Abstract
Background
Exercise and protein ingestion preserve muscle mass during moderate energy deficits.
Objective
To determine the molecular mechanisms by which exercise and protein ingestion may spare muscle mass during severe energy deficit (5500 kcal/day).
Design
Fifteen overweight, but otherwise healthy men, underwent a pre-test (PRE), caloric restriction (3.2 kcals/kg body weight/day) + exercise (45 min one-arm cranking + 8 h walking) for 4 days (CRE), followed by a control diet (CD) for 3 days, with a caloric content similar to pre-intervention while exercise was reduced to less than 10,000 steps per day. During CRE, participants ingested either whey protein (PRO, n = 8) or sucrose (SU, n = 7) (0.8 g/kg body weight/day). Muscle biopsies were obtained from the trained and untrained deltoid, and vastus lateralis.
Results
Following CRE and CD, serum concentrations of leptin, insulin, and testosterone were reduced, whereas cortisol and the catabolic index (cortisol/total testosterone) increased. The Akt/mTor/p70S6K pathway and total eIF2α were unchanged, while total 4E-BP1 and Thr37/464E-BP1 were higher. After CRE, plasma BCAA and EAA were elevated, with a greater response in PRO group, and total GSK3β, pSer9GSK3β, pSer51eIF2α, and pSer51eIF2α/total eIF2α were reduced, with a greater response of pSer9GSK3β in the PRO group. The changes in signaling were associated with the changes in leptin, insulin, amino acids, cortisol, cortisol/total testosterone, and lean mass.
Conclusions
During severe energy deficit, pSer9GSK3β levels are reduced and human skeletal muscle becomes refractory to the anabolic effects of whey protein ingestion, regardless of contractile activity. These effects are associated with the changes in lean mass and serum insulin, testosterone, and cortisol concentrations.
Similar content being viewed by others
Introduction
Although weight-loss programs are designed to eliminate fat mass (FM), 10–53% of the weight lost is fat-free mass (FFM) [1], with greater loses of FFM after interventions involving very low-calorie diets (VLCD, <800 kcal/d) or surgical procedures [1]. In the case of low-calorie diets (LCD), loss of FFM can be attenuated by concomitant exercise and by increasing the ratio of proteins to carbohydrates in the diet [2], although this effect appears to be limited to prolonged interventions eliciting a moderate energy deficit.
Essential amino acids (EAAs), and in particular leucine, are powerful stimulators of protein synthesis [3], an effect that may be potentiated by exercise [3]. However, severe energy deficit, such as during starvation, elicits a neuroendocrine response characterized by high cortisol and reduced testosterone levels in serum (raising the catabolic cortisol/testosterone index) [4]. This is accompanied by reduced insulin levels and increased peripheral insulin resistance [5], which may together attenuate the anabolic response to amino acids [6]. The mechanism by which exercise and amino acids interact to stimulate protein synthesis during severe energy deficiency remains unknown [7].
Serum leptin concentration is also markedly reduced during starvation [8]. Skeletal muscle expresses functional leptin receptors whose signaling responses share some common steps with insulin and growth factors [9]. However, the potential role that leptin may have on the regulation of muscle mass during starvation remains unknown in humans. Protein synthesis in muscle is stimulated by the pathway involving the mammalian target of rapamycin (mTor) protein kinase, which is activated through phosphorylation by Akt (protein kinase B). Activated mTor forms two distinct complexes, TORC1 and TORC2. TORC1 is sensitive to inhibition by rapamycin and propagates downstream signaling by phosphorylating p70 ribosomal S6 kinase (p70S6K, also referred to as S6K1) and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) (also referred to as PHAS-1), resulting in their activation and inhibition, respectively [10].
Phosphorylation of 4E-BP1 and p70S6K by mTor are essential for protein synthesis and hypertrophy in muscle [10]. Akt can promote protein synthesis by inhibiting glycogen synthase kinase 3 beta (GSK3β), impeding its inhibitory action on protein synthesis [11]. Protein synthesis requires sufficient and appropriate amino acid supply, and amino acid deprivation induces the phosphorylation of eukaryotic initiation factor 2 alpha (eIF2α), thereby impeding translation initiation [12]. However, little is presently known concerning the regulation of these signaling cascades by muscle contractions and plasma amino acids in humans under a severe energy deficit.
The current investigation was designed to assess the body composition and major signaling pathways regulating protein synthesis in three different skeletal muscles of overweight volunteers undergoing a severe energy deficit. For this purpose, a VLCD involving intake of solely sucrose or whey protein was combined with 45 min of one-arm cranking and 8 h of walking each day. Accordingly, in each subject one deltoid muscle remained inactive, the contralateral deltoid was exercised moderately, and the vastus lateralis was subjected to prolonged exercise. Our hypothesis was that a whey protein diet will attenuate the loss of muscle mass by activating the Akt/mTor/p70S6K/4E-BP1 pathway and, moreover, that this effect will be more pronounced in the muscles performing more exercise and potentiated by elevated levels of circulating leucine and EAAs.
