Abstract
Background:
We aimed to assess the prognostic relevance of the new Grade Groups in Prostate Cancer (PCa) within a large cohort of European men treated with radical prostatectomy (RP).
Methods:
Data from 27 122 patients treated with RP at seven European centers were analyzed. We investigated the prognostic performance of the new Grade Groups (based on Gleason score 3+3, 3+4, 4+3, 8 and 9–10) on biopsy and RP specimen, adjusted for established clinical and pathological characteristics. Multivariable Cox proportional hazards regression models assessed the association of new Grade Groups with biochemical recurrence (BCR). Prognostic accuracies of the models were assessed using Harrell’s C-index.
Results:
Median follow-up was 29 months (interquartile range, 13–54). The 4-year estimated BCR-free survival (bRFS) for biopsy Grade Groups 1–5 were 91.3, 81.6, 69.8, 60.3 and 44.4%, respectively. The 4-year estimated bRFS for RP Grade Groups 1–5 were 96.1%, 86.7%, 67.0%, 63.1% and 41.0%, respectively. Compared with Grade Group 1, all other Grade Groups based both on biopsy and RP specimen were independently associated with a lower bRFS (all P<0.01). Adjusted pairwise comparisons revealed statistically differences between all Grade Groups, except for group 3 and 4 on RP specimen (P=0.10). The discriminations of the multivariable base prognostic models based on the current three-tier and the new five-tier systems were not clinically different (0.3 and 0.9% increase in discrimination for clinical and pathological model).
Conclusions:
We validated the independent prognostic value of the new Grade Groups on biopsy and RP specimen from European PCa men. However, it does not improve the accuracies of prognostic models by a clinically significant margin. Nevertheless, this new classification may help physicians and patients estimate disease aggressiveness with a user-friendly, clinically relevant and reproducible method.
Similar content being viewed by others
Introduction
Since its introduction 50 years ago, the Gleason grading system has been widely adopted as one of the major prognostic factors in prostate cancer (PCa).1 Its use in several prognostic models, usually as a three-tier grading system (Gleason sore ⩽6, 7 or 8–10) helps risk stratify patient outcomes and guide therapeutic options.2 Several revisions of the initial grading system have been proposed to improve its prognostic performance and interpretation by physicians and patients.3, 4, 5 The International Society of Urological Pathology (ISUP) based on a 2014 consensus conference now recommends a new grading system based on its prognostic value ranging from 1 to 5: Grade Group 1=Gleason score ⩽6, Grade Group 2=Gleason score 3+4, Grade Group 3=Gleason score 4+3, Grade Group 4=Gleason score 8 and Grade Group 5=Gleason scores 9 and 10.5 The World Health Organization now proposed to use these new groupings all together to provide a better tool for physicians and patients.6 Editors of the major journals in the field of uro-oncology have recently called all potential contributors for using it.7
To date, the prognostic performance of this grading system has been assessed in several single-center studies8, 9, 10, 11 and three large multicentric cohorts of patients treated with radical prostatectomy (RP) and/or radiation therapy.12, 13, 14, 15 Epstein et al.12 first proposed this new grading system, based on data from US academic centers with final cohorts of 20 845 men undergoing RP and 5501 men treated with radiation therapy, and demonstrated the independent prognostic value of this classification to predict biochemical recurrence (BCR) in both cohorts. Recently, a validation of the prognostic performance of the new Grade Groups was reported in a nationwide population-based cohort from The National Prostate Cancer Register of Sweden with men undergoing RP and radiation therapy.15 To date, no external validation of its prognostic value has been proposed in multicentric study with European patients from different countries, who present potentially different outcomes.
To provide further evidence for the clinical relevance of these new Grade Groups in European patients, we assessed its prognostic performance in a large multicentric cohort of European men treated with RP. In addition, we assessed its differential prognostic value compared to the three-tier system with regards to predictive accuracy.
Materials and methods
Patient selection and data collection
Data from 30 711 patients with Pca, treated with RP at seven European academic institutions between 2005 and 2014, were reviewed after institutional review board approvals from each center. No patient had distant metastatic disease at the time of RP. Patients with preoperative androgen deprivation therapy or chemotherapy, preoperative PSA >50 ng ml−1, missing data regarding follow-up, preoperative PSA, biopsy and RP Gleason score, surgical margin status or pathological stage were excluded from the analysis. Patients (27 122) were considered for analysis. No patient received immediate postoperative radiotherapy, chemotherapy or androgen deprivation therapy.
