Abstract
Background:
Although early reports have suggested an association between circumcision and prostate cancer (PCa) development, results of subsequent epidemiological studies have been conflicting. Here we examine published articles that explore this association.
Methods:
We searched MEDLINE through PubMed and Embase for articles reporting on the association between PCa and circumcision, and performed a meta-analysis of qualifying studies.
Results:
On the basis of seven reports of case–control studies published from 1971 to 2014, overall findings showed nonsignificant reduced risk (odds ratio (OR) 0.88, P=0.19) of PCa in circumcised men compared with uncircumcised men, obtained under heterogeneous conditions (I2=65%). Heterogeneity and nonsignificance were erased when the overall effect was subjected to outlier treatment and three studies omitted (OR 0.90, P=0.04, I2=0%). Furthermore, subgroup analysis showed significantly reduced risks in the following subgroups: (i) post-PSA testing publications (OR 0.88, P=0.01), (ii) population-based studies (OR 0.84, P=0.05), (iii) studies that collected data by personal interview (OR 0.83, P=0.03) and (iv) studies in black race (OR 0.59, P=0.02). The strengths of these summary effects lie in the robustness revealed by sensitivity analysis.
Conclusions:
Stability of the reduced risks observed in key subgroups suggests that the protective feature of circumcision status against PCa is best seen in the context of the post-PSA testing and population-based studies as well as in the black race subgroup.
Similar content being viewed by others
Introduction
Although prostate cancer (PCa) is the most common cancer in men worldwide1 and the second leading incident cancer among men in the USA,2 its etiology remains unclear. Unmodifiable risk factors are black race, advancing age and a family history of PCa.3 A potential modifiable risk factor that might help prevent PCa is male circumcision. Historically, PCa was observed to be rare in Jewish men, who, with Muslims, compose the majority of circumcised males.4 Circumcision is typically performed for religious or cultural reasons in the neonatal stage or in early adolescence.5, 6 Most circumcisions in adulthood are done to treat pathologic conditions of the foreskin or penile glans.7, 8 The wide array of medical benefits from circumcision, including better hygiene and prevention of syphilis, led to a rise in its popularity in western populations in the nineteenth century.5, 9 Today in the USA, the majority of infant boys are circumcised.10
Most epidemiological studies have shown that both increased sexual activity and a history of sexually transmitted infections (STIs) are associated with an increased risk of PCa. Although findings regarding specific STIs are inconsistent,11, 12 circumcised men are less likely to acquire an STI compared with men with foreskin.13, 14 In addition, circumcision has been found to be protective against the development of penile cancer, which is also believed to have an infectious etiology.15 Consistent with these findings is a highly significant (P<0.0001) inverse correlation between prevalence of circumcision and PCa incidence in 181 countries.16 Taken together, these findings support the hypothesized protective association between circumcision and PCa. Although earlier epidemiological studies,17, 18, 19, 20, 21 which examined the circumcision–PCa association, have been limited by small sample sizes (cases and controls in hundreds), more recent ones22, 23, 24 have sample sizes in thousands. They have therefore been able to address potential confounding by such factors as race, education, family history of PCa, PCa screening (PSA screening or digital rectal examinations) and STI history. This accumulating evidence prompted us to perform a meta-analysis to understand the role of circumcision in the development of PCa.
Materials and Methods
Selection of studies
We searched MEDLINE using PubMed and Embase till 15 December 2014 to retrieve papers examining the relationship of circumcision with PCa using the following query: ‘circumcision’ and ‘PCa’. The electronic search was supplemented with checking the reference lists of the identified articles. To be included in the meta-analysis, the studies had to be epidemiological case–control or cohort studies with cases or outcome being PCa, and with both circumcised and uncircumcised subjects. Reviews, case reports, clinical trials, and genetic and cell studies were excluded.
Data extraction
Two investigators (NP and ES) independently reviewed the retrieved papers, extracted data and reached consensus on all the items: first author’s name, published year, country of origin, study design and size, and reported or calculated odds ratios (ORs) and 95% confidence intervals (CIs).
