Abstract
Intestinal epithelial stem cells are highly sensitive to differentiation induced by endoplasmic reticulum (ER) stress. Colorectal cancer develops from mutated intestinal epithelial stem cells. The most frequent initiating mutation occurs in Apc, which results in hyperactivated Wnt signalling. This causes hyperproliferation and reduced sensitivity to chemotherapy, but whether these mutated stem cells are sensitive to ER stress induced differentiation remains unknown. Here we examined this by generating mice in which both Apc and ER stress repressor chaperone Grp78 can be conditionally deleted from the intestinal epithelium. For molecular studies, we used intestinal organoids derived from these mice. Homozygous loss of Apc alone resulted in crypt elongation, activation of the Wnt signature and accumulation of intestinal epithelial stem cells, as expected. This phenotype was however completely rescued on activation of ER stress by additional deletion of Grp78. In these Apc-Grp78 double mutant animals, stem cells were rapidly lost and repopulation occurred by non-mutant cells that had escaped recombination, suggesting that Apc-Grp78 double mutant stem cells had lost self-renewal capacity. Although in Apc-Grp78 double mutant mice the Wnt signature was lost, these intestines exhibited ubiquitous epithelial presence of nuclear β-catenin. This suggests that ER stress interferes with Wnt signalling downstream of nuclear β-catenin. In conclusion, our findings indicate that ER stress signalling results in loss of Apc mutated intestinal epithelial stem cells by interference with the Wnt signature. In contrast to many known inhibitors of Wnt signalling, ER stress acts downstream of β-catenin. Therefore, ER stress poses a promising target in colorectal cancers, which develop as a result of Wnt activating mutations.
Similar content being viewed by others
Introduction
Colorectal cancer forms from precursor lesions known as adenomas. These lesions develop as the result of oncogenic mutations that occur in intestinal epithelial stem cells.1, 2, 3 Tight control over intestinal stem cell differentiation is thus required to maintain homeostasis and to protect from cancer development.
We have previously shown that stress emanating from the endoplasmic reticulum (ER) and subsequent activation of the unfolded protein response (UPR) results in differentiation of intestinal epithelial stem cells into transit amplifying cells.4 Under homeostatic conditions, the ER chaperone Grp78 resides at the ER membrane and inhibits activity of three transmembrane proteins: IRE1, ATF6 and PERK. When misfolded proteins accumulate, chaperones dissociate from these transmembrane proteins and UPR signalling is initiated. PERK, phosphorylates eIF2α resulting in a temporary halt of global protein translation. IRE1α and ATF6 signalling result in activation of a transcriptional programme that expands the capacity of the ER to meet increased demands for protein processing.
Besides its regulatory role in ER homeostasis, UPR activity has increasingly been recognised in cell fate decisions.5, 6
Using mice in which ER stress was induced in the intestinal epithelium specifically by deletion of chaperone Grp78, we have previously found that mutant stem cells were lost and replaced by new stem cells derived from non-recombined cells, a process called repopulation. We identified Perk-eIF2α signalling as responsible for ER stress induced stem cell loss. In addition to our work in the intestine, an identical role for ER stress signalling was observed in oesophageal epithelium and in hematopoietic stem cells.6, 7
Intestinal epithelial stem cells thus exhibit marked sensitivity to ER stress, which can trigger their differentiation. It is however unknown whether this sensitivity applies to cells that have already acquired oncogenic mutations that prime them towards malignancy. We hypothesize that intestinal epithelial stem cells that have obtained tumourigenic mutations are still sensitive to ER stress induced differentiation.