Methods
Participants
A detailed description of our study population and general procedures, as well as part of the neuroendocrine effects, have been reported previously [13, 14]. The sample size was calculated to reveal any significant difference ≥1.5-fold larger than the coefficient of variation (which was <10% in most cases) between the mean values for any individual variable, with a significance level of P < 0.05 and statistical power of 0.8. Table 1 summarizes the background characteristics of our 15 overweight men assigned randomly (simple randomization) and double-blinded to ingest a diet consisting solely of sucrose (SU; n = 7) or whey protein (Whey PRO; n = 8) during caloric restriction (CRE) phase. After being informed of potential risks and benefits, all subjects provided their written consent to participate. Ethical approval was obtained from the Regional Ethical Review Board of Umeå University (Umeå, Sweden), as well as the ethical committee of the University of Las Palmas de Gran Canaria (Canary Islands, Spain).
Experimental protocol
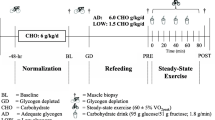
The protocol consisted of a pre-test phase (PRE), CR combined with exercise for 4 days (CRE), followed by a control diet (CD) with reduced exercise for 3 days (Fig. 1). During the PRE, and at the end of the CRE and CD phases, body composition was assessed (Lunar iDXA, GE Healthcare, Madison, WI, USA) [15]; 20 mL blood samples were drawn (in the supine position); and muscle biopsies were then taken. All of these procedures were performed in the morning following a 12-h overnight fast.
Schematic illustration of the experimental protocol. PRE: pre-test; CRE: 4 days of caloric restriction (3.2 kcal/day) and exercise (45 min of single-arm cranking and 8 h of walking each day); CD: 3 days on a diet isoenergetic with that observed during the PRE phase with reduced exercise (<10,000 steps per day); DXA: dual-energy X-ray absorptiometry; IPAQ: International Physical Activity Questionnaire
During CRE, participants ingested either whey protein (PRO, n = 8) or sucrose (SU, n = 7) (0.8 g/kg body weight/day). The solution of whey protein also contained Na+ (308 mg/L) and K+ (370 mg/L), as did the sucrose solution (160 mg of Na+ and 100 mg of K+ per liter). The subjects drank 0.5 L of this solution in the morning (immediately before arm-cranking) and again at midday and at 20:00 h. In addition, both groups were allowed to drink a hypotonic rehydrating solution containing Na+ (160 mg/L), Cl− (200 mg/L), K+ (100 mg/L), citrate (700 mg/L), and sucrose (3 g/L) ad libitum.
Every day during the CD phase, the participants ate three standardized meals containing their normal daily intake of energy (as assessed by weighing all food ingested during the 7-day pre-test period) and were not allowed to walk more than 10,000 steps. This phase was designed to allow replenishment of body water and stabilization of body weight.
Assessment of physical activity, nutrition, and body composition
The short version of the International Physical Activity Questionnaire was employed to assess daily energy expenditure due to physical activity [16]. During the 7-day PRE phase, the participants kept a dietary record and their food intake was analyzed (Dietist XP, Kost & Näringsdata, Bromma, Sweden). During the CD phase, all participants were provided a diet with the same energy content as that recorded during PRE, and the food ingested weighed. Energy intake was also calculated employing the Dietist XP program. During this phase, the sucrose and whey protein groups ingested 2256 ± 513 and 2086 ± 450 kcal/d (mean ± SD), respectively.
Biochemical, hormonal, and muscle signaling-related variables
Detailed information on the procedures used to determine biochemical variables, plasma amino acids, hormones, protein concentration in the muscle biopsies, and signaling kinases can be found in the “Materials and Methods Online Supplement”.
Statistical analyses
All values were examined for normal distribution using the Shapiro–Wilks test and, when necessary, transformed logarithmically before analysis. A repeated-measures ANOVA with time and the two different diets (sucrose vs. whey protein) was applied for analysis of the mean responses. Mauchly’s test of sphericity was run before the ANOVA, and in the case of violation of the sphericity assumption the degrees of freedom were adjusted according to the Huynh–Feldt test. When a significant effect of diet or interaction between diet and time was indicated, pairwise comparisons at specific time points were adjusted for multiple comparisons with the Holm–Bonferroni procedure. The relationship between variables was examined by simple linear regression. The changes in the levels of phosphorylated kinases from PRE to CRE ((CRE-PRE) × 100/PRE) (average of all three muscles) were analyzed for potential correlations with the plasma concentrations of hormones and amino acids. Unless otherwise stated, the values reported are mean ± standard deviations. Statistical significance was set at a P-value ≤ 0.05 and all statistical analyses were performed using SPSS v.21 (SPSS Inc., Chicago, IL, USA).
