ICV were included in the regression model simultaneously it was only group affiliation that was significantly associated with HT volume (P < 0.001).
After correction for ICV neither WM 0.34 (0.28–0.40) vs. 0.36 (0.31–0.40) (P = 0.35) nor GM 0.48 (0.40–0.57) vs. 0.48 (0.42–0.54) (P = 0.63) were significantly different compared to controls.
Qualitative analyses of the HT and comparison to the neurosurgeons assessment
The results of both quantitative (HT MR volumetry) and qualitative description of HT damage in patients with TGTV (n = 18) are presented in Table 3. One discrepancy was found where a CP patient who had non-TGTV according to the neurosurgeon was assumed to have TGTV according to volume measurements (473 mm3, BMI = 42). In addition qualitative description revealed bilateral indentation along the third ventricle wall lining in medial HT region. Accordingly, that individual was assigned to the TGTV group. In addition, six CP patients had well-preserved HT volume (range: 754–998) despite clinical signs of serious weight problems after surgery and were identified by the neurosurgeons grading as a TGTV. These patients were assigned to a separate intermediate group in Table 3, representing CP patients assumed to have subtle HT involvement. In 3 out of 6 CP patients in the intermediate group a qualitative description of a subtle HT involvement corresponded with the neurosurgeons assessment of TGTV leaving an overall discrepancy in only 3 out of 35 CP patients. None of these three patients showed any signal changes in the hypothalamus on clinical sequences available for review (axial T2 and T2 FLAIR sequences).
No remarks were found in the qualitative assessment of CP patients with non-TGTV verifying the results of the HT volumetry and the neurosurgeons assessment reflected in a well preserved HT volume (range: 699–1168 mm3) and 13 out of 17 CP patients had BMI ≤ 29.
Associations between metabolic risk factors, neuropeptides, treatment data, and HT volume among CP patients (n = 35) and controls (n = 31)
Serum leptin (Fig. 3a) and leptin/kg fat mass were significantly correlated with the HT volume (rs = −0.53; P = 0.001; rs = −0.35; P = 0.041, respectively) only in CP patients. No correlation was found between fasting ghrelin and HT volume. Fat mass in percent as well as in kg (Fig. 3b) were significantly correlated with total HT volume (rs = −0.56; P < 0.001; rs = −0.67; P < 0.001, respectively) only in CP patients.
Associations with fat mass (kg) in 35 CP patients
The HT volume versus fat mass (kg) correlation among CP patients was from a linear regression model estimated to explain 39 % of the variation in fat mass. For every 100 mm3 increase in HT volume fat mass decreased by 2.7 kg (CI: 1.5–3.9; P < 0.001). Including age at investigation and radiation in the model did not change the estimates.
Physical activity during work and leisure time and fatigue among CP patients (n = 41) and matched controls (n = 32)
The CP patients had similar self-rated physical activity during work hours compared to matched controls. During leisure time CP patients had less physical activity compared to matched controls (mean 2.41 vs. 2.02, P = 0.04).
The CP patients presented with more physical fatigue compared to healthy matched controls (12 (4–19) vs. 9 (4–18), P = 0.009). No difference was found on other fatigue subscales.
Anthropometric, biochemical, and hormone measurements in CP patients with TGTV (n = 23) vs. non-TGTV (n = 18)
Comparisons of TGTV and non-TGTV showed that the anthropometric measures BMI, fat mass in % and in kg, but not fat free mass, were significantly higher (Table 2). Serum fasting ghrelin levels were significantly lower whereas insulin and leptin were higher in TGTV. Leptin/kilogram fat mass, fasting p-glucose and insulin/kilogram fat mass did not differ. No differences were found in the levels of thyroid hormones, serum IGF-I levels or sex steroid hormones.
Discussion
In this study a novel reproducible method accredited for HT volume measurement was applied in CP patients. The study provides new data on how a decrease in HT volume is associated with an increase in fat mass and leptin. The method also has a qualitative component allowing identification of patients with subtle HT involvement. Our data show that HT volume may explain 39% of the variation in fat mass. Other possible contributors includes subtle HT involvement with a well preserved HT volume, disturbed vascular blood supply of HT nuclei, unknown metabolic derangements of HT peptides, lifestyle, and genetic background.
