Abstract
Background
In women, adhering to an overall healthy lifestyle is associated with a dramatically reduced risk of cardio-metabolic disorders. Whether such a healthy lifestyle exerts an intergenerational effects on child health deserves examination.
Methods
We included 5701 children (9–14 years old at baseline) of the Growing Up Today Study 2, and their mothers, who are participants in the Nurses’ Health Study II. Pre-pregnancy healthy lifestyle was defined as a normal body mass index, no smoking, physical activity ≥150 min/week, and diet in the top 40% of the Alternative Healthy Eating Index-2010. Obesity during childhood and adolescence was defined using the International Obesity Task Force age- and sex-specific cutoffs. Multivariable log-binominal regression models with generalized estimating equations were used to evaluate the association of pre-pregnancy healthy lifestyle and offspring obesity.
Results
We identified 520 (9.1%) offspring who became obese during follow-up. A healthy body weight of mothers and no smoking before pregnancy was significantly associated with a lower risk of obesity among offspring: the relative risks [RRs; 95% confidence intervals (CIs)] were 0.37 (0.31–0.43) and 0.64 (0.49–0.84), respectively. Eating a healthy diet and regular moderate-to-vigorous physical activities were inversely related to offspring obesity risk, but these relations were not statistically significant. Compared to children of mothers who did not meet any low-risk lifestyle factors, offspring of women who adhered to all four healthy lifestyle factors had 75% lower risk of obesity (RR: 0.25, 95% CI: 0.14–0.43).
Conclusion
Adherence to an overall healthy lifestyle before pregnancy is strongly associated with a low risk of offspring obesity in childhood, adolescence, and early adulthood. These findings highlight the importance of an overall healthy lifestyle before pregnancy as a potential strategy to prevent obesity in future generations.
Similar content being viewed by others
Introduction
Obesity during childhood has become a major public health challenge in the United States [1,2,3]. Childhood obesity increases the risk of several health conditions, including high blood pressure, dyslipidemia, and insulin resistance [4,5,6]. In addition, childhood obesity increases the likelihood of entering adulthood with obesity [7], which in turn contributes significantly to the development of chronic diseases, such as type 2 diabetes, cardiovascular disease, and certain types of cancer [8,9,10]. Effective strategies for preventing childhood obesity are critically needed to halt the rising rates of chronic health conditions in adulthood.
A growing body of evidence suggests that maternal lifestyle before or during pregnancy contributes to the risk of obesity in offspring [11,12,13,14,15,16,17,18,19]. Among modifiable lifestyle factors, maternal obesity and smoking are known to have adverse effects on pregnancy outcomes [20, 21] and long-term consequences among offspring through increasing the risk of obesity during childhood [11,12,13,14]. In addition, studies focusing on offspring obesity have also suggested that unhealthy dietary pattern and physical inactivity during pregnancy are associated with an increased BMI in offspring [15,16,17,18,19]. However, no studies have determined the effects of adopting an overall healthy lifestyle before pregnancy that consists of multiple healthy lifestyle and dietary practices, such as having a healthy body weight, no smoking, regular physical activity, and consumption of a healthful diet, that could jointly produce greater health benefits than any individual factors [22]. In women, adhering to such an overall healthy lifestyle was associated with a dramatically reduced risk of developing cardio-metabolic conditions [23,24,25]. Whether such a healthy lifestyle exerts any intergenerational effects on child health deserves examination.
Evaluation of an overall lifestyle before pregnancy in relation to offspring obesity risk is biologically meaningful. Accumulating evidence shows that lifestyle factors (e.g., smoking) have long-lasting effects on DNA methylation, which in turn might lead to changes in gene expression and ultimately to the development of diseases later in life [26, 27]. Since epigenetic modifications remain relatively intact even after the interruption of environmental exposures (i.e., smoking cessation) [26] and epigenetic traits can be passed from mothers to their offspring [28], a process named intergenerational epigenetic inheritance [29], it is likely that mothers’ lifestyle before pregnancy may have lasting effects on children’s health. Of note, a survey showed that women were willing to change their behaviors to adopt a healthier lifestyle during pregnancy [30], although an overall long-term pre-pregnancy lifestyle may also play a critical role in children’s health.