Gleason scoring and pathological evaluation
Gleason score was assessed in all biopsy and RP specimen by uro-pathologists according to ISUP guidelines.4 However, tertiary Gleason score evaluation was not considered for analysis, as it was not routinely reported in some institutions. All surgical specimens were processed according to standard pathologic procedures. Pathologic stage was assigned according to the 2007 American Joint Committee on Cancer Tumor Node and Metastasis staging system. Lymphatic tissue removed during RP was submitted for histological examination. Positive pathological margin was defined as tumor cells in contact with the inked surface of the prostatectomy specimen.
Follow-up
Follow-up was performed according to institutional protocols in agreement with guidelines at the time. In general, patients were seen postoperatively quarterly for the first year, semi-annually in the second year and annually thereafter. PSA evaluation were performed at each visit. The primary endpoint was BCR, defined as PSA value >0.2 ng ml−1 on two consecutive visits. The date of BCR was attributed to the day of the first PSA. In few cases, a radiation or an androgen deprivation therapy was initiated for rising PSA before the cut-off of 0.2 ng ml−1 was reached. In these cases, the date of BCR was attributed to the first day of androgen deprivation therapy or radiation therapy.
Statistical analysis
Biopsy and RP new Grade Groups were analyzed separately as categorical variables. We used the χ2 and Kruskall–Wallis tests to assess the differences in categorical and continuous variables between each groups, respectively. BCR-free survival curves were plotted and compared using Kaplan–Meier method and log-rank test; univariable and multivariable Cox regression models addressed the associations of each group with BCR after RP. Adjusted pairwise comparison between groups were performed. All P-values were two-sided and statistical significance was defined as a P<0.05. Harrel C-index was performed to assess the prognostic discrimination of the models. Statistical analyses were performed using Stata 11.0 statistical software (Stata, College Station, TX, USA).
Results
Descriptive characteristics and association with clinico-pathological features
Baseline characteristics of the 27 122 patients according to biopsy and RP new Grade Groups are listed in Table 1. Higher new Grade Groups assessed on biopsy and RP specimen were associated with age, higher PSA, extracapsular extension, seminal vesicle invasion, positive surgical margins and lymph node metastases (P⩽0.001).
Association of new Grade Groups with BCR
Median follow-up was 29 months (interquartile range, 13–54) for patients without BCR at last follow-up.
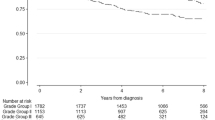
Significant differences regarding BCR-free survival (bRFS) were observed in the cohort between new Grade Groups based on biopsy (log rank test, P<0.001; Figure 1a) and prostatectomy specimen (log rank test, P<0.001; Figure 1b). The 4-year estimated bRFS for biopsy Grade Groups 1–5 were 91.3% (95% confidence interval (CI): 0.907–0.918), 81.6% (95% CI: 0.803–0.827), 69.8% (95% CI: 0.676–0.719), 60.3% (95% CI: 0.571–0.633) and 44.4% (95% CI: 0.399–0.488), respectively. The 4-year estimated bRFS for RP Grade Groups 1–5 were 96.1% (95% CI: 0.954–0.966), 86.7% (95% CI: 0.859–0.875), 67.0% (95% CI: 0.653–0.687), 63.1% (95% CI: 0.582–0.677) and 41.0% (95% CI: 0.371–0.449).
Compared with Gleason score⩽6 (Group 1), all prognostic new Grade Groups based both on biopsy and RP specimen were independently associated with a higher risk of BCR on univariable and multivariable analyses (all P<0.001; Tables 2 and 3). In these models, new Grade Groups were the strongest predictors of BCR.
The discriminations of the multivariable prognostic pre-operative and post-operative models based on the current three-tier classification and the new five-tier system (2015 ISUP groups) were not clinically different (Table 4).
Adjusted pairwise comparisons revealed significant differences between all new Grade Groups (all P<0.001), except for groups 3 and 4 on RP specimen (P=0.10). Further adjusted pairwise comparisons considering primary pattern for tumors in group 4 demonstrated that patients with tumors 3+5 on biopsy did not have a higher risk of recurrence than patients in group 3 (hazard ratio: 0.97, 95% CI: 0.71–1.35, P=0.89). On RP specimen, patients with 3+5 tumors had a lower risk of BCR than patients in group 3 (hazard ratio: 0.57, 95% CI: 0.37–88, P=0.011). In the highest risk group, patients with tumors 4+5 on RP had similar risk of BCR compared with patients from Group 4 (hazard ratio: 1.16, 95% CI: 0.97–1.39, P=0.101).
