Abstract
Background:
Multiparametric magnetic resonance imaging (mpMRI) has been increasingly used for prostate cancer (PCa). Recent studies identified distinct molecular subclasses of PCa with recurrent genomic alterations. However, the associations between molecular alterations in PCa and characteristics on mpMRI are unknown. Therefore, the objective of this study was to investigate recurrent molecular alterations in PCa and their associations with mpMRI features.
Methods:
Sixty-two PCa nodules >0.5 cm had a preoperative mpMRI. Nodules were evaluated for ERG rearrangement, PTEN deletion, SPINK1 overexpression, SPOP mutation and CHD1 deletion. Each PCa focus was matched to the corresponding location on mpMRI. Lesions were scored by single observer according to the PI-RADSv2 scale.
Results:
Of the 62 nodules, 22 (35.5%) were ERG positive, 6 (9.7%) had SPINK1 overexpression, 6 (9.7%) had SPOP mutations, 4 (6.5%) had CHD1 deletions and 1 (1.6%) had PTEN deletion. All of the nodules with CHD1 deletions were not visible on mpMRI (P=0.037). All of the nodules with SPINK1 overexpression were visible on mpMRI, although the association was not statistically significant (P=0.06). There were no significant associations between any molecular alteration with the severity of the PI-RADS scores (all P>0.05).
Conclusions:
This investigation represents the first description of an association between recurrent molecular alterations and the characterization of PCa nodules on mpMRI. This study can be considered hypothesis-generating for future studies to rigorously evaluate the association of specific PCa molecular subclasses with imaging features and potentially define specific subsets of PCa for which the utility of MRI is higher or lower.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is a common but clinically and molecularly heterogeneous disease. Many of these men will have aggressive disease, with up to 40% experiencing disease progression despite primary treatment.1 For other men, PCa will not affect their normal lifespan,2 and they may experience significant morbidity and compromised quality of life after PCa treatment.3
The significant public health burden of overtreatment has led to increased interest in prognostic biomarkers. Recent integrative analyses of gene expression, copy number alterations and chromosomal rearrangements have confirmed distinct molecular subclasses4, 5 with mutually exclusive genomic and transcriptomic events within PCa,6, 7, 8 especially between ETS fusion-positive and ETS fusion-negative PCa.7 The most common alteration in PCa involves gene fusions of androgen-regulated genes and ETS transcription factors, most commonly TMPRSS2-ERG fusion,8 present in 30–50% of cases.9, 10, 11, 12, 13 Consistent with results from population-based cohorts,14 a recent study of patients on active surveillance found that the presence of ERG translocation in the biopsy specimen was the most significant predictor of progression.15 We first reported somatic mutations in the Speckle-Type POZ Protein (SPOP) gene in 6–15% of PCas.7 SPOP mutations define a distinct subclass of PCa: SPOP mutations and ETS rearrangements are mutually exclusive, SPOP mutant prostate tumors generally lack lesions in the PI3K pathway, and they are also independent of mutations in the tumor suppressor gene TP53.7, 16 Therefore, molecular subtyping in PCa may help improve risk-prognostication, and also facilitate more personalized and directed therapies.
Recent advances in multiparametric magnetic resonance imaging (mpMRI) have also improved PCa risk stratification. Numerous studies have found that the application of mpMRI can improve the sensitivity and specificity of detecting clinically significant PCa,17 and MRI-guided targeting may improve prostate biopsy performance.18 However, little is known about the associations between molecular and genomic alterations and characteristics on mpMRI. The goal of this study was to characterize recurrent molecular alterations in PCa and their associations with features on mpMRI.
Materials and methods
Patient population
Forty-eight radical prostatectomy (RP) specimens from a single institution between 2008 and 2012 were included in the study (Weill Cornell Medicine/New York-Presbyterian Institutional Review Board protocol# IRB 1007011157). These cases are part of the well-characterized Early Detection Research Network (EDRN) cohort.19, 20 A total of 98 discrete PCa nodules were identified. Patient characteristics are listed in Table 1. Patients were included in the study if they had a corresponding preoperative mpMRI performed. None of the authors have any relevant disclosure or conflicts of interest to report. Sources of funding are provided in the acknowledgement section.