To address this novel research question, we prospectively examined the association of an overall maternal lifestyle before pregnancy with risk of obesity in their children using data from mother–child pairs enrolled in the Nurses’ Health Study II (NHSII) and Growing Up Today Study 2 (GUTS2), respectively.
Subjects and methods
Study population
The study was conducted among NHSII participants [31] and their children who participated in the GUTS2 cohort (http://www.gutsweb.org). NHSII is an ongoing prospective cohort study established in 1989 with the recruitment of 116,430 female registered nurses aged 25–42 years. A baseline questionnaire was administered to collect detailed information on medical history, lifestyle characteristics, and medications. In 2004, participants of NHSII who had children aged 8–15 years received an invitation letter for enrolling their children to participate in GUTS2. Invitation letters and questionnaires were mailed to 17,280 children and a total of 10,918 returned completed questionnaires. Follow-up questionnaires of GUTS2 were sent in 2006, 2008, 2011, and 2013. The study was approved by the Human Subjects Committees of the Harvard T. H. Chan School of Public Health and Brigham and Women’s Hospital. In NHSII and GUTS2 return of the questionnaire was considered as informed consent.
Children in the GUTS2 cohort were born between January 1989 and December 1995. Their mothers reported lifestyle factors on the NHSII questionnaires in 1989, 1991, 1993, and 1995 (Supplementary Figure 1). Using the return date of the NHSII questionnaire, we selected the questionnaire proceeding to the birthday of each child. For women who were pregnant when filling out the questionnaire, we used data assessed in the previous questionnaire cycle if available, or otherwise excluded such women from the analyses. Of 7820 mothers of 10,918 children of GUTS2, 5421 returned a questionnaire before pregnancy corresponding to 6623 children. Of those, we excluded women who reported chronic diseases (diabetes, cardiovascular disease, or cancer; 63 mothers and their 78 children) or had no information in lifestyle factors (625 mothers and their 794 children). Among offspring we excluded those who did not report any measure of body weight throughout follow-up (50 children and their 35 mothers). After these exclusions, 5701 children born to 4698 mothers were included in the final analysis.
The first diet survey was conducted in 1991 and was updated every 4 years. For all births before 1991, we computed cumulative averages between 1991 and 1995 to better assess mothers’ long-term diet quality [32]. We examined changes in diet before and after pregnancy in 2831 mothers who were pregnant between 1991 and 1995, and found no significant difference (p = 0.51) in overall diet quality (Supplementary Figure 2).
Assessment of lifestyle factors in mothers
Dietary information was obtained through validated Food Frequency Questionnaires (FFQs), which use a structured list of over 130 food items and are designed to measure diet over the past year [33, 34]. We calculated an Alternate Healthy Eating Index-2010 (AHEI) score to assess the overall dietary quality [35]. The AHEI diet score summarizes information of the following dietary factors: higher intakes of vegetables, fruits, nuts, whole grains, polyunsaturated fatty acids, and long chain omega-3 fatty acids and lower intakes of red and processed meats, sugar-sweetened beverages, trans-fats, and sodium. Alcohol was not considered in the AHEI score because of the adverse effects of alcohol on fetal outcomes. Physical activity was assessed with a validated questionnaire [36], which inquired about the average time per week participants spent in moderate/vigorous activities during the preceding year. Participants reported height and weight and whether they were current, past, or never smokers on each biennial questionnaire. We computed participants’ body mass index (BMI) by dividing weight in kilograms by the square of height in meters (kg/m2).
Definition of low-risk group
Based on the abundant evidence for health benefits of lifestyle factors in the prevention of chronic diseases among women [23,24,25], we considered four low risk lifestyle practices including eating a healthy diet (top 40% of distribution), having a health body weight (BMI 18.5–25.0), engaging in regular moderate/vigorous physical activity (>150 min/week), and no cigarette smoking.