Discussion
To accurately reflect tumor behavior and improve patient risk stratification, modifications to the Gleason grading system have been proposed during 2005 and 2014 ISUP expert conferences. Deletions of the Gleason scores 2–5 were the highlights of the first recommendations. The 2014 ISUP conference led to the upgrading of cribiform glands to Gleason pattern 4 and the adoption of a new grading system that ranges from 1 to 5 based on Gleason score. In our multicentric international cohort, we confirmed the prognostic value of these new groupings, both on needle biopsies and RP specimen from European men.
We first demonstrated new Grade Groups correlated with adverse pathological features on RP specimen. More interestingly, we confirmed significant differences in bRFS outcomes after RP between new Grade Groups. In group 1, the 4-year bRFS estimates were 91.3% and 96.1% for biopsy and RP grading, respectively. These results are in line with previous studies that reported 4- and 5-year bRFS estimates over 95% after RP.10, 12 These excellent outcomes reflect a better and homogenous definition of the tumors that are integrated to this very-low-risk group. One of the indirect missions of the 2005 recommendation was to shift aggressive therapies towards the Gleason 7 groups. In this latter group, we confirmed the need to consider Gleason 3+4 (group 2) and Gleason 4+3 (group 3) as distinct groups. In the RP cohort, BCR was twofold higher for Gleason 4+3 than for 3+4. In a recent study that assessed new Grade Groups with long-term follow-up, Group 3 was associated with a threefold higher risk of BCR and a sevenfold higher risk of distant metastasis or cancer-specific mortality compared with group 2.14 These results are in accordance with several studies, published before 2014 ISUP recommendations, that already demonstrated splitting Gleason 7 group was prognostically relevant. Indeed, 4+3 tumors were associated with adverse pathological features on RP specimen such as extraprostatic extension, seminal vesicle invasion or positive surgical margins.16, 17 After RP or radiation therapy, a primary pattern of 4 in this group was associated with worse bRFS and cancer-specific survival.18, 19, 20, 21 In our study, Group 3 tumors were even more likely to behave like group 4 tumors. Therefore, 4-year estimated bRFS in these RP Grade Groups were 67.0% and 63.1%, respectively. Similarly, in the study from the Johns Hopkins that assessed these new groupings for the first time, 5-year bRFS on biopsy Grade Groups 3 and 4 were 65% and 63%, respectively.9 Conversely, patients in the group 5 have a significantly worse prognosis compared with those in group 4; 4-year bRFS were 44.4% and 41% for biopsy and RP, respectively. These results confirm the lethal prognostic value of Gleason score 9–10 PCa.22
The question of whether this new classification can improve the prognostic use of established predictors of cancer outcome requires more than the conventional univariable and multivariable analyses of its association with disease outcomes. It must be established that its use adds information that improves by a statistically significant margin, or at least equals, the performance of a predictive model constructed without the new characteristics. One of our aims was to test the prognostic value of the new classification using concordance index. We found that, despite an independent association with bRFS, the new prognostic grouping failed to add prognostically relevant information beyond the three-tier grading (improvements of C-index <0.01). Interestingly, we demonstrated bRFS for patients with Gleason score 3+5 and 4+5 may be underestimated with this new system that assigns these patients to group 4 and 5, respectively. Such heterogeneity within the Grade Group 4 has been previously reported.11 Therefore, in higher risk patients, physicians should probably pay attention to the primary pattern of the initial Gleason score, in order to accurately risk stratify patients and avoid inaccurate risk estimation in patient counseling and decision-making.
