Abstract
Background/Objectives
Although obesity is considered an independent risk factor of nephrolithiasis, little is known about the effect of obesity on nephrolithiasis according to metabolic health status. We investigated the association between body mass index (BMI) category and the incidence of nephrolithiasis in metabolically healthy and unhealthy individuals.
Subjects/Methods
The cohort consisted of 270,190 Korean adults free of nephrolithiasis at baseline, who were followed-up annually or biennially for a median of 4.1 years. Nephrolithiasis were determined based on ultrasonographic findings. Being metabolically healthy was defined as not having any metabolic syndrome component. A parametric Cox model was used to estimate the adjusted hazard ratios (HRs) with 95% confidence intervals (CIs).
Results
During 1,415,523.0 person-years of follow-up, 13,450 participants developed nephrolithiasis (incidence rate, 9.5 per 1000 person-years). Obesity was positively associated with an increased risk of incident nephrolithiasis in dose-response manner, but the association was stronger in metabolically healthy individuals. Among metabolically healthy individuals, the multivariable-adjusted HRs (95% CIs) for incident nephrolithiasis comparing BMIs 23–24.9, 25–29.9, and ≥30 with a BMI of 18.5–22.9 kg/m2 as the reference were 1.02 (0.95–1.10), 1.12 (1.03–1.22), and 1.72 (1.21–2.44), respectively, whereas corresponding HRs (95% CIs) in metabolically unhealthy individuals were 1.10 (1.04–1.17), 1.27 (1.20–1.34), and 1.36 (1.22–1.51), respectively. The association between obesity and incident nephrolithiasis was stronger in men and current smokers.
Conclusions
Obesity was associated with a higher incidence of nephrolithiasis in both metabolically healthy and unhealthy individuals, indicating obesity per se as an independent risk factor for nephrolithiasis.
Similar content being viewed by others
Introduction
Nephrolithiasis, also called renal stones, refers to mineral deposits in the renal calyces and pelvis that are found free or attached to the renal papillae [1, 2]. The prevalence and incidence of nephrolithiasis are increasing worldwide, posing significant health and economic burdens [3, 4]. More than half of all patients with nephrolithiasis have symptoms and complications such as renal colic, infection, and renal tract obstruction that require surgical intervention. Nephrolithiasis recurs at a rate of 75% in the first 20 years after the first stone [5, 6]. While nephrolithiasis onset is influenced by age, sex, ethnicity, and geography [4], its pathogenesis is not fully elucidated.
The importance of nephrolithiasis as a systemic metabolic disorder is being increasingly recognized. Nephrolithiasis is closely associated with obesity, diabetes, hypertension, and metabolic syndrome and is considered a feature of renal manifestation of insulin resistance [7]. Epidemiologic studies have suggested that obesity and higher body mass index (BMI) are associated with increased risk of symptomatic renal stones [8,9,10]. However, in patients with obesity, an increased risk of nephrolithiasis can be mediated by insulin resistance and other obesity-associated metabolic abnormalities [10]. The obesity-related metabolic disturbance varies among obese phenotype. A subset of obese phenotype, referred to as metabolically healthy obese (MHO), are relatively insulin-sensitive and do not have any accompanying metabolic abnormalities such as dyslipidemia, insulin resistance, hypertension, or an unfavorable inflammatory profile [11]. No previous cohort studies have evaluated the effect of obesity on nephrolithiasis according to the metabolic health status; it is not yet clear whether obesity per se or its associated conditions affect renal stone formation. While there is no general agreement on the criteria to define MHO [12, 13], many studies have allowed two or fewer metabolic components, but the risk of cardiovascular disease increases progressively with the number of metabolic syndrome components, beginning with one component [14, 15]. Indeed, each metabolic syndrome criterion is an established cardiovascular risk factor [16] and has been associated with increased risk of diabetes [17].
Therefore, we examined the association of BMI with the development of nephrolithiasis in a large cohort of metabolically healthy and unhealthy adults free of nephrolithiasis at baseline who underwent a health screening examination program while using a stricter criterion with zero metabolic abnormalities and with no insulin resistance to define metabolically healthy phenotype, as previously applied [18, 19].