In accordance with previous studies [5, 6, 13, 21], we present a study population of CP patients with a positive energy balance compared to controls reflected by higher BMI, high leptin levels with leptin resistance, high insulin levels and low fasting ghrelin levels. We verify previous findings [5, 42] of less physical activity during leisure time and now we also record a new finding of increased fatigue. That combined with lower basal metabolic rate [6] most likely contributes to the persistent obesity among patients.
Disturbed satiety signaling, hyperinsulinemia, leptin resistance, and a deficiency of downstream mediators of leptin signaling seen in CP patients are key features in HO pathogenesis [13]. High circulating leptin levels have been shown in general obesity, but CP patients have a greater leptin resistance [6, 13]. In line with prior studies serum leptin levels correlated significantly with fat mass among patients and controls [43]. Our findings of increased levels of both leptin and leptin/kg fat mass with a reduced HT volume among CP patients provides further support of the important role of HT involvement in the disturbed metabolism as such a correlation was not evident among controls. Our finding of a low fasting ghrelin level is in line with previous findings in studies involving CP patients [6, 23, 44].
High correspondence was found between the HT volumetry and the neurosurgeon's assignment of patients to the non-TGTV and TGTV group. On the other hand HT volumetry lacked sensitivity in identifying a subgroup of patients within the TGTV group (n = 6) who had a well preserved HT volume despite having HT involvement according to the neurosurgeon (assigned as intermediate group, Table 3). In 3 of these patients a qualitative description agreed with HT involvement leaving only 3 out of 18 patients with TGTV with a well-preserved HT volume and a qualitative assessment without remarks. Interestingly, none of these three patients showed any signal abnormalities on other clinical sequences acquired (T2 and FLAIR), although this might have been expected reflecting early or subtle tissue changes not yet despicable with T1-weighted sequences. We therefore conclude that our combined method of volumetry and qualitative description of HT damage was not applicable in only 3/35 patients. Why some patients with HT damage restrain from obesity and some CP patients without HT lesions become obese is a key question. By combining quantitative and qualitative measurements we present a new method which can possibly facilitate the identification of this special subgroup of patients.
A grading system for the assessment of HT damage was first presented with the study of De Vile et al. [25]. with emphasis on postoperative HT morbidity followed by Puget et al. [26]. who focused on individualized treatment strategies based on preoperative assessment of HT damage. Since then several grading systems have emerged [26,27,28,29,30,31] either based on the work of De Vile et al. [25] or Puget et al. [26]. In this context, detailed selection of anatomical landmarks is very important. Roth et al. [13] presents a new type of scoring system and relates damage in different HT areas to specific HT nuclei. While Roth et al. [13] focused on children we present results when these children have entered adulthood and thus provide information currently lacking in the literature. In addition we provide more detailed qualitative information with structured terminology regarding the spectrum of HT involvement from low to high. Our method is unique in the sense that it is originally based on landmarks identified in postmortem human HT tissue. It requires relatively few landmarks minimizing the risk of measurement bias and its robustness is reflected in a high intraclass correlation coefficient (0.94), comparable data is missing in the study by Roth et al. [13]. Thus, we conclude that the method has a potential of being clinically applicable.
Histological HT analysis like in Gabery et al. [40] illustrates a nonhomogenous variation of the neuroanatomy of HT nuclei. Thus, we are careful in our discussion at nuclei level but our delineation approach allows us to include the majority of HT nuclei (PVN, supraoptic nucleus, infundibular stalk, VMH, DMH, and LH). By adding a qualitative component to our method (Table 3) we have a detailed description of the anterior, middle and posterior which along with the volume analyses indicates which part of the HT is damaged. Our method gives us a three-dimensional MR analyses of the HT as compared to Roth et al. [13] where a two-dimensional analyses involving four standard T1-weighted images was used. Importantly, our method can be applied by raters without any previous radiology background and the added sequences (see Methods) can easily be included in a formal pituitary–hypothalamus MRI and will take six to eight additional minute to perform. Furthermore, the majority of prior studies involving FreeSurfer adjust for any variation in brain size and interindividual head size by using ICV. However, we realize that the effect of radiation needs a special consideration in our study population as 14/23 in the TGTV group had CRT, and as CRT can cause brain atrophy, and possibly may have a larger effect on white matter volume.