Besides these weaknesses, the new grading system has several benefits that support its use in daily practice. This new system offers an easier and more comprehensible classification for physicians and patients alike. The previous 2–10 scale led to multiple combinations of Gleason patterns and a related score from 6 to 10 that may be misunderstanding. Indeed, patients may inadequately consider their disease as intermediate to high risk disease by referring to the widely used 1–10 scales from daily life. This misinterpretation may compromise the compliance to active surveillance that is now largely proposed to patients who have few positive biopsies with Gleason 6 PCa. Introducing the new 1–5 grading system with a first and lowest grade labeled ‘1’ may help address these concerns and allay some fears. Loeb et al.23 clearly demonstrated that from the patient’s point of view, traditional Gleason grading was confusing and the new Grade Groups more comprehensible. Almost 80% of the patients considered a 1–5 scale would be helpful in active surveillance decision making. Adopting this new classification may also help improve risk estimation and consequent treatment decision-making. Indeed, despite the evidence that supports the difference between Gleason 3+4 and 4+3 PCa regarding prognosis, the distinction between both groups still rarely impacts treatment decision and is not considered in the guidelines from the European Association of Urology, National Comprehensive Cancer Network or European Society for Medical Oncology. Zumsteg et al.24 recently proposed a new risk stratification for intermediate risk PCa patients treated with radiation therapy based on several unfavorable criteria including 3+4 and 4+3 distinction. This new classification may guide treatment decision regarding the duration of androgen deprivation therapy among these different groups. A similar controversy exists regarding the clinical value of differentiating Gleason 8 tumors from Gleason 9–10 tumors. Nevertheless, new paradigms may arise from better prognostication based on the new Grade Groups and guide active surveillance or androgen deprivation treatment decision. Finally, this new classification may help homogenize and standardize pathologic grade reporting for clinical and research use, leading to reproducibility.7
Our validation study has several limitations. First and foremost limitation is inherent to its retrospective nature that may introduce selection bias. In a multi-institutional study, variations in pathological workup and pathologist skills may additionally confound the results. However, all data were collected at high-volume centers with high expertise in uropathology and we only considered patients whose biopsies and RP specimen were assessed after adoption of the 2005 ISUP recommendations. Several pathological features that may increase the prognostic accuracy of the models such as the presence of a tertiary pattern, the number of positive cores and the percentage of involvement per core were not considered since not available at the time of data collection. Finally, our study has a limited follow-up with only bRFS as an endpoint. Assessment of an association with more meaningful endpoints such as metastasis-free, cancer-specific or overall survival would have been probably more useful to guide discussion about patient information and decision-making. To date, to our knowledge, only one study confirmed the prognostic accuracy of the present grading system with PCa death as an endpoint, in a cohort of patients treated conservatively.25 However, early BCR within two years should be considered as a surrogate for biologically and clinically aggressive PCa as it has a high likelihood to be related to micrometastasis.26, 27
Conclusions
The recently proposed new Grade Groups performed on biopsy and RP specimen is associated with adverse pathological features and strongly predicts bRFS after RP. Although it does not improve accuracy of the established prognostic models by a significant margin compared with the previous three-tier grading system, this new classification is more comprehensible and user friendly. Therefore, it helps physicians and patients in the discussion regarding disease aggressiveness and treatment decision-making. Further validation of this classification system for other treatment modalities and more meaningful endpoints such as metastasis is necessary.
Ethical standards
This study has been approved by the appropriate ethics committee.
References
Brimo F, Montironi R, Egevad L, Erbersdobler A, Lin DW, Nelson JB et al. Contemporary grading for prostate cancer: implications for patient care. Eur Urol 2013; 63: 892–901.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 2014; 65: 124–137.
Gleason DF, Mellinger GT . Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974; 111: 58–64.
Epstein JI, Allsbrook WC Jr., Amin MB, Egevad LL . The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol 2005; 29: 1228–1242.
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA . The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 2016; 40: 244–252.
Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE . The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: prostate and bladder tumours. Eur Urol 2016; 70: 106–119.
Zietman A, Smith J, Klein E, Droller M, Dasgupta P, Catto J . Consensus guidelines for reporting prostate cancer Gleason Grade. BJU Int 2016; 117: 849.
Spratt DE, Jackson WC, Abugharib A, Tomlins SA, Dess RT, Soni PD et al. Independent validation of the prognostic capacity of the ISUP prostate cancer grade grouping system for radiation treated patients with long-term follow-up. Prostate Cancer Prostatic Dis 2016; 19: 292–297.
Pierorazio PM, Walsh PC, Partin AW, Epstein JI . Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int 2013; 111: 753–760.
Spratt DE, Cole AI, Palapattu GS, Weizer AZ, Jackson WC, Montgomery JS et al. Independent surgical validation of the new prostate cancer grade groupingsystem. BJU Int 2016; 118: 763–769.
Samaratunga H, Delahunt B, Gianduzzo T, Coughlin G, Duffy D, LeFevre I et al. The prognostic significance of the 2014 International Society of Urological Pathology (ISUP) grading system for prostate cancer. Pathology 2015; 47: 515–519.
Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C et al. A contemporary prostate cancer grading system: a validated alternative to the gleason score. Eur Urol. 2016; 69: 428–435.
van den Bergh RC, van der Kwast TH, de Jong J, Zargar H, Ryan AJ, Costello AJ et al. Validation of the novel ISUP-2014 5-tier Gleason grade grouping: biochemical recurrence rates of 3+5 disease may be overestimated. BJU Int 2016; 118: 502–505.
Delahunt B, Egevad L, Srigley JR, Steigler A, Murray JD, Atkinson C et al. Validation of International Society of Urological Pathology (ISUP) grading for prostatic adenocarcinoma in thin core biopsies using TROG 03.04 ‘RADAR’ trial clinical data. Pathology 2015; 47: 520–525.