Materials and methods
Study population
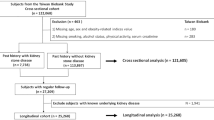
All participants in this study were enrolled in the Kangbuk Samsung Health Study, which is a cohort study of Korean individuals aged ≥18 years who underwent a comprehensive annual or biennial examination at Kangbuk Samsung Hospital Total Healthcare Center in Seoul or Suwon, South Korea [19, 20]. The present analysis included all study participants who underwent a comprehensive health examination between January 2002 and December 2015 and had at least one follow-up visit through December 31, 2015 (n = 294,418).
Among these participants, 24,228 were excluded at baseline for the following reasons (Fig. 1): missing information on uric acid (UA), BMI, high-sensitivity C-reactive protein (hsCRP), glucose, insulin, high-density lipoprotein cholesterol (HDL-C), triglyceride, blood pressure (BP), or abdominal ultrasonography (US) data (n = 9,879); polycystic kidney disease, deformity, hypoplasia, dysgenesis, renal tumor, kidney transplantation, or post-surgical status on abdominal US at baseline (n = 869); estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2 at baseline (n = 4,370); a history of kidney disease including renal stones or a pharmacologic history for urinary stone (n = 656); and nephrolithiasis on abdominal US at baseline (n = 9,942). Finally, a total of 270,190 participants were eligible for this study.
The study was approved by the Institutional Review Board of Kangbuk Samsung Hospital, which waived the requirement for informed consent due to the use of de-identified data obtained as part of routine health screening exams.
Measurements
Data on demographic characteristics, such as smoking status, alcohol consumption, physical activity, educational level, medical history of hypertension, diabetes, dyslipidemia, and kidney disease including kidney stones, and medication use were collected by standardized, self-administered questionnaires as previously described [19, 20]. Alcohol consumption was categorized into ≤20 g/day or >20 g/day. Smoking status was categorized into never, former, or current smokers. The weekly frequency of moderate- or vigorous-intensity physical activity was assessed and categorized into at least 3 times per week or <3 times per week.
Weight, height, and sitting BP were assessed by trained nurses. Weight was measured to the nearest 0.1 kg using a multi-frequency bioelectrical impedance analyzer (InBody 3.0 and InBody 720, Biospace Co., Seoul Korea) that was calibrated every morning before testing. Height was measured to the nearest 0.1 cm using a stadiometer with the participant standing barefoot. BMI was calculated as weight in kilograms divided by height in meters squared and was categorized according to Asian-specific criteria [21]: underweight, BMI < 18.5 kg/m2; normal weight, BMI of 18.5–22.9 kg/m2; overweight, BMI of 23–24.9 kg/m2; obese I, BMI of 25–29.9 kg/m2, and obese II, BMI ≥ 30 kg/m2.
Blood samples were collected from the antecubital vein after an at least 10-h fast. The serum levels of fasting glucose, UA, total cholesterol, low-density lipoprotein cholesterol (LDL-C), HDL-C, triglycerides (TG), alanine aminotransferase (ALT), hsCRP, and creatinine (Cr) were measured as previously described [19, 20]. Serum Cr was measured using the kinetic alkaline picrate (Jaffe) method in an automated chemistry analyzer (from 2002 to 2009, Advia 1650 Autoanalyzer; Bayer Diagnostics, Leverkusen, Germany; and from 2010 to 2015, Modular D2400, Tokyo, Roche) and eGFR was calculated using the CKD-EPI equation. Since we did not use an isotope dilution mass spectrometry (IDMS) method to determine serum Cr from 2002 to 2009, we reduced the Cr levels by 5%, the calibration factor used to adjust non-standardized MDRD study samples to IDMS [22, 23]. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as follows: fasting insulin (mg/dL) × fasting glucose (mg/dL)/405 [24].
Metabolically unhealthy persons were defined as those having at least one of the following metabolic abnormalities [19]; (1) fasting glucose level ≥100 mg/dL or current use of glucose-lowering agents; (2) BP ≥ 130/85 mm Hg or current use of BP-lowering agents; (3) elevated TG level (≥150 mg/dL) or current use of lipid-lowering agents; (4) low HDL-C (<40 mg/dL in men or <50 mg/dL in women); or (5) insulin resistance, defined as HOMA-IR score ≥2.5 [24] Otherwise, being metabolically healthy was defined as none of the metabolic syndrome components described above.