We recorded no global effect of radiation on parenchymal brain volumes as no differences compared to controls were recorded between GM and WM volumes after correction for ICV. Thus the reduction in HT volume is rather judged as a local effect than to be associated to global atrophy.
The study has limitations, e.g., the study population is small, however, against a rare disease and the background population of 2.5 million we included 64% of the eligible group of adult survivors of childhood-onset CP, during 52 years in this area of Sweden. This study presents the longest follow up so far (22 years) of adult patients with a childhood-onset CP and includes a control group with similar gender, age, and smoking habits, lending us to control for several confounders. We chose not to match for BMI to be able to include the measurement as an outcome variable. As this cohort is a cross-section of survivors, we cannot exclude that non-survivors may have exhibited a different demographic profile. The study design does not allow for any conclusions regarding the pathogenesis behind the volumetric results. Displacement of the HT may possibly affect the vascularity in the HT area. However, as long as the anatomical landmarks are visible it is possible to measure the HT volume regardless of displacement. The reproducibility of the method is based on the fact that no individual adjustments are made. Furthermore, if remnant tumors or cysts were within the vicinity, these structures were not included in the volume measurements. To estimate the effect of radiation on the HT volume is very difficult as radiation is closely interrelated with numerous important variables including tumor size, number of operations and number of pituitary insufficiencies. Separating these variables is difficult as it will result in small subpopulations with low-statistical power.
In conclusion, a standardized definition of HT damage is currently missing. Our method is an important contribution in the analyses of HT damage and offers a new approach in exploring the role of HT damage in obesity. The method may serve as a risk assessment of HO which is pivotal for the optimal treatment of all diseases involving HT damage. Understanding the pathogeneses behind HO will presumably contribute to obesity research in a larger context.
References
Schneeberger M, Gomis R, Claret M. Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. J Endocrinol. 2014;220:T25–46.
van Swieten MM, Pandit R, Adan RA, van der Plasse G. The neuroanatomical function of leptin in the hypothalamus. J Chem Neuroanat. 2014;61-62:207–20.
Horvath T, Diano S, Sotonyi P, Heiman M, Tschöp M. Minireview: ghrelin and the regulation of energy balance—a hypothalamic perspective. Endocrinology. 2001;142:4163–9.
Bülow B, Attewell R, Hagmar L, Malmström P, Nordström CH, Erfurth EM. Postoperative prognosis in craniopharyngioma with respect to cardiovascular mortality, survival, and tumor recurrence. J Clin Endocrinol Metab. 1998;83:3897–904.
Holmer H, Ekman B, Björk J, Nordstöm CH, Popovic V, Siversson A, et al. Hypothalamic involvement predicts cardiovascular risk in adults with childhood onset craniopharyngioma on long-term GH therapy. Eur J Endocrinol. 2009;61:671–9.
Holmer H, Pozarek G, Wirfält E, Popovic V, Ekman B, Björk J, et al. Reduced energy expenditure and impaired feeding-related signals but not high energy intake reinforces hypothalamic obesity in adults with childhood onset craniopharyngioma. J Clin Endocrinol Metab. 2010;95:5395–402.
Müller HL, Gebhardt U, Teske C, Faldum A, Zwiener I, Warmuth-Metz M, et al. Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: Results of the multinational prospective trial kraniopharyngeom 2000 after 3-year follow-up. Eur J Endocrinol. 2011;165:17–24.
Bunin GR, Surawicz TS, Witman PA, Preston-Martin S, Davis F, Bruner JM. The descriptive epidemiology of craniopharyngioma. J Neurosurg. 1998;89:547–51.
Erfurth EM, Holmer H, Fjalldal SB. Mortality and morbidity in adult craniopahryngioma. Pituitary. 2013;16:46–55.
Müller HL. Childhood craniopharyngioma. Pituitary. 2013;16:56–67.