MRI acquisition and analysis
All MRI examinations were performed on MRI units at field strengths of 1.5 or 3 Tesla and featured the use of a pelvic phased-array coil with four channels with or without an endorectal coil. The dedicated MRI protocol included a T2-weighted sequences in axial, coronal and sagittal planes, diffusion-weighted sequence with b-values of 0 and 1000 s mm−2 (single-shot spin-echo EPI sequence; TR=3500–5600, TE=70.3/105.6; slice thickness: 3 mm, no interslice gap; field of view: 14 × 14–24 × 24 cm; matrix: 128–128) and a T1-weighted dynamic contrast-enhanced sequence (TR=3.6–4.9 ms, TE=1.3–1.7 ms; slice thickness: 5 mm, no interslice gap; field of view: 24 × 24 cm; matrix: 256x128–160, mean temporal resolution: 10 s). ADC maps were generated from diffusion-weighted images on a voxel-wise basis using a monoexponential model. Image acquisition was begun after intravenous injection of 0.1 mmol of gadopentetate dimeglumine per kilogram of body weight (Magnevist; Berlex Laboratories, Montville, NJ, USA) at a rate of 2 ml s−1 using an automatic injector (Medrad, Indianola, IA, USA).
Lesion rating system
Prostate lesions detected on MRI were graded according to the Prostate imaging, reporting and data system scoring, version 2 (PIRADSv2),21 which scores the radiologist’s opinion regarding the likelihood of the presence of PCa on a scale from 1 to 5, with 5 carrying a high-diagnostic likelihood of PCa. As the PIRADS grading system changed in 2015, which is after the dates of the pre-prostatectomy mpMRI’s, all the images were re-reviewed and regraded according to the PIRADSv2 system. The radiologist is a trained genitourinary radiologist with experience in mpMRI. The radiologist identified each individual nodule that was visible on the mpMRI and applied a PIRADSv2 score, but was blinded to the location of the nodules on the prostatectomy specimen. MRI examinations were evaluated by a trained genitourinary radiologist with 6 years of experience in interpreting prostate MRI.
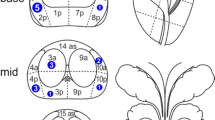
Each prostate was sectioned into 5 mm thick slices from base to apex, and the location of each nodule carefully annotated according to the sextant locations (Figure 1). Each prostate tumor nodule that was identified by the genitourinary pathologist was then matched to the corresponding location on the mpMRI. After all the mpMRI’s for all the patients in the study were read and scored by the radiologist, each pathological nodule and nodule on MRI were reviewed as a group between the pathologists, urologists and radiologist to confirm the location or absence of MRI finding of each nodule in question. If the lesion was not visible on the mpMRI, it was marked as not visible. Visible lesions were defined as lesions with a PIRADSv2 score of 2 or more. Owing to the variations in the resolution of the MRI used for the patients, the analysis was limited to PCa nodules >5 mm as measured on RP specimens. All patients in this cohort had their mpMRI’s performed and interpreted at New York-Presbyterian Hospital with an endorectal coil, and 92% (44/48) of the patients had a 3 T MRI.
Schematic of anatomic correlation between pathologic processing and MRI. (a) Pathologic processing and biobanking protocol at Weill Cornell Medicine, consisting of alternate 0.5 cm sections, quartered along right-left, anterior-posterior axes.31 (b) Representative MRI images of the prostate in axial and coronal views, with divisions into right-left, anterior-posterior and base-mid-apex. (c) Resulting 12 segment representation of prostate.
Pathologic evaluation
Slides of the formalin-fixed paraffin embedded (FFPE) tissue from all RP specimens were reviewed by study pathologists with genitourinary expertise (JF, KP, JMM) to confirm the pathologic characteristics (size, Gleason score, margin status, TNM stage) and the location of each tumor nodule. Frozen section slides from the institutional Biobank (EDRN protocol) were also examined. Tissue microarrays (TMAs) were constructed using 0.6 mm cores from the FFPE blocks, with each sample represented in triplicate.
Immunohistochemical analysis of ERG rearrangement and SPINK1 overexpression
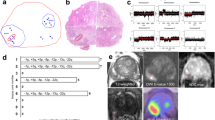
Dual ERG/SPINK1 immunohistochemical (IHC) staining was applied using a commercially available antibody for ERG protein expression (ChromoMap DAB detection kit, 1:100 dilution, Ventana Medical System, Inc., Tucson, AZ, USA) and for SPINK1 (clone EPR3864 for ERG 4D4, 1:100 dilution, Abnova, Tapei City, Taiwan) on the Discovery XT biomarker platform (Ventana Medical Systems). Semi-quantitative evaluation of ERG protein nuclear expression was determined using a four-tier grading system as previously described.22 Same approach was used to evaluate cytoplasmic SPINK1 expression. Moderate or strong staining of ⩾5% of tumor cells was considered positive for each case (Figure 2).