Outcome assessment
BMI in offspring was calculated by using self-reported weight and height. Previous studies found that self-reported weight and height are reasonably accurate in children and adolescents, although there is a potential underestimation of weight in children less than 14 years of age [37, 38]. To define obesity in childhood and adolescence, we used the age- and sex-specific cutoffs of BMI from the International Obesity Task Force [39], and for offspring aged 18 years and older we defined obesity by using World Health Organization cutoff points (i.e., BMI ≥30) [40]. Our main outcome is being obese at least once during the follow-up in GUTS2 (2004–2013) [41]. Our secondary outcome is persistent obesity, which we defined as being obese in at least two consecutive self-reports during follow-up.
Assessment of the covariates and risk factors of offspring obesity
Information on covariates and other risk factors for offspring obesity were collected through the GUTS2 baseline questionnaire in 2004, biennial questionnaires of NHSII, and NHSII pregnancy questionnaire in 2009 [42]. The GUTS2 baseline questionnaire inquired about the date of birth, gender, duration of breastfeeding, and other characteristics for each child. Maternal race/ethnicity, pre-pregnancy alcohol intake, parity, educational attainment of spouse/partner, and other information were recorded in NHSII questionnaires. Maternal age at delivery was calculated as the difference between children’s and mothers’ date of birth. The NHSII pregnancy questionnaire administered in 2009 assessed lifetime pregnancy information for each child on the type of delivery, hypertensive disorders during pregnancy, pre-eclampsia, gestational diabetes, offspring birth weight, and gestational age at delivery.
Statistical analysis
We assessed associations with risk of offspring obesity for each maternal lifestyle factor independently, as well as for the overall maternal lifestyle before pregnancy, by calculating the relative risks (RRs) and 95% confidence intervals (CIs) using multivariable log-binominal regression models with generalized estimating equations (GEE) and specifying an exchangeable correlation structure. Correlations of outcomes between siblings born to the same mother were accounted for using the GEE model. Covariates in multivariable models included maternal factors such as age at delivery, mom’s race/ethnicity, parity, and pre-pregnancy alcohol intake; and educational attainment of spouse/partner; and offspring gender. We generated a missing category for covariates, including parity (0.8%) and educational attainment of spouse/partner (4.8%). Given the small proportion of missing in parity, we assumed parity of 1 to avoid unstable estimates caused by sparse missing parity.
We calculated the population attributable risk (PAR) percentages and 95% CIs to estimate the proportion of offspring obesity that would be potentially avoided if all women were in low-risk group [43]. We used multivariable log-binominal GEE models to calculate RRs comparing participants in the low-risk category with the rest of the GUTS2 participants when calculating the PARs.
We performed secondary analyses to assess the robustness of our findings. We evaluated effect modification by risk factors for offspring obesity including pre-pregnancy BMI, pregnancy complications, gestational age, birth weight, breastfeeding, and parity. P values for interaction were assessed by testing the significance of multiplicative interaction term in the multivariable regression model. Moreover, we conducted the analysis separately in boys and girls to explore potential gender differences. Given that maternal BMI may mediate the effects of maternal lifestyle and diet on offspring health, we performed a secondary analysis by including the other three low-risk lifestyle factors to define the overall healthy lifestyle and adjusted for BMI to evaluate whether maternal BMI accounted for the association for other low-risk lifestyle factors. Finally, to analyze time-varying data we used GEE to take into consideration of all available information in obesity status through follow-up, allowing obesity measures missing at random.
All statistical tests were two-sided and were considered statistically significant at p < 0.05. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline characteristics of the 5701 offspring and their 4698 mothers are presented in Table 1. Women were on average 33 years old at the delivery, had a healthy body weight (mean BMI 23.0), and did not smoke (92.6%) before pregnancy. The offspring population included more girls (53.1%) than boys.