Loeb S, Folkvaljon Y, Robinson D, Lissbrant IF, Egevad L, Stattin P . Evaluation of the 2015 Gleason Grade Groups in a Nationwide Population-based Cohort. Eur Urol 2015; 69: 1135–1141.
Lau WK, Blute ML, Bostwick DG, Weaver AL, Sebo TJ, Zincke H . Prognostic factors for survival of patients with pathological Gleason score 7 prostate cancer: differences in outcome between primary Gleason grades 3 and 4. J Urol 2001; 166: 1692–1697.
Koontz BF, Tsivian M, Mouraviev V, Sun L, Vujaskovic Z, Moul J et al. Impact of primary Gleason grade on risk stratification for Gleason score 7 prostate cancers. Int J Radiat Oncol Biol Phys 2012; 82: 200–203.
Stark JR, Perner S, Stampfer MJ, Sinnott JA, Finn S, Eisenstein AS et al. Gleason score and lethal prostate cancer: does 3+4=4+3? J Clin Oncol 2009; 27: 3459–3464.
Alenda O, Ploussard G, Mouracade P, Xylinas E, de la Taille A, Allory Y et al. Impact of the primary Gleason pattern on biochemical recurrence-free survival after radical prostatectomy: a single-center cohort of 1,248 patients with Gleason 7 tumors. World J Urol 2011; 29: 671–676.
Tollefson MK, Leibovich BC, Slezak JM, Zincke H, Blute ML . Long-term prognostic significance of primary Gleason pattern in patients with Gleason score 7 prostate cancer: impact on prostate cancer specific survival. J Urol 2006; 175: 547–551.
Burdick MJ, Reddy CA, Ulchaker J, Angermeier K, Altman A, Chehade N et al. Comparison of biochemical relapse-free survival between primary Gleason score 3 and primary Gleason score 4 for biopsy Gleason score 7 prostate cancer. Int J Radiat Oncol Biol Phys 2009; 73: 1439–1445.
Tsao CK, Gray KP, Nakabayashi M, Evan C, Kantoff PW, Huang J et al. Patients with biopsy Gleason 9 and 10 prostate cancer have significantly worse outcomes compared to patients with Gleason 8 disease. J Urol 2015; 194: 91–97.
Loeb S, Curnyn C, Sedlander E . Perspectives of prostate cancer patients on Gleason Scores and the new Grade Groups: initial qualitative study. Eur Urol 2016; 70: 1083–1085.
Zumsteg ZS, Spratt DE, Pei I, Zhang Z, Yamada Y, Kollmeier M et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol 2013; 64: 895–902.
Berney DM, Beltran L, Fisher G, North BV, Greenberg D, Moller H et al. Validation of a contemporary prostate cancer grading system using prostate cancer death as outcome. Br J Cancer 2016; 114: 1078–1083.
Shariat SF, Kattan MW, Erdamar S, Nguyen C, Scardino PT, Spencer DM et al. Detection of clinically significant, occult prostate cancer metastases in lymph nodes using a splice variant-specific rt-PCR assay for human glandular kallikrein. J Clin Oncol 2003; 21: 1223–1231.
Shariat SF, Gottenger E, Nguyen C, Song W, Kattan MW, Andenoro J et al. Preoperative blood reverse transcriptase-PCR assays for PSA and human glandular kallikrein for prediction of prostate cancer progression after radical prostatectomy. Cancer Res 2002; 62: 5974–5979.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mathieu, R., Moschini, M., Beyer, B. et al. Prognostic value of the new Grade Groups in Prostate Cancer: a multi-institutional European validation study. Prostate Cancer Prostatic Dis 20, 197–202 (2017). https://doi.org/10.1038/pcan.2016.66
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2016.66
- Springer Nature Limited
This article is cited by
-
Prediction of disease progression indicators in prostate cancer patients receiving HDR-brachytherapy using Raman spectroscopy and semi-supervised learning: a pilot study
Scientific Reports (2022)
-
Comparative Effectiveness of Radiotherapy versus Focal Laser Ablation in Patients with Low and Intermediate Risk Localized Prostate Cancer
Scientific Reports (2020)
-
The new ISUP 2014/WHO 2016 prostate cancer grade group system: first résumé 5 years after introduction and systemic review of the literature
World Journal of Urology (2020)
-
Der aktuelle Stand beim Gleason-Score
Uro-News (2019)
-
Prostate cancer grading: a decade after the 2005 modified system
Modern Pathology (2018)