Quality assessment of the studies
Methodological quality of the included studies was evaluated using the Newcastle–Ottawa Score Quality Scale.25 Each study was assessed based on three broad perspectives: selection, comparability and exposure with scores ranging from 0 to 9. Scores for high-, medium- and poor-quality studies were ⩾7, 4–6 and <4, respectively.
Meta-analysis
We estimated summary ORs and 95% CI of PCa associated with circumcision status. Raw data, without adjustment, were used to calculate study-specific OR estimates. Pooled ORs were obtained using either the fixed- (in the absence of heterogeneity26) or random- (in its presence27) effect models. Heterogeneity among studies was addressed in a number of ways: (i) it was estimated using the χ2-based Q-test28 with significance set at P<0.10 (ref. 29, ii) it was quantified using the I2 statistic, which measures the degree of inconsistency among studies30 and (iii) sources of heterogeneity were explored using the Galbraith plot method31 to identify potential outlier studies after which their influence on pooled effects and/or heterogeneity was graphically examined.32 Outlier treatment was applied in the overall analysis. Pooled ORs were subjected to sensitivity analysis, which involved omitting one study at a time and recalculating the pooled OR, to test for robustness of the summary effects.
We also performed subgroup analyses for geography (USA vs other), type of controls (population based vs hospital/cancer center based), data collection instrument (personal interview vs self-administered questionnaire), race (white vs black) and date of case diagnosis (before 1988 vs after 1988, which was about the time of introduction of PSA testing). Data were analyzed using Review Manager 5.3 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark) and SigmaStat 2.3 and SigmaPlot 11.0 (Systat Software, San Jose, CA, USA). Significance was set at P⩽0.05 in all calculations except heterogeneity estimation. Publication bias was not investigated because of the low sensitivity of the qualitative and quantitative tests when the number of studies is lower than 10.33
Results
Included studies
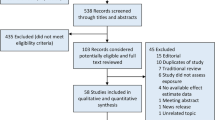
Figure 1 outlines the study selection process in a flowchart following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.34 Of the 100 titles identified, 84 were eliminated for not meeting the inclusion criteria. The remaining 16 articles were retrieved as full-text articles. From these 16, 8 articles were excluded on the basis of not being about circumcision or lacking data relating circumcision with PCa. An additional article22 included data repeated in a subsequent report23 and therefore was eliminated. The remaining seven were deemed eligible for inclusion in the meta-analysis.17, 18, 19, 20, 21, 23, 24
Table 1 shows the qualitative features of the seven case–control studies; no cohort study was identified. The studies used incident cases of PCa (histologically confirmed or clinical cancers) and therefore, test the association between development of PCa and circumcision. These studies were conducted in the USA, Canada and England. Most of the subjects were white, although some studies had racial admixture (up to 16%).17, 23, 24 Two studies19, 24 analyzed whites and blacks separately; another two18, 20 were limited to whites. Ages of subjects ranged from 35 to 89 years. Controls were individually or frequency matched to cases on age (within 5 years) in all but one study.17 Four studies were population based;18,19,23,24 the others hospital/cancer center based.17, 18, 20, 21 Data on circumcision were obtained by in-person interview in all but one study20 in which a self-administered questionnaire was completed. Newcastle–Ottawa Scale scores ranged from 6 to 9 with means and s.d. of 6.4±0.55 and 9.0±0.00 for the studies pre-PSA testing17, 18, 19, 20, 21 and post-PSA testing,23, 24 respectively.
Table 2 outlines the quantitative features (number of cases and controls, percent circumcised, study-specific OR) of the seven studies. Two studies presented separate data for whites and blacks.19, 24 Although Ross et al.19 did not present circumcision frequencies for cases, these were estimated based on the reported OR and the prevalence of exposure in controls. All but two studies17, 20 reported a lower risk of PCa in circumcised men although the risk was statistically significant only in three.19, 21, 24 Three studies22, 23, 24 reported ORs adjusted for potential confounders.