We identified 520 (9.1%) offspring who were ever obese during follow-up. Table 2 shows the associations between individual maternal lifestyle factors and risk of offspring obesity during childhood through early adulthood. In multivariable analysis, offspring born to women with obesity (BMI ≥30) had an RR of 4.15 (95% CI: 3.36–5.13) of developing obesity, compared to children born to mothers with a BMI of 18.5–25. Offspring of women who smoked before pregnancy were also at a higher risk (RR: 1.64, 95% CI: 1.25–2.15) of having obesity than children of mothers who never smoked. Pre-pregnancy AHEI diet score and moderate-vigorous physical activity were not associated with the risk of offspring obesity.
Table 3 presents the association between low-risk lifestyle factors in women and risk of obesity in offspring. The risk of offspring obesity was 63% (RR: 0.37, 95% CI: 0.31–0.43) lower in children of women with a healthy weight compared to the rest. Compared to offspring of women who smoked before pregnancy, children of non-smoking mothers had 36% (RR: 0.64, 95% CI: 0.49–0.84) lower risk of obesity. Adherence to a healthy diet and regular physical activity were not significantly associated with offspring obesity risk; the RRs (95% CIs) were 0.98 (0.82, 1.17) and 0.90 (0.76, 1.08), respectively.
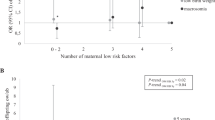
When we grouped women based on the number of low-risk factors, a higher number of low-risk factors was significantly associated with a lower risk of offspring obesity (Fig. 1 and Supplementary Table 1). Offspring born to women who adhered to all four low-risk factors had 75% lower risk (RR: 0.25, 95% CI: 0.14–0.43) of obesity, compared to children born to mothers who did not adhere to any low-risk factor. The corresponding PAR was 55.1% (95% CI: 41.9–63.2%). These associations did not change materially when analyses were stratified by gender (Supplementary Figure 3 and Supplementary Table 2) and were strengthened when we evaluated the low-risk factors in relation to persistent obesity in offspring (Supplementary Table 3). The risk of persistent obesity in offspring of mothers who adhered to four low-risk factors was 84% (RR: 0.16, 95% CI: 0.04–0.59) lower than in offspring of women who did not adhere to any low-risk factor. Adherence to three low-risk factors (healthy diet, ≥150 min/week of moderate/vigorous exercise, and non-smoking) was marginally associated with offspring obesity risk (RR: 0.64, 95% CI: 0.41–1.00), in comparison with offspring of women with no low-risk factors. Adjusting for BMI led to attenuated risk reduction of 12% (RR: 0.76, 95% CI: 0.49–1.16) and the mediation analysis showed that 47.9% of this association was explained by BMI (Supplementary Table 4).
Association between pre-pregnancy low-risk factors and risk of obesity in offspring. PAR population-attributed risk. Adjusted for mother’s age at birth (years), mom’s race/ethnicity (white, others), parity (null-parity, 1, 2, 3, ≥4 previous pregnancies), pre-pregnancy alcohol intake (0, 0–5, 5–15, ≥15 g/day), educational attainment of spouse/partner (high school/college, graduate school), and gender of the offspring (boy, girl). Reference group for relative risk calculation is all other women with no low-risk factor. Table 1 in supplement shows the numeric estimates of relative risk and population-attributed risk
We did not observe effect modification by established childhood obesity risk factors (all p > 0.05 for interaction) (Table 4). Compared with offspring born to women who did not adhere to any low-risk factor, the effect estimates of obesity risk in offspring of women who adhered to all low-risk factors did not materially change by strata of gestational age, pregnancy complications, and pre-pregnancy BMI.
The time-varying analysis showed that compared to children born from women with any low-risk factor, offspring of women with four low-risk factors had 80% (RR: 0.20, 95% CI: 0.09–0.42) lower risk of obesity (Supplementary Table 5).