We investigate this using VillinCreERT2-Apcfl/fl(IEC) animals in which both alleles of the tumour suppressor gene Apc can be deleted at the same time from the intestinal epithelium on injection with tamoxifen. Apc is a gatekeeper gene that constrains Wnt signalling activity. In humans, homozygous mutations in APC are found in 90% of sporadic colorectal carcinomas and those tumours that do not carry APC mutations often have activating mutations in Wnt signalling transcription factor β-catenin.8 Heterozygosity of Apc both in mice and humans results in development of multiple adenoma’s throughout the intestine, a syndrome in humans known as familial adenomatous polyposis. Homozygous deletion of Apc from the intestinal epithelium, results in prompt progenitor cell expansion in the crypt, eventually causing adenomatous tissue to develop throughout the intestine.9 Moreover, hyperactivated Wnt signalling protects against chemotherapeutics.10 We set out to determine the effect of ER stress on intestinal epithelium in which we homozygously delete Apc.
Results
ER stress abrogates Wnt signalling in vitro and ex vivo
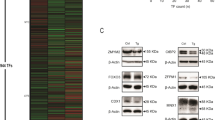
The starting point of these studies was the observation that a large number of Wnt signalling target-genes are downregulated upon induction of ER stress by subtilase cytotoxin (SubAB) treatment in vitro.4 We had previously performed messenger RNA (mRNA) micro-array analysis on LS174T colorectal cancer cells that were treated with SubAB to induce ER stress compared with cells that were treated with enzymatically inactive SubAA272B as control. Treatment with SubAB results in specific proteolysis of GRP78 inside the ER, resulting in UPR activation.11, 12 Upon SubAB treatment we observed downregulation of a large number of Wnt target-genes, such as LGR5, AXIN2, CD44, EPHB2 and EPHB3 (Figure 1a).
ER stress signalling represses the Wnt signalling signature in vitro. (a) Relative mRNA levels of a panel of Wnt target-genes from mRNA expression arrays of LS174T cells on 20 h treatment with SubAB (200 ng/ml) or the enzymatically inactive SubAA272B as a control. Values are extracted from mRNA micro-array. (b) GSEA of Wnt signalling pathway target-genes on LS174T cells that were treated with either SubAB or SubAA272B. (c) mRNA expression in Apcfl/fl(IEC) organoids that were either treated with vehicle (veh) or 4OHT for 24 h followed by either 200 ng/ml SubAB or SubAA272B treatment for 20 h. *P<0.05, ***P<0.001.
To further analyse Wnt signalling pathway activity, we performed geneset enrichment analysis (GSEA) for the Wnt signature in these cells. We extracted the LS174T specific Wnt signature from mRNA expression arrays performed with cells in which Wnt signalling was blocked by dominant negative expression of effector transcription factor TCF4.13 We found that shortly after induction of ER stress in LS174T cells using SubAB, the Wnt signalling gene signature was significantly lost (Figure 1b).
We additionally analysed the effect of ER stress on Wnt signalling in organoids. To this end we generated Apcfl/fl organoids in which treatment with tamoxifen metabolite 4OHT resulted in homozygous deletion of Apc. Upon recombination, these organoids exhibit highly activated Wnt signalling and become hyperproliferative. We treated non-recombined (wild type) and recombined (Apc−/−) organoids with SubAB and tested a panel of Wnt target-genes. Both in wild type and Apc mutant organoids, we found that SubAB induced ER stress results in downregulation of Wnt target-genes (Figure 1c).
To assess the extent of UPR activation upon loss of Grp78, we performed microarray analysis of Grp78fl/fl organoids in which treatment with 4OHT had resulted in knockout of Grp78. By GSEA we found significant induction of the UPR signature (Supplementary Figure. 1). Further GSEA showed profound loss of both the Wnt signalling geneset and the intestinal epithelial stem cell geneset on deletion of Grp78. These results show that induction of ER stress, by loss of Grp78, compromises the Wnt signalling gene signature in vitro and ex vivo.
Effects of ER stress on the intestinal epithelium are dominant over the phenotype of hyperactive Wnt signalling
In Grp78fl/fl mice, we previously showed that intestinal epithelial stem cells are lost as soon as 24 h after recombination. In the following days, crypts become hypoplastic and crypt epithelium becomes thin.4 Conversely, loss of Apc leads to increased proliferation and stem cell expansion resulting from hyperactive Wnt signalling. This causes crypt enlargement, which is visible most clearly from day 3 post induction (p.i.).9, 14, 15 In a number of days thereafter, the intestine fills with adenomatous tissue, proving that homozygous loss of Apc is sufficient for adenomagenesis.16 To investigate whether the Apc−/− intestinal epithelium is sensitive to ER stress mediated stem cell differentiation, we crossed Apcfl/fl animals to Grp78fl/fl and VillinCreERT2 mice.