Results
Body composition and lean mass
The changes in body composition, performance, blood lipids, and neuroendocrine status have been reported previously [13, 14, 17]. After CRE, lean mass was reduced by 6 and 4% in the arms and the legs, respectively (both, P < 0.001; P < 0.05 for time × extremity interaction), with no significant difference between the two diets (P = 0.34). Accordingly, after the 3 days on the control diet, the relative lean mass losses from the legs and trained arm were 57 and 29% less than the losses from the control arm (P < 0.05) [14]. At baseline, the arms and leg muscles had a similar protein concentration (181.7 ± 15.4 and 188.8 ± 12.9 g/kg wet muscle−1, mean of both arms and the vastus lateralis, respectively, P = 0.19). After CRE the protein concentration in the muscle biopsies (mean of the three muscles) was increased by 4.9% (P = 0.010), remaining non-significantly elevated (2.2%, P = 0.34 compared to baseline values) after the 3 days on a control diet (P = 0.84 for extremity × time interaction).
Metabolic and hormonal changes
The changes in plasma concentrations of amino acids and hormones have also been previously documented [14]. In brief, plasma concentrations of asparagine, the three branched-chain amino acids (BCAA) and the EAA were elevated after CRE, with more pronounced increases in the whey protein group [14]. Serum concentrations of insulin and free testosterone, as well as the free androgen index were reduced similarly in both groups, whereas cortisol and the catabolic index (cortisol/total testosterone and cortisol/free testosterone) rose after CRE and CD [14], without significant differences between groups (see Table 4 in reference 14).
Signaling related to protein synthesis in skeletal muscle
Representative immunoblots are presented in Supplementary Fig. 1. In comparison to the PRE period, the level of total Akt (Fig. 2a) was increased to a similar extent in both groups following CD, but only in the arm muscles (P < 0.05 for extremity × time interaction). However, neither pSer473Akt (Fig. 2b) nor the ratio pSer473Akt/total Akt (Fig. 2c) were altered significantly during the intervention (Fig. 2).
Protein expression levels of total and phosphorylated Akt (A-C) and mTOR (D-F) as well as total p70S6K (G) in skeletal muscle following the different experimental stages with ingestion of sucrose or whey protein during severe energy deficit. For a detailed explanation of the various phases, see Fig. 1. PRE: before the intervention; CRE: caloric restriction and exercise; CD: control diet. C. Arm control arm, T. Arm training arm, Legs legs, Means (C. Arm + T. Arm + Legs)/3, Arms (C. Arm + T. Arm)/2, Exercised (T. Arm + Legs)/2. The values shown are mean ± standard errors (n = 7 and 8, for the sucrose and protein groups, respectively) expressed in arbitrary units (a.u.). *P < 0.05 compared to PRE; †P < 0.05 compared to CRE; $P < 0.05 compared to the legs
The changes observed in the total level of mTor were not statistically significant (Fig. 2d), while pSer2448mTor (Fig. 2e) was elevated following CD compared with PRE and CRE (P < 0.05 for time effect), primarily due to the increase in the trained arm (P < 0.05). This response was more marked in the sucrose than in the protein-supplemented group (P < 0.05 for extremity × supplementation). pSer2448mTor did not change significantly in the legs. Concomitantly, pSer2448mTor/total mTor (Fig. 2f) and total p70S6K (Fig. 2g) were unchanged, while pThr389p70S6K was undetectable.
Regardless of the type of supplementation, the mean levels of total 4E-BP1 (Fig. 3a) and Thr37/464E-BP1 (Fig. 3b), a downstream target of mTor, were elevated after CRE and remained so after CD, although Thr37/464E-BP1/total 4E-BP1 did not change significantly (Fig. 3c).
Protein expression levels of total and phosphorylated 4E-BP1 (A-C), GSK3β (D-F), and eIF2α (G-I) in skeletal muscle following the different experimental stages with ingestion of sucrose or whey protein during severe energy deficit. For a detailed explanation of the various phases, see Fig. 1. PRE: before the intervention; CRE: caloric restriction and exercise; CD: control diet. C. Arm control arm, T. Arm training arm, Legs legs, Means (C. Arm + T. Arm + Legs)/3, Arms (C. Arm + T. Arm)/2, Exercised (T. Arm + Legs)/2. The values shown are mean ± standard errors (n = 7 and 8, for the sucrose and protein groups, respectively) expressed in arbitrary units (a.u.). *P < 0.05 compared to PRE; †P < 0.05 compared to CRE; $P < 0.05 compared to the legs
The total GSK3β (Fig. 3d) was reduced in all muscles after CRE and remained so after CD. Ser9GSK3β phosphorylation (Fig. 3e) was also lowered after CRE, but returned to PRE levels after CD, resulting in higher pSer9GSK3β/total GSK3β ratio (Fig. 3f). These changes in pSer9GSK3β were more pronounced in the protein than the sucrose group (P < 0.05 for extremity × supplementation). Total eIF2α (Fig. 3g) did not change significantly, while pSer51eIF2α (Fig. 3h) and the pSer51eIF2α/total eIF2α ratio (Fig. 3i) were reduced in all muscles after CRE, and remained so after CD.