Recurrent molecular alterations in prostate cancer. (a) Representative case of ERG expression by immunohistochemistry. (b) Fluorescence in situ hybridization (FISH) break-apart assay showing ERG rearrangement. (c) FISH assay for PTEN showing hemizygous deletion. (d) Representative case of SPINK1 overexpression by immunohistochemistry. (e) Sanger sequencing of SPOP exons 6 and 7, showing mutation F133L. (f) FISH assay for CHD1 showing hemizygous deletion.
SPOP mutation analysis
Direct Sanger sequencing of putative SPOP somatic mutations was performed by standard methods following PCR amplification using specific primers as previously described.7 Using additional tissues cores from the same archival FFPE blocks selected for TMA construction, DNA was extracted using phenol–chloroform and purified by ethanol precipitation method as previously described.23
Fluorescence in situ hybridization analysis of ERG rearrangement, PTEN deletion and CHD1 deletion
Five μm–thick tissue sections from the TMA blocks were used for CHD1 fluorescence in situ hybridization (FISH) analysis, whereas full sections were utilized for ERG rearrangements and PTEN deletions. ERG rearrangement was identified by performing a dual-color break-apart FISH assay, using red-labeled probe (BAC clone RP11-24A11) and a green-labeled probe (BAC clone RP11-372O17), which span the centromeric and telomeric regions of ERG, respectively, and as previously described.8, 22 For detection of PTEN deletion, a gene specific probe (BAC clone CTD-2047N14) and a reference probe located at 10q25.2 (RP11-431P18) were used. For detection of CHD1 deletion, a gene specific probe (RP11-58M12) and reporter probe that corresponded to pericentromeric sequence on chromosome 5 (RP11-429D13) were used. Deletion was defined per nucleus as fewer than two copies of the gene specific probe in the presence of two reference signals. At least 50 nuclei were evaluated per tissue core using a fluorescence microscope (Olympus BX51; Olympus Optical, Tokyo, Japan) (Figure 2).
Statistical analysis
Differences in variables with a continuous distribution across categories were assessed using the Mann–Whitney U test. The Fisher’s exact test and the χ2 test were used to evaluate the association between categorical variables. All tests were two-sided, with a P-value of <0.05 considered to be statistically significant. All analyses were performed with STATA SE, v13.0 (StataCorp, College Station, TX, USA).
Results
Clinical characteristics
Overall, 48 men underwent RP, with a median age of 63 years (IQR 58-69), and a median preoperative PSA of 4.95 ng dl−1 (IQR: 3.75–7.1, Table 1). The majority of the patients (67%) had pT2 disease, with 81.3% (39/48) having Gleason score 7 or above disease. Six percent of the men (3/48) had nodal metastasis at the time of prostatectomy.
In total, 98 PCa nodules were identified on the 48 RP specimens, with a median size of 0.7 cm (IQR: 0.3–1.3 cm, see Table 2). Of these 98 tumor nodules, 62 (63.3%) were >0.5 cm in size. Of the lesions >0.5 cm, 63% (39/62) of the nodules were Gleason 7, and 16.1% (10/62) were Gleason 8 or above. Of the 36 nodules ⩽0.5 cm, 13 (36.1%) were Gleason 7 and 23 (63.9%) were Gleason 6.
Molecular alterations
ERG expression was evaluated on 95% (60/62) of the nodules from the RP specimens, with 2 IHC failures (3%, Table 2). Of the 60 nodules that were evaluated, 35.5% (22/60) were positive for ERG overexpression. SPINK1 IHC was performed on 95% (60/62) of the nodules also, of which 10% (6/62) were positive for SPINK1 overexpression. ERG expression and SPINK1 overexpression were mutually exclusive at all times. Forty-two of the nodules (68%) were successfully evaluated for SPOP mutation, of which 6 (10%) were positive. FISH was performed to detect CHD1 or PTEN deletions on 23% (23/62) and 43.5% (27/62) of the specimens, respectively, with positive findings for CHD1 and PTEN deletions found on 17% (4/23) and 3.7% (1/27) of the specimens, respectively. These data are consistent with the reported prevalence of these alterations in other cohorts.6, 7, 8, 22, 24, 25
ERG translocations were present in about 36% (17/47) of the nodules that were Gleason grade 7 or higher compared to 39% (5/13) of the nodules that were Gleason grade 6 (P=0.34, Table 3). SPOP mutations were more frequent in Gleason 7 (8%) and 8+ (30%) disease and absent in Gleason 6 disease (P=0.04). Although SPINK1 overexpression, CHD1 deletions and PTEN deletions were only found in nodules with Gleason grade 7 or higher, there was no significant association between Gleason grade and these molecular alterations.