In these animals, injections with tamoxifen result in recombination restricted to the small intestinal and colonic epithelium.17 Recombination occurs to a similar extent in stem cells, transit amplifying cells and all differentiated cell types, including Paneth cells that reside at the crypt base and which are reported to play a critical role in maintenance of the stem cell niche.18 We generated animals in which intestinal epithelial cell (IEC) specific deletion of Grp78, Apc or both Grp78 and Apc alleles occurred on injections with tamoxifen. In this fashion we could induce IEC specific ER stress (Grp78−/−(IEC)), Wnt signalling pathway hyperactivation (Apc−/−(IEC)) or both ER stress and Wnt signalling hyperactivation (Apc−/−Grp78−/−(IEC)). We compared these animals to littermate controls that either carried the wild type alleles of both Apc and Grp78 or lacked the VillinCreERT2 allele. To monitor Cre-mediated recombination on tissue sections we crossed Rosa26LacZ or Rosa26ZsGreen reporter alleles into all animals. We killed animals on day 3 p.i., as effects of both ER stress and Wnt signalling hyperactivation are clearly observed at that time point.
In concordance with previous reports we observed that Apc−/−(IEC) animals displayed crypt elongation and densely packed crypt cells.9 Conversely, Grp78−/−(IEC) animals displayed epithelial thinning and crypt hypoplasia, similar to our previous findings in Ah1Cre-Grp78−/−(IEC) animals.4 Combined knockout in Apc−/−Grp78−/−(IEC) animals resulted in crypts that mostly resembled Grp78−/−(IEC) crypts with reduced length and marked thinning of crypt cells (Figures 2a and b).
Induction of ER stress by loss of Grp78 causes Apc−/−Grp78−/−(IEC) crypts to resemble Grp78−/−(IEC) crypts. (a) Haematoxylin and eosin staining on representative small intestinal sections of animals of indicated genotypes on day 3 after tamoxifen injections. Arrows depict a representative morphometric measurement. (b) Relative crypt length of animals of indicated genotype. (c) Grp78 staining on small intestinal sections of animals of indicated genotypes. (d) Quantitative RT-PCR analysis of Apc and Grp78 from intestinal epithelial fractions. (e) BrdU staining on small intestinal sections of animals of indicated genotypes. (f) Quantification of BrdU-positive cells per crypt. (g) Cleaved caspase-3 staining on representative small intestinal sections of animals of indicated genotypes. (h) Quantification of cleaved caspase-3-positive cells in either crypts or villi. Quantifications were performed in 30 crypts per animal, N=5 animals per genotype. Magnifications × 200. *P<0.05, **P<0.01, ***P<0.001.
Analysis of expression of the ZsGreen and LacZ reporter alleles, showed that recombination efficiency was excellent (>95% of all crypts, Supplementary Figure. 2). Also, staining for Grp78 confirmed absence of the protein in Grp78−/−(IEC) and Apc−/−Grp78−/−(IEC) mice (Figure 2c). To confirm recombination on the mRNA level we performed quantitative reverse transcription PCR (RT-PCR) analysis of Grp78 and Apc on isolated epithelial fractions. In Apc−/−(IEC) and Apc−/−Grp78−/−(IEC) animals, Apc mRNA levels were significantly reduced and in Grp78−/−(IEC) and Apc−/−Grp78−/−(IEC) animals, Grp78 mRNA levels were significantly reduced (Figure 2d).