Relationships between changes in plasma amino acids, hormones, lean mass, and muscle signaling
A detailed description of the associations observed between signaling, amino acids, and hormones is reported in the Supplementary Results. Briefly, associations were observed between Akt, pSer473Akt, mTor, 4E-BP1, GSK3β, pSer9GSK3β, eIF2α, and pSer51eIF2α on the one hand, and leptin, insulin, EAA (or BCAA), and cortisol/total testosterone ratio, on the other hand, when both groups were pooled together. Likewise, the change in the Thr37/464E-BP1/total 4E-BP1 ratio from PRE to CRE and from PRE to CD was associated with the corresponding change in cortisol (r = 0.37, P = 0.043; both groups pooled together, n = 30).
The mean regional lean mass was correlated positively with both the mean Thr37/464E-BP1/total 4E-BP1 (r = 0.85, P = 0.004, n = 9) and the mean pSer9GSK3β/total GSK3β (r = 0.75, P = 0.021, n = 9, each point is the mean of the 15 subjects). An association was also observed between the change from PRE to CD in lean mass (all muscles) and the corresponding changes in pSer473Akt (r = −0.33, P = 0.028, n = 45) and pSer473Akt/total Akt (r = −0.34, P = 0.023, n = 45).
Discussion
In contrast to our hypothesis, protein ingestion during a severe energy deficit did not facilitate protein synthesis signaling. Although protein synthesis in skeletal muscle is known to be stimulated by amino acids to a greater extent when the muscle has been exercised, the current study shows that when the energy deficit is severe, protein synthesis becomes resistant to the anabolic effect of amino acids in manner that cannot be counteracted by moderate or large amounts of contractile activity. This study also shows that during a severe energy deficit the serum concentration of the anabolic hormones insulin and testosterone are reduced, blunting the translation initiation due to insufficient inactivation of GSK3β through phosphorylation at serine 9. Ser9GSK3β phosphorylation is catalyzed by Akt, whose own phosphorylation in skeletal muscle is stimulated by insulin, testosterone, and leptin [18,19,20] (Fig. 4). The associations observed here between the reductions in serum insulin, leptin, and pSer9GSK3β support a mechanistic link between the drop in these hormones and the lack of activation of protein synthesis, despite the fact that the circulating concentrations of amino acids were increased in both groups. Consequently, circulating amino acids cannot be incorporated into muscle proteins during a severe energy deficit and are left available for gluconeogenesis, which is essential to maintain the glycaemia under these conditions. This interpretation is supported by cell experiments showing that insulin, via Akt activation, phosphorylates GSK3β [21], while leptin can signal in skeletal muscle through ERK1/2, which is also able to elicit Ser9GSK3β phosphorylation [22]. Moreover, we found that pSer9GSK3β was also associated with total testosterone, which via Akt activation elicits GSK3β phosphorylation [20].