All participants underwent abdominal US at baseline and at each visit. Experienced radiologists who were unaware of the aim of the study performed abdominal US using a Logic Q700 MR 3.5 MHz transducer (GE, Milwaukee, WI, USA). Images were captured in a standard position with the subjects in the supine position with the right arm raised above the head. Diagnosis of renal stones was made when hyperechoic structures causing acoustic shadowing were seen in the collecting system on US [25]. US diagnosis of fatty liver was determined based on known standard criteria, including presence of a diffuse increase of fine echoes in the liver parenchyma compared with kidney or spleen parenchyma, deep beam attenuation, and bright vessel walls [26].
Statistical analyses
The characteristics of the study participants were explored according to the previously mentioned BMI categories (<18.5, 18.5–22.9, 23.0–24.9, 25.0–29.9, or ≥30 kg/m2). To compare results among different BMI categories, one-way analysis of variance was used for continuous variables, and a Chi-square test was used for categorical variables.
The primary end point was development of nephrolithiasis. Each participant was followed from the baseline exam until either development of nephrolithiasis or the last health exam conducted prior to December 31, 2015, whichever came first. The incidence rate was calculated as the number of incident cases divided by the number of person-years of follow-up. Since nephrolithiasis was known to have developed between the two visits but the precise time at which it developed was unknown, a parametric proportional hazard model was used to account for this type of interval censoring (stpm command in Stata) [27]. In these models, the baseline hazard function was parameterized with restricted cubic splines in log time with four degrees of freedom.
Hazard ratio (HR) and 95% confidence interval (CI) were calculated for incident nephrolithiasis according to BMI in total, metabolically healthy, and unhealthy individuals. Data that were initially adjusted for age and sex were then further adjusted for center (Seoul or Suwon), year of screening exam, smoking status (never, past, current, or unknown), alcohol intake (0, <20, ≥20 g/day, or unknown), regular exercise, and education level (high school graduate or less, community college or university graduate, graduate school or higher, or unknown) (Model 1). Model 2 was further adjusted for total cholesterol, HDL, TG, glucose, systolic BP, HOMA-IR, and hsCRP. The proportional hazards assumption was assessed by examining graphs of estimated log (−log) survival. To determine linear trends of incidence, the number of categories was used as a continuous variable and tested on each model. To further explore the shape of dose–response relationship of BMI level with the development of nephrolithiasis, restricted cubic splines with knots were used at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles of BMI distribution.
Additional analyses were performed in pre-specified subgroups as previously applied in studies on metabolically healthy obesity [18, 19]: age (<50 vs. ≥50 years), sex (female vs. male), smoking status (never vs. current smokers), alcohol intake (<20 vs. ≥20 g/day), vigorous exercise (<3 vs. ≥3 time per week), HOMA-IR (<2.5 vs. ≥2.5), hsCRP (<1.0 vs. ≥1.0 mg/L), and the presence of fatty liver (No vs. Yes) were performed. Interactions between BMI categories and subgroup characteristics were tested using likelihood ratio tests comparing models with vs. without multiplicative interaction terms.
It is possible for a participant to have developed a stone and pass it, leaving no changes on US between baseline and follow-up. Unfortunately, the information on medical utilization or hospitalization was not available in this study. However, via self-administered questionnaire, the information on history of kidney/bladder disease including urolithiasis was collected using a single question; thus the history of nephrolithiasis could not have been differentiated from other kidney/bladder disease. We performed the analysis on the association between BMI category and self-report of physician-diagnosed kidney/bladder disease including urolithiasis.
Statistical analyses were carried out using STATA version 14.0 (StataCorp LP, College Station, TX, USA). All P values <0.05 were considered statistically significant.