Discussion
In this prospective investigation among US women and their children, we observed that a pre-defined overall healthy lifestyle before pregnancy characterized by maintenance of a healthy body weight, avoidance of cigarette smoking, moderate/vigorous exercise, and consumption of a healthful diet, was strongly associated with a lower risk of obesity in offspring. Offspring born to women who adhered to all four healthy lifestyle factors had 75% lower risk of developing obesity in childhood through early adulthood, in comparison with children born to mothers without any low-risk factors. We found that maternal lifestyle factors could explain 55% of offspring obesity during childhood through early adulthood. The majority of this relationship was explained by maternal pre-pregnancy BMI alone. These findings demonstrate the potentially important role of maternal lifestyle, especially weight control, before entering pregnancy in the development of obesity in offspring.
Several studies have been conducted to evaluate the role of lifestyle factors during pregnancy or the period proximal to pregnancy on the risk of offspring obesity. A meta-analysis evaluating the association of pre-pregnancy BMI with offspring obesity showed that children born to mothers who were obese had three times higher risk of obesity during childhood [11]. Findings from another meta-analysis including 236,687 children revealed that offspring of mothers who smoked during pregnancy had 55% greater risk of becoming obese during childhood than children of mothers who did not smoke [13]. In contrast, the evidence is limited regarding the role of maternal physical activity and dietary patterns in relation to offspring obesity, and such evidence is primarily generated from investigations that focused on these lifestyle practices during pregnancy [15,16,17,18,19]. In 5125 Greek mother-offspring pairs, higher physical activity levels during pregnancy were associated with a lower risk of offspring obesity at age 8 years [19]. Regarding maternal diet, findings from a Danish study showed that maternal “western diet” consisted of high intakes of red and processed meat and high-fat dairy products was associated with higher risk of lower birth weight, which is a risk factor of childhood obesity [44]. In a US study, a higher AHEI score of maternal diet during pregnancy was not significantly associated with birth weight [45]. Lastly, a prospective study of Dutch mother-offspring pairs failed to demonstrate significant associations between either a healthy diet pattern or an unhealthy diet pattern consumed during pregnancy and body composition in children at age six after adjustment for other childhood obesity risk factors [15]. Apparently, more evidence is needed to elucidate whether long-term physical activity levels and healthy diet practiced before pregnancy is beneficial for children’s metabolic health.
The data collected in the NHSII and GUTS2 allowed us to evaluate the joint effects of major lifestyle factors in women before pregnancy in relation to the risk of offspring obesity. We confirmed the findings of previous studies that maternal obesity is one of the most influential risk factors for obesity in offspring. Likewise, in line with previous reports, we found that pre-pregnancy smoking was independently associated with the risk of offspring obesity. The lack of significant association for physical activity and diet are unexpected, although lack of long-term repeated assessments of these factors before pregnancy may play a role in these null associations. Moreover, the adjustment of maternal BMI may account for partially the beneficial effects of eating a healthy diet and being physically active because maternal body weight control may likely operate in the causal pathways linking diet and physical activities with offspring health. Nonetheless, our results further suggest that the benefits of adhering to an overall healthy lifestyle exceed those of any single lifestyle factors.