Müller HL, Emser A, Faldum A, Bruhnken G, Etavard-Goris N, Gebhardt U, et al. Longitudinal study on growth and body mass index before and after diagnosis of childhood craniopharyngioma. J Clin Endocrinol Metab. 2004;89:3298–305.
Grant DB, Kendall BE, Neville BG, Stanhope R, Watkins KE, Hayward RD. Management of childhood craniopharyngioma: can the morbidity of radical surgery be predicted. J Neurosurg. 1996;85:73–81.
Roth CL. Hypothalamic obesity in craniopharyngioma patients: disturbed energy homeostasis related to extent of hypothalamic damage and its implication for obesity intervention. J Clin Med. 2015;4:1774–97.
Babinski J. Tumeur du corps pituitaire sans acromégalie et avecarrêt de développement des organes génitaux. Rev Neurol. 1900;8:531–3.
Frohlich A. Ein fall von tumor der hypophysis cerebri ohne akromegalie. Wien Klin Rdsch. 1901;15:883–6.
Hochberg I, Hochberg Z. Expanding the definition of hypothalamic obesity. Obes Rev. 2010;11:709–21.
Roth CL, Hunneman DH, Gebhardt U, Stoffel-Wagner B, Reinehr T, Müller HL. Reduced sympathetic metabolites in urine of obese patients with craniopharyngioma. Pediatr Res. 2007;61:496–501.
Lustig RH. Hypothalamic obesity: the sixth cranial endocrinopathy. Endocrinologist. 2002;12:210–7.
Bray GA, Gallagher TF Jr. Manifestations of hypothalamic obesity in man: a comprehensive investigation of eight patients and a review of the literature. Medicine (Baltimore). 1975;54:301–30.
Bray GA, Inoue S, Nishizawa Y. Hypothalamic obesity. The autonomic hypothesis and the lateral hypothalamus. Diabetologia. 1981;20:366–77.
Roth C, Wilken B, Hanefels F, Schröter W, Leonhardt U. Hyperphagia in children with craniopharyngioma is associatedwith hyperleptinaemia and a failure in the downregulation of appetite. Eur J Endocrinol. 1998;138:89–91.
Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–9.
Roth CL, Gebhardt U, Müller HL. Appetite-regulating hormone changes in patients with craniopharyngioma. Obesity. 2011;19:36–42.
Bray GA. Syndromes of hypothalamic obesity in man. Pediatr Ann. 1984;13:525–36.
De Vile CJ, Grant DB, Kendall BE, Neville BG, Stanhope R, Watkins KE, et al. Management of childhood craniopharyngioma: can the morbidity of radical surgery be predicted? J Neurosurg. 1996;85:73–81.
Puget S, Garnett M, Wray A, Grill J, Habrand JL, Bodaert N, et al. Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. J Neurosurg. 2007;106:3–12.
Elliott RE, Sands SA, Strom RG, Wisoff JH. Craniopharyngioma Clinical Status Scale: a standardized metric of preoperative function and posttreatment outcome. Neurosurg Focus. 2010;28:E2.
Elowe-Gruau E, Beltrand J, Brauner R, Pinto G, Samara-Boustani D, Thalassinos C, et al. Childhood craniopharyngioma: hypothalamus-sparing surgery decreases the risk of obesity. J Clin Endocrinol Metab. 2013;98:2376–82.
Van Gompel JJ, Nippoldt TB, Higgins DM, Meyer FB. Magnetic resonance imaging-graded hypothalamic compression in surgically treated adult craniopharyngiomas determining postoperative obesity. Neurosurg Focus. 2010;28:E3.
Mortini P, Gagliardi F, Bailo M, Spina A, Parlangeli A, Falini A, et al. Magnetic resonance imaging as predictor of functional outcome in craniopharyngiomas. Endocrine. 2016;51:148–62.
Mallucci C, Pizer B, Blair J, Didi M, Doss A, Upadrasta S, et al. Management of craniopharyngioma: the Liverpool experience following the introduction of the CCLG guidelines. Introducing a new risk assessment grading system. Childs Nerv Syst. 2012;28:1181–92.