We did not detect morphological differences between VillinCreERT2-Grp78−/−(IEC) animals and our previously published Ah1Cre-Grp78−/−(IEC) animals although recombination in Ah1Cre excludes Paneth cells. To assess effects of Grp78 deletion on Paneth cell presence and distribution, we performed staining for lysozyme and found that similar to control animals, Paneth cells were present in all crypts of animals lacking Grp78 (VillinCreERT2-Grp78−/−(IEC) and VillinCreERT2-Apc−/−Grp78−/−(IEC)) with distribution similar to wild type (Supplementary Figure. 3). We therefore concluded that the phenotype obtained upon deletion of Grp78 is not dependent on its signalling in Paneth cells or on presence of these cells.
Hyperactivation of Wnt signalling results in progenitor cell proliferation and stem cell expansion, whereas deletion of Grp78 induces stem cell differentiation at 24 h, followed by loss of proliferation at ~72 h.4, 9 We therefore next analysed intestinal proliferation. As expected, Apc−/−(IEC) mice had increased amounts of BrdU-positive cells per crypt (18.4 vs 10.3 in controls, P<0.001) whereas the intestines of Grp78−/−(IEC) mice displayed reduced proliferation (3.1 vs 10.3 in controls, P<0.001). In Apc−/−Grp78−/−(IEC) mice, BrdU incorporation was reduced to the level of Grp78−/−(IEC) mice (2.7 vs 10.3 in controls, P<0.001; Figures 2e and f).
It has previously been described that homozygous deletion of Apc results in increased apoptosis of crypt cells.19 Analysis of cleaved caspase-3 indeed showed increased numbers of apoptotic cells in crypts of Apc−/−(IEC) mice (0.878 vs 0.386 in controls, P<0.05), whereas apoptosis was unaltered in Grp78−/−(IEC) mice (0.267 vs 0.386 in controls, NS). In Apc−/−Grp78−/−(IEC) animals, numbers of apoptotic cells were low, similar to numbers observed in in Grp78−/−(IEC) mice (0.233 vs 0.386 in controls, NS; Figures 2g and h). Taken together, simultaneous deletion of Grp78 to induce ER stress and Apc to induce hyperactive Wnt signalling from the intestinal epithelium results in a phenotype that completely rescues the Apc−/−(IEC) phenotype in terms of crypt length, proliferation and apoptosis, and highly resembles the phenotype of Grp78−/− epithelium. We thus conclude that activation of the UPR leads to loss of both normal and Apc mutant stem cells in vivo.
Wnt signalling pathway target-genes are not increased in Apc−/−Grp78−/−(IEC) epithelium while high expression of ER stress markers is maintained
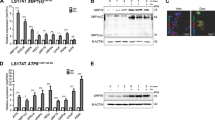
As intestines of Apc−/−Grp78−/−(IEC) mice did not exhibit a histological phenotype of Wnt signalling activation, we next evaluated gene expression levels of Wnt signalling target-genes in epithelium of these mice. To this end we used intestinal organoids of primary epithelium from mice of all three different genotypes and compared all organoids to non-recombined controls. Organoids were recombined by 4OHT treatment for 24 h, and harvested 24 h later. Recombination was excellent, as judged by mRNA levels of Apc and Grp78 after deletion (Figure 3a). All organoids carried the Rosa26LacZ reporter allele which enabled verifying recombination efficacy by lacZ staining (Figure 3b). mRNA expression analysis confirmed that all tested Wnt target-genes (Axin2; CD44; Ephb2; Ephb3) were upregulated in Apc−/−(IEC) organoids (Figure 3c). In Apc−/−Grp78−/−(IEC) organoids these genes were downregulated. To test whether downregulation of Wnt targets extended from mRNA to the level of protein expression we performed immunohistochemical staining for CD44 and Ephb2 and indeed found upregulation of these proteins in Apc−/−(IEC) epithelium and downregulation in both Grp78−/−(IEC) and Apc−/−Grp78−/−(IEC) epithelium (Figure 3d and Supplementary Figure. 4). Next, we analysed presence of ER stress and UPR activity in organoids of all genotypes. We found significant upregulation of UPR activation markers Chop, Xbp1(s), Perk, Atf4 and Erdj4 in Grp78−/−(IEC) and Apc−/−Grp78−/−(IEC) organoids. In Apc−/−(IEC) organoids, UPR target-genes were not upregulated, in contrast, Xbp1(s) was downregulated (Figure 3e). Thus, depletion of Grp78 from the epithelium results in UPR activation, regardless of Apc status. In addition, Wnt signalling is abrogated phenotypically and on the level of target-genes in Apc−/−Grp78−/−(IEC) epithelium.