Association between molecular alterations and MRI findings
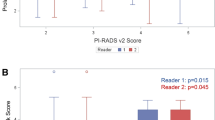
Overall, of the 62 nodules >0.5 cm, 40 (64.5%) were visible on mpMRI. Although a higher proportion of nodules with Gleason 7 or 8+ were visible on mpMRI compared to Gleason 6 nodules (61.5% and 90 vs 53.9%, respectively, the differences were not statistically significant (P=0.14). Of the visible nodules, 92% (35/38) had PIRADS scores of 4 or 5 (Table 4), with a median minimum ADC value of 662.5 (IQR: 560–797). One hundred percent of the nodules that had overexpression of SPINK1 were visible on mpMRI compared to 61% of those without SPINK1 overexpression, although the differences were not statistically significant (P=0.06). However, there was no significant association between the PIRADS score or ADC values and SPINK1 overexpression (P=0.99). All tumor nodules that had CHD1 deletions were not visible on MRI, whereas 63% of those without CHD1 deletions were visible on mpMRI (P=0.037). There were no significant associations between ERG translocation, SPOP mutation, or PTEN deletion and characteristics on mpMRI.
On a logistic regression model predicting visibility on mpMRI, Gleason grade (odds ratio (OR)=8.6, P=0.01) and size of the nodule on the pathological specimen (OR=5.2, P<0.01) were associated with increased odds of visibility. None of the molecular or genomic alterations were associated with visibility on mpMRI on a logistic regression model. On multivariable regression model controlling for tumor size, Gleason grade, molecular changes and tumor location, there were no significant independent predictors for visibility, although there was a trend towards significance for Gleason grade (OR=4.8, P=0.09).
Discussion
Advances in mpMRI have improved the detection, sampling and stratification of PCa.17, 18 Significant progress has been made in understanding the molecular basis for PCa tumorigenesis and progression, with the development of multiple biomarkers that are associated with PCa progression and biochemical recurrence-free survival. One important study found that functional and morphological features of mpMRI, such as diffusion-weighted imaging and tumor size, had a low but significant correlation with cell cycle progression scores.26 Another more recent study found that gene expression profiles from prostate biopsy specimens were strongly associated with features on mpMRI, especially in genes associated with androgen receptor signaling.27 However, these studies did not evaluate the most common genomic and molecular alterations in PCa, such as SPOP mutations and SPINK1 overexpression, that represent common and clinically important subtypes in PCa. To the best of our knowledge, this is the first study to evaluate any potential associations between the most common molecular alterations in PCa and their characteristics on mpMRI.
This study suggests a significant association between CHD1 deletions and detection on mpMRI, and a possible association with SPINK1 overexpression. Both SPINK1 and CHD1 are important alterations in tumorigenesis and PCa progression. SPINK1 is a protein that is overexpressed in a subset of ETS-negative PCas, and has been found to be associated with increased growth and invasion in PCa models through its interactions with the epidermal growth factor receptor (EGFR).28, 29 Recent studies have also found that SPINK1 positive tumors represent a particular aggressive subtype in PCa, and has been independently associated with biochemical recurrence-free survival and progression.24 All SPINK1 overexpressing tumors were visible and appeared suspicious for malignancy on mpMRI, compared to a rate of 61% for those who were SPINK1 negative. This distinction may be important to help further risk-stratify PCa lesions on initial diagnosis or progression on active surveillance, as SPINK1 positive PCa represents an aggressive subtype, but may also serve as a potential marker for targeted therapy for patients with SPINK1 overexpression, towards either the SPINK1 protein or against EGFR.28, 29 Early results from clinical trials in lung cancer and PCa support the use of targeted therapy defined by distinct molecular subtypes (that is, ERG, ETV1, EGFR status), and methods to define the molecular subtypes are becoming increasingly important. If diagnostic imaging can provide further granularity in risk-prognostication by identifying molecular subclasses, then mpMRI can be an even more essential component in personalizing PCa treatment. However, further studies are needed to clarify these important molecular subtypes and their respective imaging on mpMRI.