Results
Table 1 show the baseline characteristics of participants according to BMI category. Comparisons between metabolically healthy and unhealthy phenotypes are also shown (Supplementary Table 1). The overall prevalence of metabolically unhealthy phenotype was 52.4%, and its prevalence increased with increasing BMI category as follows: 19.9% in BMI < 18.5 kg/m2; 36.7% in 18.5–22.9 kg/m2; 59.8% in BMI of 23–24.9 kg/m2; 76.5% in BMI of 25–29.9 kg/m2; and 91.7% in BMI ≥ 30 kg/m2. Participants in higher BMI categories were more likely to be older and male; to exercise and drink alcohol; and to have higher levels of BP, glucose, UA, total cholesterol, LDL-C, TG, ALT, hsCRP, and HOMA-IR than normal-weight or underweight participants. Participants in higher BMI categories were also more likely to have lower levels of education and HDL-C.
During 1,415,523.0 person-years of follow-up, 13,450 participants developed nephrolithiasis (incidence rate, 9.5 cases per 1000 person-years). The median follow-up period for participants was 4.1 years (interquartile range, 2.1–7.8). The association between BMI category and development of nephrolithiasis was examined separately in metabolically healthy and unhealthy persons (Table 2). In both groups, obesity was positively associated with an increased risk of incident nephrolithiasis in a dose–response manner (P for trend < 0.001), but the association was stronger in the metabolically healthy group (P for interaction < 0.001). Among metabolically healthy persons, the multivariable adjusted HRs (95% CIs) for incident nephrolithiasis comparing BMIs 23–24.9, 25–29.9, and ≥30 with a BMI of 18.5–22.9 kg/m2 (as in the normal-weight category) were 1.02 (0.95–1.10), 1.12 (1.03–1.22), and 1.72 (1.21–2.44), respectively, whereas the corresponding HRs (95% CIs) in metabolically unhealthy persons were 1.10 (1.04–1.17), 1.27 (1.20–1.34), and 1.36 (1.22–1.51), respectively (Model 1). This association persisted after adjustment for total cholesterol, HDL, TG, glucose, hsCRP, and HOMA-IR (Model 2). When we analyzed using metabolically healthy normal-weight category as a single reference, multivariable-adjusted HRs (95% CIs) comparing BMIs 23–24.9, 25–29.9, and ≥30 with metabolically healthy normal-weight category were 1.00 (0.93–1.08), 1.08 (1.00–1.17), and 1.61 (1.14–2.29), respectively, in the metabolically healthy group and 1.12 (1.05–1.19), 1.27 (1.20–1.35), and 1.33 (1.19–1.49) in the metabolically unhealthy group (Supplementary Table 2). In spline regression analyses, there was a dose–response relationship between BMI level and development of nephrolithiasis in both metabolically healthy and unhealthy individuals (Figs. 2 and 3). In the analysis on the association between BMI category and self-report of physician-diagnosed kidney/bladder disease including urolithiasis, the results were consistent with the main findings using nephrolithiasis based on US (Supplementary Table 3).
Multivariable-adjusted hazard ratios for nephrolithiasis by BMI level among metabolically healthy individuals. Curves represent adjusted hazard ratios for nephrolithiasis based on restricted cubic splines with knots at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles of BMI distribution. Models were adjusted for age, center, year of screening exam, smoking status, alcohol intake, physical activity, and education level
Multivariable-adjusted hazard ratios for nephrolithiasis by BMI level among metabolically unhealthy individuals. Curves represent adjusted hazard ratios for nephrolithiasis based on restricted cubic splines with knots at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles of BMI distribution. Models were adjusted for age, center, year of screening exam, smoking status, alcohol intake, physical activity, and education level
In the pre-specified subgroup analyses, the associations between BMI categories and nephrolithiasis were similar across study participant subgroups, except for sex and smoking status. The association was stronger in men (P for interaction < 0.001) and current smokers (P for interaction = 0.024). Otherwise, there were no significant interactions by age (<50 vs. ≥50 years), alcohol intake (<20 vs. ≥20 g/day), vigorous exercise (<3 vs. ≥3 time per week), hsCRP (<1.0 vs. ≥1.0 mg/L), the presence of fatty liver (No vs. Yes) or HOMA-IR (<2.5 vs. ≥2.5) (Table 3).
Discussion
In this large cohort study of metabolically healthy and unhealthy individuals, the risk of new-onset nephrolithiasis increased in a dose–response manner as BMI increased. This association persisted after adjusting for measured confounders and residual levels of metabolic factors. These findings suggest that excess adiposity per se can contribute to the development of nephrolithiasis, even in the absence of metabolic abnormalities.