Overall and subgroup findings
Table 3 summarizes the overall and subgroup findings of the meta-analysis. The forest plot in Figure 2 shows the contribution of each individual study giving the overall nonsignificant summary OR of 0.88. This was obtained under heterogeneous conditions (I2=65%) and using Galbraith plot analysis, we identified three studies19, 20, 21 to be outliers (Figure 3). Omitting these studies followed by re-analysis erased heterogeneity (I2=0%) and generated a significant OR of 0.90 (P=0.04; Figure 4).
Overall meta-analysis. Diamond denotes the pooled odds ratio (OR). Squares indicate the OR in each study, with square sizes directly proportional to the weight contribution (%) of the study. Horizontal lines on each side of the squares represent 95% confidence intervals (CI). The χ2-test P-value is <0.10 indicating presence of heterogeneity, necessitating use of the random-effect model.
Meta-analysis with outlier omitted. Diamond denotes the pooled odds ratio (OR). Squares indicate the OR in each study, with square sizes directly proportional to the weight contribution (%) of the study. Horizontal lines on each side of the squares represent 95% confidence intervals (CI). The χ2-test P-value is >0.10 indicating lack of heterogeneity, necessitating use of the fixed-effect model.
Table 3 also shows the results of the subgroup analysis. Risks were reduced (6–17%) in both pre- and post-PSA testing groups, in population-based studies, in studies using personal interviews, in both USA and other geographic areas. Risk was reduced by 41% in blacks, but no effect was seen in whites. Likewise, risk was reduced by 31% in studies with older subjects, but not in those with younger (mean/median=65 years) subjects. Risks were increased in hospital-based studies and in the one study using self-administered questionnaires. Heterogeneity (I2) of these summary effects ranged from 0 to 77%. As we used estimated rates of circumcision for cases for the Ross et al. data, we compared findings with and without this study and found no material difference in any comparison except the white race subgroup. With Ross et al.,19 the observed OR was 1.00 (P=0.99) and increased to 1.19 (P=0.29) without it.
Sensitivity treatment did not alter the pooled effects of the overall and outlier-omitted analyses as well as the significant reduced risk findings of the post-PSA testing, population-based and interview-based studies conferring robustness to the findings. In contrast, the ORs for four subgroups (pre-PSA testing, hospital based, USA and white race) were not robust with the ORs closer to 1.00 and heterogeneity lowered with serial omission of two studies.19, 20
Discussion
With a sample size of 8633 subjects from seven published case–control studies, this meta-analysis demonstrates an overall small (12%) nonsignificant reduced risk of PCa in circumcised men. Outlier/heterogeneity identification and subgroup analyses modified this effect. Examined in greater detail, significant protective effects (10–17%) were generated in the subgroups of studies that were post-PSA testing,23, 24 population based18, 23, 24 and interview based,17, 18, 21, 23, 24 as well as overall without the three outlying studies19, 20, 21 omitted. The strengths of these individual subgroups lie in the following: (i) absence of heterogeneity (I2=0%) pointing to combinability of the studies, (ii) large sample sizes translating to increased statistical power, (iii) close matching on age and predominantly white subjects with minimal admixture (3–7%) reducing selection bias and (iv) population-based design being more representative of the general population.
Examined in combination, the significant 12% protective and nonheterogeneous effect of the post-PSA testing studies all used personal interviews to collect data, had age-matched controls with fairly large sample sizes. In contrast, the 6% nonsignificant protective effect of the pre-PSA studies was heterogeneous (I2=77%). These included the hospital-based studies in which controls may have been sick and not representative of the general population.