CHD1 encodes a protein that is essential for remodeling chromatin states and transcriptional control across the genome, and is found in about 10-25% of primary and metastatic PCa lesions.6, 7 All of the lesions with CHD1 deletions were not visible on mpMRI, compared to a rate of 37% for those without CHD1 deletions. In light of the recent studies that supported the use of MR fusion biopsy techniques to improve the sampling and sensitivity of prostate biopsies to detect high grade PCas,18 this group with CHD1 deletions (and possibly tumors with SPINK1 overexpression) may represent an important subclass of aggressive PCa that may not be initially discovered, followed or evaluated by mpMRI. With increasing utilization of mpMRI and reliance on imaging modalities in the diagnosis and management of PCa,30 it is important for future studies to substantiate these findings and identify potential aggressive subtypes that may be missed during their window of curability.
It is interesting to note that ERG rearrangements and SPOP mutations, two of the early clonal events in PCa tumorigenesis that represent distinct subclasses of primary PCa,4, 5, 7 had no significant association with visibility on mpMRI or with any lesion characteristics on mpMRI. These results could suggest that the early driver lesions may not confer any visibility on mpMRI, and that secondary mutations and alterations, such as CHD1 deletions and SPINK1 overexpression, are required to characterize the PCa with mpMRI.
Our study has several limitations that should be considered. The inter- and intratumoral heterogeneity in PCa can confound the association of specific endpoints with molecular subclasses; the use of TMAs may exacerbate this limitation. The inherent resolution limitation of mpMRI is also a limitation; it is difficult to definitively identify the presence or absence of small nodules, and so those samples were excluded a priori. With future improvements in mpMRI, hopefully we can study the impact of molecular alterations in a cohort of smaller nodules. In addition, whole-mount sectioning was not used to map out the exact locations of each nodule to the mpMRI, which could represent an ascertainment bias. The overall sample size of this cohort is small; although significant differences were found in mpMRI characteristics, these findings should be best viewed as hypothesis-generating for future studies. In addition, the samples were obtained from a single institution and were reviewed retrospectively, with its inherent biases.
Conclusions
This investigation represents the first description of an association between molecular alterations and the characterization of PCa on mpMRI. These molecular alterations may help identify different subclasses in PCa by imaging modalities and potentially image-guided personalized therapies. Further investigations are required to substantiate these findings.
References
Shariat S, Kattan M, Vickers A, Karakiewicz P, Scardino P . Critical review of prostate cancer predictive tools. Future Oncol 2009; 5: 1555–1584.
Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS et al. Outcomes of localized prostate cancer following conservative management. JAMA 2009; 302: 1202–1209.
Resnick MJ, Penson DF . Functional outcomes after treatment for prostate cancer. N Engl J Med 2013; 368: 1654.
Kaffenberger SD, Barbieri CE . Molecular subtyping of prostate cancer. Curr Opin Urol 2016; 26: 213–218.
Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 2015; 163: 1011–1025.
Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012; 487: 239–243.
Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet 2012; 44: 685–689.
Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005; 310: 644–648.
Soller MJ, Isaksson M, Elfving P, Soller W, Lundgren R, Panagopoulos I . Confirmation of the high frequency of the TMPRSS2/ERG fusion gene in prostate cancer. Genes Chromosomes Cancer 2006; 45: 717–719.
Yoshimoto M, Joshua AM, Chilton-Macneill S, Bayani J, Selvarajah S, Evans AJ et al. Three-color FISH analysis of TMPRSS2/ERG fusions in prostate cancer indicates that genomic microdeletion of chromosome 21 is associated with rearrangement. Neoplasia 2006; 8: 465–469.
Lapointe J, Kim YH, Miller MA, Li C, Kaygusuz G, van de Rijn M et al. A variant TMPRSS2 isoform and ERG fusion product in prostate cancer with implications for molecular diagnosis. Mod Pathol 2007; 20: 467–473.
Mehra R, Tomlins SA, Shen R, Nadeem O, Wang L, Wei JT et al. Comprehensive assessment of TMPRSS2 and ETS family gene aberrations in clinically localized prostate cancer. Mod Pathol 2007; 20: 538–544.
Perner S, Mosquera JM, Demichelis F, Hofer MD, Paris PL, Simko J et al. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol 2007; 31: 882–888.
Demichelis F, Setlur SR, Beroukhim R, Perner S, Korbel JO, Lafargue CJ et al. Distinct genomic aberrations associated with ERG rearranged prostate cancer. Genes Chromosomes Cancer 2009; 48: 366–380.