The association between obesity and nephrolithiasis is well established. In a cohort study by Taylor et al., BMI (based on self-reported weight and height) was positively associated with the risk of self-reported stones in both men and women; as BMI increased, risk of kidney stones increased [10]. Semins et al. reported a positive association between obesity (BMI of ≥30 kg/m2) and higher risk of kidney stone disease based on medical claim data in men and women, but the risk of stone disease remained stable as the degree of obesity increased [9]. Recently, Yoshimura et al. reported a positive association between BMI tertile and risk of nephrolithiasis in Japanese men, where risk increased as BMI tertile increased [8]. To our knowledge, the effect of excess adiposity on the risk of nephrolithiasis in metabolically healthy and unhealthy individuals has not previously been examined. The mechanism of stone formation in obese phenotype is believed to involve insulin resistance [8, 10]. Insulin resistance leads to urine acidification, which increases the risk of nephrolithiasis [8, 10]. However, in our study, the association between increased BMI and nephrolithiasis was observed in metabolically healthy phenotype.
The association between BMI and new-onset nephrolithiasis was more evident in metabolically healthy individuals than in unhealthy ones even though the absolute incidence of nephrolithiasis was higher in the metabolically unhealthy group than in the metabolically healthy group. The reason for this is unclear, but the relative contribution of obesity per se on nephrolithiasis is lower in the metabolically unhealthy population compared to metabolically healthy counterparts given that metabolic abnormalities are also risk factors of nephrolithiasis [28].
While the mechanisms by which obesity per se contribute to nephrolithiasis are not yet fully understood, oxidative stress and inflammation might explain the association between obesity and nephrolithiasis. Obesity alters adipokine expression and has been shown to increase levels of inflammatory molecules, including tumor necrosis factor-α and interleukin-6 [29]. Several markers of oxidative stress and inflammation have been detected in both the kidneys and urine of patients with kidney stones [30, 31]. The association between nephrolithiasis and obesity has been attributed to an increase in total caloric intake or a lithogenic diet [32, 33]. Furthermore, obesity alters urine chemistry, thereby increasing the risk of nephrolithiasis [32]. Unfortunately, in our study, information on urine chemistry, markers of inflammation or oxidative stress, and diet was not available; future studies that assess these factors will be required to confirm our hypothesis that oxidative stress and inflammation may be responsible for the association between obesity and nephrolithiasis.
In subgroup analysis, the association between obesity and incident nephrolithiasis was stronger in men or current smokers. Interaction by smoking status may be affected by high prevalence of current smoking in men. Several plausible mechanisms can explain the interaction by sex. First, even though 43.4% of the study population was female, the prevalence of overweight and obesity was much lower in females than in males, resulting in lack of power to detect an association between BMI and nephrolithiasis in women. Second, estrogen might have a protective effect against the formation of kidney stones [34]. In human adipose tissue, aromatase converts adrenal androgen to estrogen; therefore, estrogen production is expected to rise as body weight increases [29]. Third, women are often more aware of their body weight than men [35]. This increased awareness of overweight or obesity might be related to strong personal motivation and implementation of lifestyle changes, which can affect the incidence of nephrolithiasis. Fourth, sex differences in fat distribution can affect the development of nephrolithiasis. Men predominantly store fat in the visceral area, while women tend to store fat predominantly in the gluteal–femoral region [36]. In a recent study by Akarken et al., visceral fat was an independent risk factor of nephrolithiasis, but subcutaneous fat was not [37]. Several studies focusing on the different roles of upper-body and lower-body fat in metabolism have suggested disease-protective effects of lower-body fat [38, 39]. Unfortunately, data on fat distribution were unavailable in our study. Further studies using detailed fat distribution phenotyping of obese phenotype will be helpful in understanding the differential effect of BMI on nephrolithiasis risk between men and women.