Schematic representation of the proposed mechanisms regulating translation initiation and protein synthesis in human skeletal muscle under a severe energy deficit. In a state with low serum concentrations of insulin, testosterone, and leptin, anabolic signaling activated by insulin-like growth factor-1 signaling (IGF1), acting via its receptor (IGFR) and the insulin receptor substrate 1 (IRS1), is negatively regulated blunting Akt activation. Akt and other protein kinases modulate the GTPase activity of tuberous sclerosis proteins 1 and 2 (TSC1/2), repressing their GTP activity and thus stimulating mTOR-derived signaling. Such stimulation is likely to be lacking during severe energy deficit as reflected by the unchanged levels of phosphorylated Akt observed here. The amino acid induction of protein synthesis via mTOR activation (through various intermediary proteins) is blunted in such a high energy-deficient state, likely due to the proteolytic effect resulting from the increased cortisol and the reduced testosterone plasma concentrations. mTOR regulates protein synthesis by phosphorylating p70 ribosomal protein S6 kinase (p70S6K) and eIF4E-binding protein 1 (4E-BP1). A partial activation of the pathway is seen with the presence of: (1) increased levels of phosphorylated 4E-BP1, which allows the release of the mRNA cap-binding protein eIF4E, required for the formation of the eIF4F complex, essential for the global increase in translation and (2) a reduction in the phosphorylation of the alpha subunit of eIF2 (eIF2α) on serine 51 induces eIF2B activation and thereby partial stimulation of translation initiation. However, p70S6k remains dephosphorylated (inactivated) impeding the phosphorylation of its target (ribosomal protein S6; rpS6), leading to downregulation of mRNA translation. Phosphorylation of the GDP-GTP exchange factor eIF2B by glycogen synthase kinase 3β (GSK3β) leads to its inhibition downregulating translation initiation. Upon anabolic stimulation GSK3β is phosphorylated (inactivated) by Akt allowing translation initiation; however, the severe energy deficit observed here blunts Akt-mediated inactivation of GSK3β. The arrows inside dashed gray boxes presented besides protein markers depict overall outcomes in the present investigation. Green (total form) and black (phosphorylated form) thin arrows represent the overall direction of the results (increase/decrease) after the intervention (mean of all muscles). Blue thick arrows represent the overall effect on protein synthesis (stimulation/inhibition). The length of the arrow is representative of the magnitude of the change. Dashed blue connecting lines indicate indirect effect (mediator proteins not shown)
The acute effects of severe energy deficit on the signaling cascades that regulate protein synthesis
No significant changes in the Akt/mTor/p70S6K signaling pathway were observed here after 4 days of a severe energy deficit close to 5500 kcal/d, regardless of the level of contractile activity or protein supplementation. However, since our measurements were performed in a fasted state and after a 12-h rest period, a transient activation of Akt/mTor/p70S6K during the first hours after the ingestion of supplements may have escaped detection. In fact, the levels of total 4E-BP1 and Thr37/464E-BP1, a downstream target of mTor, were elevated in all muscles after CRE, indicating that this part of the pathway leading to translation initiation was activated. This conclusion is also supported by the reductions of pSer51eIF2α and pSer51eIF2α/total eIF2α observed in all muscles after CRE, which should have facilitated translation initiation [12].
However, this stimulating effect elicited by the elevated Thr37/464E-BP1 and the reduced pSer51eIF2α and pSer51eIF2α/total eIF2α ratio was counteracted by the decrease of pSer9GSK3β after CRE. GSK3β intervenes in the regulation of protein synthesis, cell proliferation, and survival [23]. Upon phosphorylation, GSK3β activity is inhibited what causes its substrate, eukaryotic initiation factor 2B (eIF2B), to become dephosphorylated and activated [11]. Thus, the lowered levels of pSer9GSK3β might have increased the inhibitory action of GSK3β on protein synthesis. In contrast to our expectations, the reduction of pSer9GSK3β was more marked in the protein group, which could have caused a greater inhibition of protein synthesis in this group despite the increased levels of BCAA and leucine [14]. Consequently, the balance between protein synthesis and breakdown was not influenced significantly by the ingestion of whey protein, and both groups lost a similar proportion of lean mass [14].
Since 4E-BP1 inhibits protein synthesis, the increase of total 4E-BP1 observed here might have attenuated protein synthesis. However, this potential effect was counteracted by a commensurate increase in Thr37/464E-BP1 after CRE. Interestingly, there was an inverse association between the levels of total 4E-BP1 on the one hand, and testosterone and free testosterone on the other hand, as well as between the levels of pSer9GSK3β and total testosterone. This suggests that testosterone may downregulate 4E-BP1 and upregulate GSK3β to facilitate protein synthesis, as has been reported in animal and cell culture studies [20]. Likewise, the rise in the level of pSer51eIF2α from PRE to CRE, which impedes translation initiation, was associated with the change in serum cortisol. Altogether, these findings confirm that the serum cortisol/testosterone ratio plays a critical role in the regulation of protein synthesis.
The preservation of muscle mass by exercise cannot be explained by changes in protein synthesis signaling
Despite failing to enhance protein synthesis signaling, exercise resulted in a remarkable preservation of muscle mass [14]. Therefore, our data point toward an attenuation of protein breakdown as the potential main mechanisms by which exercise spares muscle mass during a severe energy deficit. Unexpectedly, administration of whey protein, which is rich in leucine and stimulates protein synthesis while attenuating protein breakdown in humans [24], did not preserve lean mass, regardless of the level of exercise. The amount of protein ingested (0.8 g/kg body weight), as well as the timing (every 8 h), should have maximally stimulated protein synthesis in both the rested and exercised muscles [25]. It remains unknown whether some stimulation of protein synthesis could have been achieved by administering 1.6 or even 2.4 g/kg body weight of dietary protein per day, as reported during LCDs [2, 7]. However, Hector et al. have recently reported a reduction of protein synthesis during a 10-day LCD (40% reduced energy intake), which was not altered by doubling the daily protein intake to 2.4 g/kg body weight [26]. Thus, the present and Hector et al. studies indicate that during moderate [26] and severe energy deficits the skeletal muscle becomes refractory to the stimulation of protein synthesis by the ingestion of proteins, even when three times the RDA for protein is administered [26].