Berg KD, Vainer B, Thomsen FB, Roder MA, Gerds TA, Toft BG et al. ERG protein expression in diagnostic specimens is associated with increased risk of progression during active surveillance for prostate cancer. Eur Urol 2014; 66: 851–860.
Lindberg J, Klevebring D, Liu W, Neiman M, Xu J, Wiklund P et al. Exome sequencing of prostate cancer supports the hypothesis of independent tumour origins. Eur Urol 2013; 63: 347–353.
Turkbey B, Mani H, Shah V, Rastinehad AR, Bernardo M, Pohida T et al. Multiparametric 3 T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol 2011; 186: 1818–1824.
Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313: 390–397.
Prensner JR, Chinnaiyan AM, Srivastava S . Systematic, evidence-based discovery of biomarkers at the NCI. Clin Exp Metastasis 2012; 29: 645–652.
Srivastava S, Rossi SC . Early detection research program at the NCI. Int J Cancer 1996; 69: 35–37.
American College of Radiology. MR prostate imaging reporting and data system version 2.0. Available at http://www.acr.org/Quality-Safety/Resources/PIRADS/. Accessed April 2017..
Park K, Tomlins SA, Mudaliar KM, Chiu YL, Esgueva R, Mehra R et al. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia 2010; 12: 590–598.
Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY et al. The genomic complexity of primary human prostate cancer. Nature 2011; 470: 214–220.
Terry S, Nicolaiew N, Basset V, Semprez F, Soyeux P, Maille P et al. Clinical value of ERG, TFF3, and SPINK1 for molecular subtyping of prostate cancer. Cancer 2015; 121: 1422–1430.
Yoshimoto M, Joshua AM, Cunha IW, Coudry RA, Fonseca FP, Ludkovski O et al. Absence of TMPRSS2:ERG fusions and PTEN losses in prostate cancer is associated with a favorable outcome. Mod Pathol 2008; 21: 1451–1460.
Renard-Penna R, Cancel-Tassin G, Comperat E, Varinot J, Léon P, Roupret M et al. Multiparametric magnetic resonance imaging predicts postoperative pathology but misses aggressive prostate cancers as assessed by cell cycle progression score. J Urol 2015; 194: 1617–1623.
Stoyanova R, Pollack A, Takhar M, Lynne C, Parra N, Lam LL et al. Association of multiparametric MRI quantitative imaging features with prostate cancer gene expression in MRI-targeted prostate biopsies. Oncotarget 2016; 7: 53362–53376.
Tomlins SA, Rhodes DR, Yu J, Varambally S, Mehra R, Perner S et al. The role of SPINK1 in ETS rearrangement-negative prostate cancers. Cancer Cell 2008; 13: 519–528.
Ateeq B, Tomlins SA, Laxman B, Asangani IA, Cao Q, Cao X et al. Therapeutic targeting of SPINK1-positive prostate cancer. Sci Transl Med 2011; 3: 72ra17.
Porten SP, Smith A, Odisho AY, Litwin MS, Saigal CS, Carroll PR et al. Updated trends in imaging use in men diagnosed with prostate cancer. Prostate Cancer Prostatic Dis 2014; 17: 246–251.
Esgueva R, Park K, Kim R, Kitabayashi N, Barbieri CE, Dorsey PJ et al. Next-generation prostate cancer biobanking: toward a processing protocol amenable for the International Cancer Genome Consortium. Diagn Mol Pathol 2012; 21: 61–68.
Acknowledgements
This study was funded by EDRN NCI U01 CA111275-09 (MAR and JMM), NCI R01 CA125612-05A1 and K08CA187417-02), the Prostate Cancer Foundation, and the Urology Care Foundation (Rising Star in Urology Research Award to CEB). CEB is a Damon Runyon Clinical Investigator supported (in part) by the Damon Runyon Cancer Research Foundation. This work was also supported in part by the Translational Research Program at WCM Pathology and Laboratory Medicine.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lee, D., Fontugne, J., Gumpeni, N. et al. Molecular alterations in prostate cancer and association with MRI features. Prostate Cancer Prostatic Dis 20, 430–435 (2017). https://doi.org/10.1038/pcan.2017.33
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2017.33
- Springer Nature Limited
This article is cited by
-
The diverse roles of SPOP in prostate cancer and kidney cancer
Nature Reviews Urology (2020)
-
A TMEFF2-regulated cell cycle derived gene signature is prognostic of recurrence risk in prostate cancer
BMC Cancer (2019)