Limitations of this meta-analysis need to be acknowledged. All the studies of PCa development and circumcision available for this meta-analysis are case–control, and hence retrospective, studies. We found no report of a prospective cohort study. A further limitation is that in all these studies circumcision was based on self-report rather than medical examinations and therefore misclassification of exposure could occur. Misclassification would likely weaken the observed associations. In addition, we were not able to investigate the relationship between circumcision and PCa in various ethnic groups and non-western countries. This is important given the interplay between circumcision, culture and race.16 Two studies19, 24 analyzed blacks separately from whites and generated a significant OR indicating 41% protection with no heterogeneity (I2=0%). Still, additional large studies in non-white and non-black populations are warranted.
Although age is a major risk factor for PCa, we were not able to adequately explore whether or not age modifies the effect of circumcision on risk. We were also unable to address the possibility of confounding factors altering our findings. However, the post-PSA testing studies adjusted for several risk factors (age, race education, family history of PCa, history of STI, history of prostatitis, PCa screening and number of sexual partners). Our overall OR for these studies (0.89) differed little from their adjusted ORs (0.86–0.89).
The findings we report here highlight some important observations. The overall observed protective effect of circumcision on PCa was small (about 12% reduced risk). Studies with small sample sizes would lack the statistical power to find such an association. The results from our meta-analysis were not apparent from an examination of the individual studies, but when the studies were combined using the meta-analysis approach, a clear protective association to PCa for circumcision status was revealed. This was enhanced by the absence of heterogeneity. For these reasons, subgroup analysis and outlier treatment such as performed here are useful.
This is, to our knowledge, the first meta-analysis that addresses the association of circumcision status with PCa. Our findings add reduced risk of PCa to the array of benefits conferred by circumcision. If the lower risk of PCa with circumcision is through the reduction in risk of STI and infection/inflammation in the prostate, then the timing of circumcision is important and needs to precede sexual activity. Three studies reported results for timing of circumcision.19, 23, 24 One found reduced risk of PCa only in those circumcised before first intercourse,23 another in those circumcised before the age of 1 year but also in those circumcised after the age of 3524 and a third found that the negative association was observed for circumcision both at birth and later.19 Previous calculations have shown that the single risk factor of lack of circumcision contributed 24% of PCa cases.35 For countries with low circumcision rates (China, Japan and other Southeast Asian countries), neonatal implementation of circumcision programs might be economically advantageous as well as life-saving,16 but studies in these population groups are needed.
Conclusion
Results of this meta-analysis point to a small but protective association of circumcision status with PCa. Future investigations in non-white non-western populations warrant close attention to design and methodological features. Well-designed epidemiological studies of large size with control for other potential confounders would help illuminate the complex interplay of other factors associated with PCa risk.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D . Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90.
Siegel R, Ma J, Zou Z, Jemal A . Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29.
Key T . Risk factors for prostate cancer. Cancer Surv 1995; 23: 63–77.
World Health Organizations, Department of Reproductive Health and Research and Joint United Nations Programme on HIV/AIDS (UNAIDS). Male Circumcision: Global Trends and Determinants of Prevalence, Safety and Acceptability. UNAIDS JUNPoHA, Department of Reproductive Health and Research: Geneva, Switzerland, 2007.
Kaicher DC, Swan KG . A cut above: circumcision as an ancient status symbol. Urology 2010; 76: 18–20.
Morris BJ, Waskett JH, Banerjee J, Wamai RG, Tobian AA, Gray RH et al. A ‘snip’ in time: what is the best age to circumcise? BMC Pediatr 2012; 12: 20.
Rickwood AM . Medical indications for circumcision. BJU Int 1999; 83: 45–51.
Hayashi Y, Kojima Y, Mizuno K, Kohri K . Prepuce: phimosis, paraphimosis, and circumcision. ScientificWorldJournal 2011; 11: 289–301.
Morris BJ . Why circumcision is a biomedical imperative for the 21(st) century. Bioessays 2007; 29: 1147–1158.
Maeda JL, Chari R, Elixhauser A . Circumcisions performed in U.S. community hospitals, 2009: Statistical Brief #126. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality: Rockville, MD, 2012, pp 1-13. (www.hcup-us.ahrq.gov/reports/statbriefs/sb126.jsp).