A healthy lifestyle and diet may share many health effects in common which in combination contribute to a dramatic decrease in the risk of chronic diseases [23,24,25]. A consumption of healthy diet, maintaining a healthy body weight, regular physical activity, and avoidance of smoking could induce favorable metabolic and molecular alterations, resulting in improved insulin sensitivity, reduced systemic inflammation, and alleviated oxidative stress which in turn will delay the organ damage and consequently decrease the risk of chronic diseases [46, 47]. In women, lifestyle factors could influence the development and programming of fetus through their effects on the intrauterine environment [14]. When the intrauterine environment is exposed to metabolic stresses induced by hyperinsulinemia, inflammation, and other metabolic changes, the developmental programming and metabolic adaptation of fetus may be altered through epigenetic changes or the disturbance of the homeostatic control mechanisms, leading to an elevated risk of childhood obesity [48]. Indeed, findings from an offspring epigenome-wide human study identified 28 CpG sites that are differentially methylated in the offspring of mothers with obesity [49]. Other potential mechanisms could be ascribed to the development and function of adipose tissue, pancreas, liver, and brain of the fetus. Studies conducted in rodents showed that maternal obesity could promote morphology alteration of adipocytes in the fetus that is related to the development of offspring obesity [50]. Similarly, a maternal diet rich in calories during pregnancy is associated with adverse programming of the hypothalamus in the fetus, which may lead to hyperphagia and obesity [51]. Furthermore, research has shown that epigenetic modifications following environmental exposures could persist for several years even after the exposures stopped [26]. For example, an epigenome-wide study of cigarette smoking showed that methylation levels of 36 CpGs (among 2568 CpGs that were differentially methylated in a meta-analysis [26] comparing former versus never smokers) annotated to 19 genes (e.g., TIAM2, PRRT1, and AHRR) in former smokers did not recover to never-smoker levels even after 30 years of smoking cessation [26]. In a review of the dynamics of epigenetic phenomena across generations, Burggren [28] has hypothesized that epigenetic traits could be transmitted from one generation to the next. These mechanisms may pertain to our novel findings that linked pre-pregnancy lifestyle with childhood obesity.
Strengths of our study include a large sample size, prospective study design with long follow-up, detailed pre-pregnancy information acquired by using validated questionnaires, and information on pregnancy which allowed us to conduct comprehensive analyses. Although our goal is to examine long-term lifestyle and dietary practices of mothers before they enter pregnancy in relation to their offspring’s health, we mainly used single assessment of lifestyle practices in this analysis to maintain a prospective temporality of the associations of interest. This may introduce misclassification of long-term lifestyle behaviors, although the impact of such a misclassification is likely to nullify the associations because of the prospective study design. Given the prospective study design, the possibility of recall bias is unlikely. Another limitation of our study is the lack of genetic information of mothers and offspring. The potential interactions between genetic predisposition and lifestyle or diet may also play a critical role in the development of obesity later in life [48, 52]. NHSII participants are predominantly white, reflecting the race/ethnic composition of the nursing profession at the time of study enrollment. Therefore our results may not apply to other race/ethnic groups. In addition, the prevalence of specific chronic disease risk factors, such as obesity, is lower in our population compared to the general US population. More studies are warranted to extend this research to more general populations. Another limitation is that we used self-reported weight and height to estimate the obesity status among children, thus introducing measurement errors in outcome ascertainments. Nevertheless, it is unlikely that misclassification of obesity status was associated with maternal lifestyle before pregnancy, and therefore the measurement errors may likely attenuate true associations. In the current analysis, we focused on pre-pregnancy lifestyle practices and did not explicitly assess lifestyle during pregnancy and therefore could not separate the roles of lifestyle before and during pregnancy in the risk of childhood obesity. This remains a question for future studies. Lastly, as in any observational studies, we cannot exclude the possibility of uncontrolled confounding such as offspring physical activity and diet quality, or residual confounding.
Conclusion
In conclusion, we found that children of mothers who adhered to an overall healthful lifestyle before pregnancy had a substantially lower risk of obesity during childhood through early adulthood. Moreover, we observed a monotonic dose–response relationship of this association in that healthier lifestyle practices predicted greater reduction in childhood obesity risk, although clearly, maintaining a healthy body weight and non-smoking of the mom were the two single most critical maternal factors to predict offspring BMI. These findings underscore the importance of adopting an overall healthy lifestyle, particularly in women who plan a pregnancy, to prevent obesity and metabolic consequences later in life of their children.
References
Burton BT, Foster WR, Hirsch J, Van Itallie TB. Health implications of obesity: an NIH consensus development conference. Int J Obes. 1985;9:155–170.
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–14.
Lobstein T, Baur L, Uauy R. Obesity in children and young people: a crisis in public health. Obes Rev. 2004;5:4–104.
Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med. 2003;157:821–7.
Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics. 1999;103:1175–82.
Invitti C, Guzzaloni G, Gilardini L, Morabito F, Viberti G. Prevalence and concomitants of glucose intolerance in European obese children and adolescents. Diabetes Care. 2003;26:118–24.
Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9:474–88.
Hu Y, Bhupathiraju SN, de Koning L, Hu FB. Duration of obesity and overweight and risk of type 2 diabetes among US women. Obesity. 2014;22:2267–73.
Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–77.
Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes. 2011;35:891–8.
Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS ONE. 2013;8:e61627.
Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes. 2008;32:201–10.
Rayfield S, Plugge E. Systematic review and meta-analysis of the association between maternal smoking in pregnancy and childhood overweight and obesity. J Epidemiol Community Health. 2017;71:162–73.
Gaillard R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur J Epidemiol. 2015;30:1141–52.
van den Broek M, Leermakers ET, Jaddoe VW, Steegers EA, Rivadeneira F, Raat H, et al. Maternal dietary patterns during pregnancy and body composition of the child at age 6 y: the Generation R Study. Am J Clin Nutr. 2015;102:873–80.
Martin CL, Siega-Riz AM, Sotres-Alvarez D, Robinson WR, Daniels JL, Perrin EM, et al. Maternal dietary patterns during pregnancy are associated with child growth in the first 3 years of life. J Nutr. 2016;146:2281–8.
Bisson M, Tremblay F, St-Onge O, Robitaille J, Pronovost E, Simonyan D, et al. Influence of maternal physical activity on infant’s body composition. Pediatr Obes. 2017;12:38–46.
Kong KL, Gillman MW, Rifas-Shiman SL, Wen X. Leisure time physical activity before and during mid-pregnancy and offspring adiposity in mid-childhood. Pediatr Obes. 2016;11:81–87.
Mourtakos SP, Tambalis KD, Panagiotakos DB, Antonogeorgos G, Arnaoutis G, Karteroliotis K, et al. Maternal lifestyle characteristics during pregnancy, and the risk of obesity in the offspring: a study of 5,125 children. BMC Pregnancy Childbirth. 2015;15:66.
Leddy MA, Power ML, Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol. 2008;1:170–8.
DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. J Fam Pract. 1995;40:385–94.
Dawson IG, Dohle S. Towards an understanding of adult judgments of synergistic health benefits. Br J Health Psychol. 2016;21:204–23.
van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;337:a1440.
Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–7.
Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22.
Joehanes R, Just AC, Marioni RE, Pilling LC, Reynolds LM, Mandaviya PR, et al. Epigenetic signatures of cigarette smoking. Circ Cardiovasc Genet. 2016;9:436–47.
Alegria-Torres JA, Baccarelli A, Bollati V. Epigenetics and lifestyle. Epigenomics. 2011;3:267–77.
Burggren WW. Dynamics of epigenetic phenomena: intergenerational and intragenerational phenotype ‘washout’. J Exp Biol. 2015;218:80–87.
Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell. 2014;157:95–109.
Crozier SR, Robinson SM, Borland SE, Godfrey KM, Cooper C, Inskip HM. Do women change their health behaviours in pregnancy? Findings from the Southampton Women’s Survey. Paediatr Perinat Epidemiol. 2009;23:446–53.
Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–96.
Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–40.
Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–67.
Willett WC, Sampson L, Browne ML, Stampfer MJ, Rosner B, Hennekens CH, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127:188–99.
Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–18.
Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–9.
Himes JH, Faricy A. Validity and reliability of self-reported stature and weight of US adolescents. Am J Hum Biol. 2001;13:255–60.
Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 2000;106:52–58.
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3.
Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452.
Yuan C, Gaskins AJ, Blaine AI, Zhang C, Gillman MW, Missmer SA, et al. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr. 2016;170:e162385.
Michels KB, Willett WC, Graubard BI, Vaidya RL, Cantwell MM, Sansbury LB, et al. A longitudinal study of infant feeding and obesity throughout life course. Int J Obes. 2007;31:1078–85.