One possible explanation for the lack of differences between our dietary groups involves their similar catabolic response during CRE. In fact, we have demonstrated that the amount of lean mass lost can be predicted from the change in the catabolic index (cortisol/free testosterone) [14], which rose similarly in both groups. We can only speculate as to why the ingestion of whey protein was associated here with an elevated catabolic index. One plausible explanation is the requirement for more pronounced activation of the hypothalamic/pituitary/adrenal axis when whey protein alone is ingested in order to avoid hypoglycemia [27]. In rodents, a diet with high proteins to carbohydrates ratio content promotes the expression of TNF-α mRNA [28], which via activation of NF-κβ, may facilitate muscle proteolysis.
The exercised arm muscles are likely more sensitive than the leg muscles to stimulation of protein synthesis by circulating amino acids during severe energy deficit
It has been reported that exercise potentiates the anabolic response to amino acids [3]. Accordingly, a positive association was observed between the changes from PRE to CRE in pSer2448mTor in the trained arm and the changes in plasma BCAA and leucine. Infusion of BCAA or leucine alone is known to enhance 4E-BP1 phosphorylation in human skeletal muscle [29]. In the present investigation, the serum levels of all three BCAA were elevated after CRE in both groups, although more markedly in the subjects ingesting whey protein, despite the fact that the blood samples were drawn 12 h after the last ingestion of approximately 26 g of whey protein. Despite a greater BCAA and leucine plasma concentration during the CRE in the group supplemented with proteins, 4E-BP1 phosphorylation was similarly increased in both groups, without clear differences between the exercised and non-exercised muscles. However, the protein synthesis-signaling cascade was blunted due to the reduced pSer9GSK3β, probably caused by the increased serum cortisol/testosterone ratio, which affected all muscles. These findings indicate that during severe energy deficit, the anabolic effect of amino acids is not potentiated by contractile activity, in the exercised muscles.
The increase in cortisol levels during fasting is associated with blunted protein synthesis signaling
The elevation of serum cortisol levels in both groups following CRE may have contributed to the inhibition of protein synthesis by lessening the increase in total 4E-BP1, leaving p70S6K in a dephosphorylated state [30] and reducing pSer51eIF2α [31]. Moreover, glucocorticoids inhibit the uptake of amino acids by muscle cells [32]; stimulate KLF-15 (Kruppel-like factor 15), which activates the transcription of the BCAT (branched-chain amino acid aminotransferase) gene [33], encoding an enzyme involved in BCAAs catabolism; and inhibit the phosphorylation of 4E-BP1 and S6K1 mediated by insulin and IGF-I (insulin-like growth factor I) [34]. Glucocorticoids may also inhibit phosphorylation of PI3K (phosphatidylinositol 3-kinase) and Akt [35], upstream kinases of mTor, and Forkhead box protein O (FOXO). Indeed, mice in which Akt is constitutively activated are resistant to the negative effects of glucocorticoids on protein synthesis [36] and muscle mass [37].
Serum leptin concentration is positively associated with protein synthesis signaling in human skeletal muscle
As another novelty, we have found a close association between serum leptin concentration and the mean protein expression of total Akt, total GSK3β, and pSer9GSK3β in the three analyzed muscles. Moreover, there was also a close association between the changes in serum leptin concentration and the changes in pSer9GSK3β. Although these associations are compatible with the proposed anabolic effect of leptin in skeletal muscle [17, 38], the prolonged administration of leptin to amenorrheic athletes has not been associated with significant changes in lean mass [39]. Moreover, no activation of protein synthesis signaling was observed in human skeletal muscle 20 min after the intravenous administration of metreleptin [40]. Since leptin receptors were upregulated [17] and the serum concentration of leptin increased from CRE to CD (i.e., with re-feeding), we think that leptin may have a role in easing a potential anabolic response of skeletal muscles upon re-feeding.
Limitations
Although muscle glycogen concentrations were not assessed, the fact that the total concentration of proteins in skeletal muscle was increased by 4.9% after CRE is compatible with a marked reduction of muscle glycogen and the associated water. In fact, we have estimated that the increase in protein concentration observed here after CRE could be explained by 11–12 g/kg wet muscle reduction in muscle glycogen [41]. Muscle protein concentration returned to baseline values after the 3 days on a control diet, compatible with at least partial recovery of muscle glycogen stores, and therefore our lean mass assessment obtained 3 days after the end of CRE should reflect more trustfully the actual changes in muscle mass, than the measurements performed after CRE. Lastly, we could not find a molecular explanation for the mechanism by which exercise attenuated the loss of muscle mass. However, since all muscles were likely exposed to the same internal neuroendocrine milieu, we think that this molecular mechanism should be specifically triggered by contractile activity inside the active muscle fibers.