There are several limitations to this study. First, while unenhanced computed tomography (CT) is considered the reference standard, our study used US to diagnose nephrolithiasis. In a recent study, US showed 70% sensitivity and 94.4% specificity for detecting nephrolithiasis compared to CT [40]. However, US has a lower sensitivity and specificity than CT but does not require use of radiation, becoming the primary alternative to CT. Furthermore, US is limited by its lower sensitivity for smaller stones <3 mm and its inability to accurately size stones [41, 42]. If participants with small stones undetected on US would be classified as those without incident nephrolithiasis, this type of misclassification might have underestimated the association between BMI and risk of nephrolithiasis. Second, while we used BMI to classify obesity, BMI does not differentiate fat tissue from lean tissue. However, BMI has been shown to correlate well with fat mass and is considered a good indicator of general fatness [43]. Third, we were unable to consider dietary information that may have affected both adiposity level and nephrolithiasis. Fourth, information on hospitalization or specific medication that could have affected nephrolithiasis was not available. When restricted analysis was performed among participants who were not under treatment for hypertension or gout, the association between BMI and nephrolithiasis was consistent with the main findings of overall study population (data not shown). Finally, the majority of subjects in our study were young to middle aged, healthy Korean adults, which may limit the generalizability of our findings to other populations.
In conclusion, in both metabolically healthy and unhealthy individuals, increasing BMI was positively associated with an increased risk of nephrolithiasis in a dose–response manner independent of metabolic health status, indicating the role of obesity per se in the pathogenesis of nephrolithiasis. Further studies are required to unravel the mechanism by which excess adiposity contributes to the pathogenesis of nephrolithiasis.
References
Alelign T, Petros B. Kidney stone disease: an update on current concepts. Adv Urol. 2018;2018:3068365.
Khan SR, Pearle MS, Robertson WG, Gambaro G, Canales BK, Doizi S, et al. Kidney stones. Nat Rev Dis Prim. 2016;2:16008.
Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12:e86–96.
Scales CD Jr., Smith AC, Hanley JM, Saigal CS, Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–5.
Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006;367:333–44.
Parmar MS. Kidney stones. BMJ. 2004;328:1420–4.
Sakhaee K, Maalouf NM, Sinnott B. Clinical review. Kidney stones 2012: pathogenesis, diagnosis, and management. J Clin Endocrinol Metab. 2012;97:1847–60.
Yoshimura E, Sawada SS, Lee IM, Gando Y, Kamada M, Matsushita M, et al. Body mass index and kidney stones: a cohort study of Japanese men. J Epidemiol. 2016;26:131–6.
Semins MJ, Shore AD, Makary MA, Magnuson T, Johns R, Matlaga BR. The association of increasing body mass index and kidney stone disease. J Urol. 2010;183:571–5.
Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–62.
Phillips CM. Metabolically healthy obesity: definitions, determinants and clinical implications. Rev Endocr Metab Disord. 2013;14:219–27.
Fruhbeck G, Toplak H, Woodward E, Halford JC, Yumuk V. Need for a paradigm shift in adult overweight and obesity management - an EASO position statement on a pressing public health, clinical and scientific challenge in Europe. Obes Facts. 2014;7:408–16.
Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: a systematic review and meta-analysis. Ann Intern Med. 2013;159:758–69.
Ho JS, Cannaday JJ, Barlow CE, Mitchell TL, Cooper KH, FitzGerald SJ. Relation of the number of metabolic syndrome risk factors with all-cause and cardiovascular mortality. Am J Cardiol. 2008;102:689–92.
Tsai SP, Wen CP, Chan HT, Chiang PH, Tsai MK, Cheng TY. The effects of pre-disease risk factors within metabolic syndrome on all-cause and cardiovascular disease mortality. Diabetes Res Clin Pract. 2008;82:148–56.
Kahn R, Buse J, Ferrannini E, Stern M, American Diabetes Association, European Association for the Study of Diabetes. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–304.
Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–72.
Chang Y, Ryu S, Choi Y, Zhang Y, Cho J, Kwon M-J, et al. Metabolically healthy obesity and development of chronic kidney disease: a cohort study. Ann Intern Med. 2016;164:305–12.
Chang Y, Kim B-K, Yun KE, Cho J, Zhang Y, Rampal S, et al. Metabolically-healthy obesity and coronary artery calcification. J Am Coll Cardiol. 2014;63:2679–86.