Wacholder S, Benichou J, Heineman EF, Hartge P, Hoover RN. Attributable risk: advantages of a broad definition of exposure. Am J Epidemiol. 1994;140:303–9.
Knudsen VK, Orozova-Bekkevold IM, Mikkelsen TB, Wolff S, Olsen SF. Major dietary patterns in pregnancy and fetal growth. Eur J Clin Nutr. 2008;62:463–70.
Poon AK, Yeung E, Boghossian N, Albert PS, Zhang C. Maternal dietary patterns during third trimester in association with birthweight characteristics and early infant growth. Science. 2013;2013:786409.
Rizza W, Veronese N, Fontana L. What are the roles of calorie restriction and diet quality in promoting healthy longevity? Ageing Res Rev. 2014;13:38–45.
Powers SK, Lennon SL, Quindry J, Mehta JL. Exercise and cardioprotection. Curr Opin Cardiol. 2002;17:495–502.
Whitaker RC, Dietz WH. Role of the prenatal environment in the development of obesity. J Pediatr. 1998;132:768–76.
Sharp GC, Lawlor DA, Richmond RC, Fraser A, Simpkin A, Suderman M, et al. Maternal pre-pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: findings from the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2015;44:1288–304.
Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010;140:387–98.
Poston L. Maternal obesity, gestational weight gain and diet as determinants of offspring long term health. Best Pract Res Clin Endocrinol Metab. 2012;26:627–39.
Tint MT, Fortier MV, Godfrey KM, Shuter B, Kapur J, Rajadurai VS, et al. Abdominal adipose tissue compartments vary with ethnicity in Asian neonates: growing up in Singapore toward healthy outcomes birth cohort study. Am J Clin Nutr. 2016;103:1311–7.
Acknowledgements
We thank the thousands of participants in the Growing Up Today Study as well as their mothers. This study was supported by grants UM1-CA176726, P30-DK046200, U54-CA155626, T32-DK007703-16, HD066963, HL096905, DK084001, OH009803, and MH087786 from the National Institutes of Health. CZ is supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. QS is supported by NIH grants, ES021372, ES022981, and HL34594. GZ is supported by a Unilever postdoctoral fellowship. ES is supported by Center for Disease Control and Prevention/The National Institute for Occupational Safety and Health.
Author contributions
KD and QS conceptualized the analysis, performed data analysis, interpreted the data, drafted the initial manuscript, and revised the manuscript; ES and AEF designed the study, obtained funding and critically reviewed and revised the manuscript; GZ and CY performed statistical analysis and critically reviewed and revised the manuscript; CZ, XW, FBH, and JEC critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study was approved by the Human Subjects Committees of the Harvard T. H. Chan School of Public Health and Brigham and Women’s Hospital. In NHSII and GUTS2 return of the questionnaire was considered as informed consent.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Dhana, K., Zong, G., Yuan, C. et al. Lifestyle of women before pregnancy and the risk of offspring obesity during childhood through early adulthood. Int J Obes 42, 1275–1284 (2018). https://doi.org/10.1038/s41366-018-0052-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0052-y
- Springer Nature Limited
This article is cited by
-
Unsupervised clustering analysis of comprehensive health status and its influencing factors on women of childbearing age: a cross-sectional study from a province in central China
BMC Public Health (2023)
-
Maternal diet quality during pregnancy is associated with biomarkers of metabolic risk among male offspring
Diabetologia (2021)
-
Associations between a maternal healthy lifestyle score and adverse offspring birth outcomes and childhood obesity in the Lifeways Cross-Generation Cohort Study
International Journal of Obesity (2020)
-
Grand-maternal lifestyle during pregnancy and body mass index in adolescence and young adulthood: an intergenerational cohort study
Scientific Reports (2020)
-
Maternal pre-pregnancy overweight and gestational diabetes and dietary intakes among young adult offspring
Nutrition & Diabetes (2020)