In summary, our present findings demonstrate that during severe energy deficit the skeletal muscles become refractory to the anabolic effects of whey protein ingestion. Increased circulating amino acids after the ingestion of proteins during severe energy deficit fail to potentiate the anabolic stimulus of exercise because the reduction of serum insulin and testosterone concentration (together with the increase in cortisol) impede a full activation of the protein synthesis signaling cascade by diminishing Ser9GSK3β, which blunts translation initiation. Future studies should examine whether inhibition of pSer9GSK3β dephosphorylation and/or stimulation of GSK3βSer9 phosphorylation facilitates an anabolic response to amino acids in humans under severe energy deficit. Finally, this study clearly shows that preservation of skeletal muscle mass by exercise during periods of severe energy deficit is not mediated by an enhanced protein synthesis signaling.
References
Chaston TB, Dixon JB, O’Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes. 2007;31:743–50.
Pasiakos SM, Cao JJ, Margolis LM, Sauter ER, Whigham LD, McClung JP, et al. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: a randomized controlled trial. FASEB J. 2013;27:3837–47.
Blomstrand E, Eliasson J, Karlsson HK, Kohnke R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J Nutr. 2006;136:269S–73S.
Cahill GF Jr. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22.
Vendelbo MH, Clasen BF, Treebak JT, Moller L, Krusenstjerna-Hafstrom T, Madsen M, et al. Insulin resistance after a 72-h fast is associated with impaired AS160 phosphorylation and accumulation of lipid and glycogen in human skeletal muscle. Am J Physiol Endocrinol Metab. 2012;302:E190–200.
Biolo G, Ciocchi B, Stulle M, Piccoli A, Lorenzon S, Dal Mas V, et al. Metabolic consequences of physical inactivity. J Ren Nutr. 2005;15:49–53.
Carbone JW, McClung JP, Pasiakos SM. Skeletal muscle responses to negative energy balance: effects of dietary protein. Adv Nutr. 2012;3:119–26.
Karl JP, Smith TJ, Wilson MA, Bukhari AS, Pasiakos SM, McClung HL, et al. Altered metabolic homeostasis is associated with appetite regulation during and following 48-h of severe energy deprivation in adults. Metabolism. 2016;65:416–27.
Guerra B, Santana A, Fuentes T, Delgado-Guerra S, Cabrera-Socorro A, Dorado C, et al. Leptin receptors in human skeletal muscle. J Appl Physiol. 2007;102:1786–92.
Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol. 2014;49:59–68.
Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16.
Baird TD, Wek RC. Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Adv Nutr. 2012;3:307–21.
Calbet JA, Ponce-Gonzalez JG, Perez-Suarez I, de la Calle Herrero J, Holmberg HC. A time-efficient reduction of fat mass in 4 days with exercise and caloric restriction. Scand J Med Sci Sports. 2015;25:223–33.
Calbet JAL, Ponce-Gonzalez JG, Calle-Herrero J, Perez-Suarez I, Martin-Rincon M, Santana A, et al. Exercise preserves lean mass and performance during severe energy deficit: the role of exercise volume and dietary protein content. Front Physiol. 2017;8:483.
Calbet JA, Dorado C, Diaz-Herrera P, Rodriguez-Rodriguez LP. High femoral bone mineral content and density in male football (soccer) players. Med Sci Sports Exerc. 2001;33:1682–7.
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95.
Perez-Suarez I, Ponce-Gonzalez JG, de La Calle-Herrero J, Losa-Reyna J, Martin-Rincon M, Morales-Alamo D, et al. Severe energy deficit upregulates leptin receptors, leptin signaling, and PTP1B in human skeletal muscle. J Appl Physiol. 2017;123:1276–87.
Garcia-Carrizo F, Nozhenko Y, Palou A, Rodriguez AM. Leptin effect on acetylation and phosphorylation of pgc1alpha in muscle cells associated with AMPK and Akt activation in high-glucose medium. J of cellular physiology. J Cell Physiol. 2016;231:641–9.
Kohn AD, Kovacina KS, Roth RA. Insulin stimulates the kinase activity of RAC-PK, a pleckstrin homology domain containing ser/thr kinase. EMBO J. 1995;14:4288–95.
White JP, Gao S, Puppa MJ, Sato S, Welle SL, Carson JA. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol Cell Endocrinol. 2013;365:174–86.
Proud CG. Regulation of protein synthesis by insulin. Biochem Soc Trans. 2006;34:213–6.
Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, et al. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159–70.
Huo X, Liu S, Shao T, Hua H, Kong Q, Wang J, et al. GSK3 protein positively regulates type I insulin-like growth factor receptor through forkhead transcription factors FOXO1/3/4. J Biol Chem. 2014;289:24759–70.
Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. 2011;93:997–1005.
Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr. 2014;99:86–95.
Hector AJ, McGlory C, Damas F, Mazara N, Baker SK, Phillips SM. Pronounced energy restriction with elevated protein intake results in no change in proteolysis and reductions in skeletal muscle protein synthesis that are mitigated by resistance exercise. FASEB J. 2018;32:265–75.
Watts AG, Donovan CM. Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation. Front Neuroendocrinol. 2010;31:32–43.
Diaz-Rua R, Keijer J, Palou A, van Schothorst EM, Oliver P. Long-term intake of a high-protein diet increases liver triacylglycerol deposition pathways and hepatic signs of injury in rats. J Nutr Biochem. 2017;46:39–48.
Liu Z, Jahn LA, Long W, Fryburg DA, Wei L, Barrett EJ. Branched chain amino acids activate messenger ribonucleic acid translation regulatory proteins in human skeletal muscle, and glucocorticoids blunt this action. J Clin Endocrinol Metab. 2001;86:2136–43.
Shah OJ, Anthony JC, Kimball SR, Jefferson LS. Glucocorticoids oppose translational control by leucine in skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1185–90.
Rannels DE, Rannels SR, Li JB, Pegg AE, Morgan HE, Jefferson LS. Effects of glucocorticoids on peptide chain initiation in heart and skeletal muscle. Adv Myocardiol. 1980;1:493–501.
Kostyo JL, Redmond AF. Role of protein synthesis in the inhibitory action of adrenal steroid hormones on amino acid transport by muscle. Endocrinology. 1966;79:531–40.
Shimizu N, Yoshikawa N, Ito N, Maruyama T, Suzuki Y, Takeda S, et al. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab. 2011;13:170–82.
Shah OJ, Kimball SR, Jefferson LS. Acute attenuation of translation initiation and protein synthesis by glucocorticoids in skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E76–82.
Zheng B, Ohkawa S, Li H, Roberts-Wilson TK, Price SR. FOXO3a mediates signaling crosstalk that coordinates ubiquitin and atrogin-1/MAFbx expression during glucocorticoid-induced skeletal muscle atrophy. FASEB J. 2010;24:2660–9.
Frost RA, Lang CH. Multifaceted role of insulin-like growth factors and mammalian target of rapamycin in skeletal muscle. Endocrinol Metab Clin North Am. 2012;41:297–322, vi.
Schakman O, Kalista S, Bertrand L, Lause P, Verniers J, Ketelslegers JM, et al. Role of Akt/GSK-3beta/beta-catenin transduction pathway in the muscle anti-atrophy action of insulin-like growth factor-I in glucocorticoid-treated rats. Endocrinology. 2008;149:3900–8.
Arounleut P, Bowser M, Upadhyay S, Shi XM, Fulzele S, Johnson MH, et al. Absence of functional leptin receptor isoforms in the POUND (Lepr(db/lb)) mouse is associated with muscle atrophy and altered myoblast proliferation and differentiation. PLoS ONE. 2013;8:e72330.
Brinkoetter M, Magkos F, Vamvini M, Mantzoros CS. Leptin treatment reduces body fat but does not affect lean body mass or the myostatin-follistatin-activin axis in lean hypoleptinemic women. Am J Physiol Endocrinol Metab. 2011;301:E99–104.
Moon HS, Huh JY, Dincer F, Schneider BE, Hasselgren PO, Mantzoros CS. Identification and saturable nature of signaling pathways induced by metreleptin in humans: comparative evaluation of in vivo, ex vivo, and in vitro administration. Diabetes. 2015;64:828–39.
Fernandez-Elias VE, Ortega JF, Nelson RK, Mora-Rodriguez R. Relationship between muscle water and glycogen recovery after prolonged exercise in the heat in humans. Eur J Appl Physiol. 2015;115:1919–26.
Acknowledgements
We offer special thanks to José Navarro de Tuero for his excellent technical assistance and to Tobias Lopez Jessen for his help editing the English version of the manuscript.
Funding
This study was financed by grants from the Ministerio de Economía y Competitividad (PI14/01509 and FEDER), ULPGC: ULPAPD-08/01-4, and Östersund municipality.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Martin-Rincon, M., Perez-Suarez, I., Pérez-López, A. et al. Protein synthesis signaling in skeletal muscle is refractory to whey protein ingestion during a severe energy deficit evoked by prolonged exercise and caloric restriction. Int J Obes 43, 872–882 (2019). https://doi.org/10.1038/s41366-018-0174-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0174-2
- Springer Nature Limited
This article is cited by
-
Caloric restriction induces anabolic resistance to resistance exercise
European Journal of Applied Physiology (2020)