Loss of Grp78 in Apc mutant epithelium dampens the increased expression of Wnt signalling target-genes. (a) Quantitative RT-PCR analysis of Apc and Grp78 on either non-recombined organoids (control) or recombined organoids carrying the indicated genotypes. (b) Recombination efficiency of LacZ reporter allele assessed in organoids by X-gal staining. (c) Quantitative RT-PCR of a panel of markers of ER stress and activation of the UPR. (d) Quantitative RT-PCR of a panel of Wnt signalling pathway target-genes. (e) CD44 staining on representative small intestinal sections of animals of indicated genotypes. Magnifications × 100 for organoids and × 200 for intestinal sections. *P<0.05, **P<0.01, ***P<0.001.
Stem cell marker expression in Apc−/−Grp78−/−(IEC) epithelium is similar to Grp78−/−(IEC) epithelium
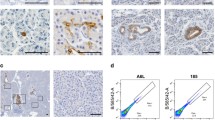
Wnt signalling drives stem cell fate and animals that lack epithelial Grp78 have profoundly reduced expression of Wnt signalling target-genes. To examine presence of stem cells at the crypt base (crypt base columnar cells), we performed in situ hybridisation for Olfm4.20 As expected, we observed increased stem cell numbers in Apc−/−(IEC) animals and stem cells were absent from crypts in Grp78−/−(IEC) animals. Similarly, in crypts of Apc−/−Grp78−/−(IEC) mice, we observed loss of all Olfm4-positive stem cells (Figure 4a). We next performed quantitative RT-PCR on organoids from these animals for stem cell markers Olfm4 and Lgr5. Indeed, mRNA levels of these stem cell markers were high in Apc−/−(IEC) organoids and reduced in both Grp78−/−(IEC) and Apc−/−Grp78−/−(IEC) organoids (Figure 4b). We next analysed alternative stem cell markers Bmi1, Hopx and mTert.21, 22, 23 We found these transcripts to be upregulated in Apc−/− organoids but expression in Grp78−/−(IEC) and Apc−/−Grp78−/−(IEC) organoids was variable (Figure 4c).
Loss of Grp78 results in loss of intestinal epithelial stem cells regardless of Apc status. (a) In situ hybridisation for Olfm4 in mice of indicated genotypes (b) Quantitative RT-PCR on recombined organoids with indicated genotypes and non-recombined organoids (control) for stem cell markers Olfm4 and Lgr5. (c) Quantitative RT-PCR of a panel of alternative stem cell markers. **P<0.01, ***P<0.001. Magnifications × 200.
Thus, presence of Grp78 is required for crypt base columnar stem cell fate. Sensitivity of these cells for ER stress is independent of Apc since cells in which Apc and Grp78 are deleted simultaneously are rapidly lost on induction of ER stress.
ER stress induced loss of Wnt signalling occurs downstream of β-catenin
Deletion of both alleles of Apc results in accumulation of transcriptionally active, nuclear β-catenin throughout all crypt and villus cells.9 To delineate the molecular basis of ER stress induced loss of the Wnt signature, we next assessed presence of β-catenin protein in intestines of animals from all four genotypes (Figure 5a). We confirmed Grp78 deletion by staining for Grp78 on consecutive sections (Figure 5b). Under homeostatic conditions β-catenin is expressed on the membrane of all epithelial cells and nuclear localisation is solely found in cells at the crypt base. As expected, in Apc−/−(IEC) intestine, nuclear localisation of β-catenin extended to all epithelial cells, including those located in the differentiated compartment. Conversely, in crypts of Grp78−/−(IEC) mice we did not observe nuclear β-catenin in transit amplifying cells and villi. Surprisingly, in Apc−/−Grp78−/−(IEC) mice, we found that although the expression of Wnt signalling target-genes was significantly reduced, nuclear β-catenin expression was detectable in all enterocytes. These data suggest that those components required for nuclear translocation of β-catenin upon deletion of Apc, are unaltered upon deletion of Grp78 and that in Apc−/−Grp−/−(IEC) epithelium, the Wnt signature is abrogated downstream of β-catenin.