Ha J, Cohen J, Aziz T, Convit A. Association of obesity-mediated insulin resistance and hypothalamic volumes: possible sex differences. Hind Public Corp. 2013;35:249–59.
Follin C, Gabery S, Petersén Å, Sundgren PC, Björkman-Burtcher I, Lätt J, et al. Associations between metabolic risk factors and the hypothalamic volume in childhood leukemia survivors treated with cranial radiotherapy. PLoS ONE. 2016;11:e0147575.
Fjalldal S, Holmer H, Rylander L, Elfving M, Ekman B, Osterberg K, et al. Hypothalamic involvement predicts cognitive performance and psychosocial health in long-term survivors of childhood craniopharyngioma. J Clin Endocrinol Metab. 2013;98:3253–62.
Follin C, Link K, Wiebe T, Moëll C, Björk J, Erfurth EM. Bone loss after childhood acute lymphoblastic leukaemia: an observational study with and without GH therapy. Eur J Endocrinol. 2011;164:695–703.
Link K, Moell C, Garwicz S, Cavallin-Ståhl E, Björk J, Thilén U, et al. Growth hormone deficiency predicts cardiovascular risk in young adults treated for acute lymphoblastic leukemia in childhood. J Clin Endrinol Metab. 2004;89:5003–12.
Wilhelmsen L, Tibblin G, Aurell M, Bjure J, Ekström-Jodal B, Grimby G. Physical activity, physical fitness and risk of myocardial infarction. Adv Cardiol. 1976;18:217–30.
Smets EM, Garssen B, Bonke B, De Haes JC. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–25.
Gabery S, Murphy K, Schultz K, Loy CT, McCusker E, Kirik D, et al. Changes in key hypothalamic neuropeptide populations in Huntington disease revealed by neuropathological analyses. Acta Neuropathol. 2010;120:777–88.
Gabery S, Georgiou-Karistianis N, Lundh SH, Cheong RY, Churchyard A, Chua P, et al. Volumetric analysis of the hypothalamus in Huntington disease using 3T MRI: the image-HD study. PLoS ONE. 2015;10:e0117593.
Mai J, Paxinos G, Voss T. Atlas of human brain. Oxford, UK: Academic Press; 2008.
Harz KJ, Müller HL, Waldeck E, Pudel V, Roth C. Obesity in patients with craniopharyngioma: assessment of food intake and movement counts indicating physical activity. J Clin Endocrinol Metab. 2003;88:5227–31.
Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5.
Goldstone AP, Patterson M, Kalingag N, Ghatei MA, Brynes AE, Bloom SR, et al. Fasting and postprandialhyperghrelinemia in Prader–Willi syndrome is partially explainedby hypoinsulinemia, and is not due to peptide YY3–36 deficiencyor seen in HT obesity due to craniopharyngioma. J Clin Endocrinol Metab. 2005;90:2681–90.
Acknowledgments
This work was supported by the Swedish Children's Cancer Foundation, the Swedish Cancer Foundation, and the Medical Faculty, Lund University, Sweden
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Eva Marie Erfurth received lecture fees from Pfizer and Eli Lilly, B.E. received lecture fees from Novartis and has received fees for consultancy from Pfizer and Shire, all other authors have nothing to disclose.
Rights and permissions
About this article
Cite this article
Fjalldal, S., Follin, C., Gabery, S. et al. Detailed assessment of hypothalamic damage in craniopharyngioma patients with obesity. Int J Obes 43, 533–544 (2019). https://doi.org/10.1038/s41366-018-0185-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0185-z
- Springer Nature Limited
This article is cited by
-
Posterior hypothalamic involvement on pre-operative MRI predicts hypothalamic obesity in craniopharyngiomas
Pituitary (2023)
-
Benign Paranasal Sinus Tumors
Current Otorhinolaryngology Reports (2023)
-
Hypothalamic syndrome
Nature Reviews Disease Primers (2022)
-
Body mass index at diagnosis of a childhood brain tumor; a reflection of hypothalamic-pituitary dysfunction or lifestyle?
Supportive Care in Cancer (2022)
-
Cognitive interference processing in adults with childhood craniopharyngioma using functional magnetic resonance imaging
Endocrine (2021)