Kim S, Chang Y, Yun KE, Jung HS, Lee SJ, Shin H, et al. Development of nephrolithiasis in asymptomatic hyperuricemia: a cohort study. Am J Kidney Dis. 2017;70:173–81.
World Health Organization. Regional Office for the Western Pacific. The Asia-Pacific perspective: redefining obesity and its treatment. Health Communications Australia: Sydney; 2000.
Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–72.
Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–51.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Schepens D, Verswijvel G, Kuypers D, Vanrenterghem Y. Images in Nephrology. Renal cortical nephrocalcinosis. Nephrol, Dial, Transplant. 2000;15:1080–2.
Mathiesen UL, Franzen LE, Aselius H, Resjo M, Jacobsson L, Foberg U, et al. Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not of fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases. Dig Liver Dis. 2002;34:516–22.
Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21:2175–97.
Wong Y, Cook P, Roderick P, Somani BK. Metabolic syndrome and kidney stone disease: a systematic review of literature. J Endourol. 2016;30:246–53.
Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf). 2006;64:355–65.
Khan SR. Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl Androl Urol. 2014;3:256–76.
Aggarwal KP, Narula S, Kakkar M, Tandon C. Nephrolithiasis: molecular mechanism of renal stone formation and the critical role played by modulators. Biomed Res Int. 2013;2013:292953.
Wollin DA, Skolarikos A, Preminger GM. Obesity and metabolic stone disease. Curr Opin Urol. 2017;27:422–7.
Lieske JC. New insights regarding the interrelationship of obesity, diet, physical activity, and kidney stones. J Am Soc Nephrol. 2014;25:211–2.
Heller HJ, Sakhaee K, Moe OW, Pak CY. Etiological role of estrogen status in renal stone formation. J Urol. 2002;168:1923–7.
Wardle J, Haase AM, Steptoe A. Body image and weight control in young adults: international comparisons in university students from 22 countries. Int J Obes (Lond). 2006;30:644–51.
Blaak E. Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care. 2001;4:499–502.
Akarken I, Tarhan H, Ekin RG, Cakmak O, Koc G, Ilbey YO, et al. Visceral obesity: A new risk factor for stone disease. Can Urol Assoc J. 2015;9:E795–9.
Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat Rev Endocrinol. 2015;11:90–100.
Pinnick KE, Nicholson G, Manolopoulos KN, McQuaid SE, Valet P, Frayn KN, et al. Distinct developmental profile of lower-body adipose tissue defines resistance against obesity-associated metabolic complications. Diabetes. 2014;63:3785–97.
Kanno T, Kubota M, Sakamoto H, Nishiyama R, Okada T, Higashi Y, et al. The efficacy of ultrasonography for the detection of renal stone. Urology. 2014;84:285–8.
Brisbane W, Bailey MR, Sorensen MD. An overview of kidney stone imaging techniques. Nat Rev Urol. 2016;13:654.
Ganesan V, De S, Greene D, Torricelli FCM, Monga M. Accuracy of ultrasonography for renal stone detection and size determination: is it good enough for management decisions? BJU Int. 2017;119:464–9.
Sun Q, van Dam RM, Spiegelman D, Heymsfield SB, Willett WC, Hu FB. Comparison of dual-energy x-ray absorptiometric and anthropometric measures of adiposity in relation to adiposity-related biologic factors. Am J Epidemiol. 2010;172:1442–54.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kim, S., Chang, Y., Yun, K.E. et al. Metabolically healthy and unhealthy obesity phenotypes and risk of renal stone: a cohort study. Int J Obes 43, 852–861 (2019). https://doi.org/10.1038/s41366-018-0140-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0140-z
- Springer Nature Limited
This article is cited by
-
Changes in metabolic overweight phenotypes over time and risk of nephrolithiasis: a cohort study
BMC Public Health (2024)
-
Machine learning models for screening clinically significant nephrolithiasis in overweight and obese populations
World Journal of Urology (2024)
-
Metabolic health status and renal disorders: a cross-sectional study
Scientific Reports (2023)
-
Obesity phenotypes related to musculoskeletal disorders; a cross-sectional study from RaNCD cohort
Archives of Public Health (2022)