Expression of c-Myc depends on Grp78 presence
In colorectal cancer cell lines, we previously identified that ER stress induced loss of stemness results from Perk-eIF2α signalling.4 The resulting protein translation attenuation depletes cells of proteins with a short half-life that rely on continuous translation such as the oncogene c-Myc.24 Moreover, c-Myc has been shown to be critical in Wnt signalling induced proliferation and for expression of the Wnt signature downstream of β-catenin.2519 We therefore hypothesized that reduced proliferation and stemness in Apc−/−Grp78−/−(IEC) animals correlated with reduced levels of c-Myc. Assessment of c-Myc levels on both mRNA and protein levels revealed increased expression in Apc−/−(IEC) mice compared with wild type, and strongly decreased expression in both Grp78−/−(IEC) and Apc−/−Grp78−/−(IEC) mice (Figures 6a and b) despite abundant nuclear β-catenin levels in the double mutant mice.
We thus show that in Apc−/−Grp78−/−(IEC) animals, suppression of Wnt signalling downstream of nuclear β-catenin results in severely compromised expression of c-Myc, potentially contributing to loss of stemness.
Loss of self-renewal capacity results in repopulation of Apc−/−Grp78−/− cells by wild-type cells that evaded recombination
In previous experiments using Ah1Cre-Grp78−/−(IEC) animals, we observed that Grp78 mutant cells are replaced by wild-type cells that have evaded recombination, establishing loss of self-renewal capacity in these animals. To investigate self-renewal capacity of Apc−/−Grp78−/− cells, we first analysed longevity of organoids to analyse functional self-renewal capacity. We found that Apc−/−Grp78−/−(IEC) organoids remained small and eventually died within 7 days after recombination, contrasting the unimpaired growth in non-recombined organoids (Figure 7a). Reseeding of either Grp78−/−(IEC) or Apc−/−Grp78−/−(IEC) organoids did not result in growth as opposed to non-recombined and Apc−/− organoids, suggesting loss of self-renewal capacity in Apc−/−Grp78−/−(IEC) epithelium ex vivo.
Rapid repopulation of the Apc−/−Grp78−/−(IEC) epithelium by wild-type cells. (a) Ex vivo culture of organoids from indicated genotypes at indicated time points. (b) Immunohistochemical detection of Grp78 on indicated time points in Apc−/−Grp78−/−(IEC) mice. (c) Relative expression of Grp78 mRNA in epithelium derived from indicated mice, detected by quantitative RT-PCR, reflecting presence of wild-type cells. N=5. Magnifications × 200. *P<0.05, **P<0.01.
We performed an additional mouse experiment in which we gave two tamoxifen injections on consecutive days instead of five injections in 3 days. We killed animals on day 2 to 4 p.i., Similar to Ah1Cre-Grp78−/−(IEC) mice, we observed that the epithelium of Apc−/−Grp78−/−(IEC) animals was repopulated by wild type, Grp78 proficient, cells (Figure 7b). At day 4 p.i., most mutant cells had been replaced by wild-type cells. We confirmed these findings by quantitative RT-PCR for Grp78 mRNA expression, which increased significantly in the days following recombination (Figure 7c). These results show that self-renewal capacity of double mutant cells is lost and that Grp78 presence is thus required for self-renewal of cells that have homozygously lost Apc.