Dennis LK, Dawson DV . Meta-analysis of measures of sexual activity and prostate cancer. Epidemiology 2002; 13: 72–79.
Taylor ML, Mainous AG 3rd, Wells BJ . Prostate cancer and sexually transmitted diseases: a meta-analysis. Fam Med 2005; 37: 506–512.
Weiss HA, Quigley MA, Hayes RJ . Male circumcision and risk of HIV infection in sub-Saharan Africa: a systematic review and meta-analysis. AIDS 2000; 14: 2361–2370.
Tobian AA, Serwadda D, Quinn TC, Kigozi G, Gravitt PE, Laeyendecker O et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med 2009; 360: 1298–1309.
Larke NL, Thomas SL, dos Santos Silva I, Weiss HA . Male circumcision and penile cancer: a systematic review and meta-analysis. Cancer Causes Control 2011; 22: 1097–1110.
Morris BJ, Waskett JH . Circumcision reduces prostate cancer risk. Asian J Androl 2012; 14: 661–662.
Wynder EL, Mabuchi K, Whitmore WF Jr . Epidemiology of cancer of the prostate. Cancer 1971; 28: 344–360.
Mandel JS, Schuman LM . Sexual factors and prostatic cancer: results from a case-control study. J Gerontol 1987; 42: 259–264.
Ross RK, Shimizu H, Paganini-Hill A, Honda G, Henderson BE . Case-control studies of prostate cancer in blacks and whites in southern California. J Natl Cancer Inst 1987; 78: 869–874.
Newell GR, Fueger JJ, Spitz MR, Babaian RJ . A case-control study of prostate cancer. Am J Epidemiol 1989; 130: 395–398.
Ewings P, Bowie C . A case-control study of cancer of the prostate in Somerset and east Devon. Br J Cancer 1996; 74: 661–666.
Rosenblatt KA, Wicklund KG, Stanford JL . Sexual factors and the risk of prostate cancer. Am J Epidemiol 2001; 153: 1152–1158.
Wright JL, Lin DW, Stanford JL . Circumcision and the risk of prostate cancer. Cancer 2012; 118: 4437–4443.
Spence AR, Rousseau MC, Karakiewicz PI, Parent ME . Circumcision and prostate cancer: a population-based case-control study in Montreal, Canada. BJU Int 2014; 114: E90–E98.
Wells GA SB, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 15 January 2015).
Mantel N, Haenszel W . Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22: 719–748.
DerSimonian R, Laird N . Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188.
Lau J, Ioannidis JP, Schmid CH . Quantitative synthesis in systematic reviews. Ann Intern Med 1997; 127: 820–826.
Berman NG, Parker RA . Meta-analysis: neither quick nor easy. BMC Med Res Methodol 2002; 2: 10.
Higgins JP, Thompson SG . Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558.
Galbraith RF . A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med 1988; 7: 889–894.
Pabalan N, Bapat B, Sung L, Jarjanazi H, Francisco-Pabalan O, Ozcelik H . Cyclin D1 Pro241Pro (CCND1-G870A) polymorphism is associated with increased cancer risk in human populations: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2008; 17: 2773–2781.
Ioannidis JP, Trikalinos TA . The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ 2007; 176: 1091–1096.
Moher D, Liberati A, Tetzlaff J, Altman DG . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097.
Morris BJ, Waskett J, Bailis SA . Case number and the financial impact of circumcision in reducing prostate cancer. BJU Int 2007; 100: 5–6.
Spitz MR, Fueger JJ, Newell GR . The development of a comprehensive, institution-based patient risk evaluation program: II. Validity and reliability of questionnaire data. Am J Prev Med 1988; 4: 188–193.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pabalan, N., Singian, E., Jarjanazi, H. et al. Association of male circumcision with risk of prostate cancer: a meta-analysis. Prostate Cancer Prostatic Dis 18, 352–357 (2015). https://doi.org/10.1038/pcan.2015.34
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2015.34
- Springer Nature Limited