Discussion
The ER chaperone Grp78 acts as a suppressor of the UPR. We previously found that activation of the UPR by depletion of Grp78 results in rapid loss of intestinal epithelial stem cells by forced differentiation to a transit amplifying cell fate in vivo. We and others subsequently made similar observations in normal oesophageal and hematopoietic stem cells and in cultures of human colorectal cancer stem cells.6, 7, 26 In the present study we used an in vivo model of intestinal epithelial adenomatous transformation to examine if activation of the UPR can correct intestinal adenomagenesis. To address this question we generated animals in which homozygous deletion of Apc and Grp78 from the intestinal epithelium occurs on tamoxifen injections. Deletion of Grp78 completely rescued the Apc mutant phenotype, resulting in an intestinal epithelium with shortened crypts, loss of stem cells and loss of proliferation. This shows that activation of the UPR is sufficient to drive loss of Apc mutant intestinal epithelial stem cells in vivo. Similar to our previous observations in normal intestinal epithelium and oesophageal epithelial cells, we found no evidence that activation of the UPR resulted in epithelial cell loss by apoptosis. Instead, we found that the increased number of apoptotic cells observed in Apc mutant crypts was reduced in Apc−/−Grp78−/−(IEC) animals. This suggests that stem cells may be lost by differentiation as was previously observed by lineage tracing in normal intestinal and oesophageal epithelial stem cells.4, 7
To induce UPR activation we use deletion of Grp78. With genetic rescue experiments we have previously shown that stem cell loss on Grp78 depletion specifically depends on UPR activation. Our current studies add to these observations by showing that in Grp78−/− epithelium, UPR pathway activity is significantly increased.
In contrast to our observations that the UPR rescues the Apc mutant phenotype, it was shown that deletion of UPR transcription factor Xbp1 increases intestinal tumourigenesis.27 This may be explained by the fact that Grp78 deletion in our experiments results in activation of all three branches of the UPR instead of isolated perturbation of Xbp1 signalling.
The definitive proof of loss of self-renewal capacity in Apc−/−Grp78−/− double stem cells was provided by the observation that mutant crypts were repopulated by new stem cells that were negative for the LacZ reporter allele and had thus escaped Cre-mediated recombination. Such repopulation has previously been described for other genes that are critical for stem cell self-renewal such as c-Myc,25 Brg1,28 and the stem-cell-specific transcription factor Ascl2.29 The most rapid repopulation was previously observed in 5 days after deletion of Grp78 in the intestinal epithelium using the β-naphtoflavone inducible Ah1Cre.4 The time course of repopulation of Apc−Grp78 double mutant stem cells is identical to that of Grp78 single-mutant stem cells, suggesting that loss of stemness is independent of the Apc status and may therefore occur at some level below the function of Apc. Interestingly, although stem cell repopulation has been shown for multiple genes using Ah1Cre promoter driven recombination in which Paneth cells are not recombined, this phenomenon had not been described using VillinCreERT2 mediated recombination, in which efficient Cre-mediated deletion occurs in Paneth cells.30, 31 This suggests that it is unlikely that Paneth cells are the major source of intestinal epithelial repopulation. Instead many different epithelial cell types may have the capability to de-differentiate in the case of massive loss of stem cells.31 Alternatively repopulating cells are derived from a non-epithelial source such as the stroma or hematopoietic cells.32
The pronounced effect of activation of the UPR on intestinal epithelial stemness seems to be related to inhibition of the Wnt signature. It has been described that ER stress can inhibit Wnt signalling by interfering with the glycosylation of Wnt ligands in vitro.33 Interestingly, however, in our experiment Wnt pathway hyperactivation that results from deletion of Apc is Wnt ligand independent. Therefore, reduced glycosylation of Wnt ligands cannot explain loss of the Wnt signature. Indeed, we observed nuclear accumulation of β-catenin throughout Apc−/−Grp78−/−(IEC) epithelium despite profound loss of the Wnt signature, intestinal epithelial stem cells and proliferation. This suggests that loss of the Wnt signature occurs at a level downstream of β-catenin. This contrasts most other strategies targeting Wnt signalling upstream of β-catenin and may be an important advantage in treatment strategies directed against colorectal cancer in which β-catenin accumulation in the nucleus is the result of genetic mutations.34 A similar phenotype to what we have observed in Apc−/−Grp78−/−(IEC) epithelium has previously been described in mice that lacked both Apc and c-Myc. These animals do not exhibit hyperactive Wnt-signalling phenotypically while maintaining nuclear accumulation of β-catenin throughout the crypt–villus axis.25 In previous in vitro experiments, we found that activation of the UPR results in rapid loss of c-Myc protein expression in a Perk-eIF2α-dependent manner. We therefore examined if c-Myc expression was lost in Apc−/−Grp78−/−(IEC) mice and found that despite intact nuclear β-catenin, c-Myc expression was lost from the same cells. Therefore, loss of c-Myc likely contributes to loss of the Wnt signature. The fact that repopulation in Grp78−/−(IEC) and Apc−/−Grp78−/−(IEC) animals occurs much more rapidly than in animals that lack c-Myc (5 vs 21 days) suggests loss of additional factors that are pivotal for stem cell fate. Although untransformed stem cells are also affected by Grp78 deletion-mediated ER stress, human colon cancer stem cells were more sensitive to chemotherapy in vitro and in vivo after induction of the UPR while healthy tissue remained unaffected putting forth ER stress signalling as a potential therapeutic target in colorectal cancer.26 In conclusion, our results show that ER stress signalling suppresses the intestinal epithelial Wnt signature and results in loss of stemness and self-renewal capacity of premalignant Apc−/− stem cells. The UPR mediates this effect below the level of nuclear β-catenin. This is an important observation as to date, limited therapeutic options target Wnt signalling in the presence of Apc or β-catenin mutations. Our data therefore suggest that modulation of the UPR may be a potent strategy in the prevention or treatment of colorectal cancer.
Materials and methods
Animal experiments
All mouse experiments were performed in the Academic Medical Center Animal Research Institute in accordance with local guidelines. VillinCreERT2, Rosa26LacZ, Rosa26ZsGreen, Apcfl/fl and Grp78fl/fl alleles were all described previously.9, 35, 36, 37, 38, 39 Group size (N=5 per genotype) was chosen based on previous experiments. Blinding and randomisation was not performed. Investigators were blinded after tissue processing. All mice had a C57BL/6 background, were between 8 and 12 weeks old. Different sexes were equally distributed among all groups.
For CreERT2 mediated recombination, mice were given 5 injections with 50 mg/kg tamoxifen (Sigma-Aldrich, St Louis, MO, USA 10 mg/ml in corn oil), during 3 consecutive days and were killed 24 h thereafter. Two hours before killing all mice received 100 mg/kg BrdU intraperitoneally (Sigma-Aldrich, 10 mg/ml in phosphate-buffered saline (PBS)). For repopulation experiments, mice were given 2 injections with tamoxifen on days 0 and 1, and were killed on day 2 to 4. After killing, intestines were immediately taken out and rinsed in cold PBS.
Immunohistochemistry and tissue preparation
Tissue was fixed in 4% buffered formaldehyde in PBS. The next day, formalin was replaced with 70% ethanol and processed according to standard protocols for paraffin embedding. For immunohistochemistry, 4 μm sections were deparaffinized and rehydrated. Endogenous peroxidase was blocked in 0.3% H2O2 in methanol. For antigen retrieval, slides were treated at 96 °C for 10 min in 0.01 M sodium citrate buffer pH 6.0, or for 20 min in 10 mM Tris 1 mM EDTA buffer pH 9.0. Slides were incubated overnight at 4 °C with primary antibody diluted in PBT (PBS, 0.1% Triton X-100, 1% w/v BSA). Primary antibodies: anti-BrdU mouse monoclonal 1:500 (Roche BMC9318), anti-GRP78 rabbit monoclonal 1:200 (Cell Signaling C50B12), anti-β-catenin mouse monoclonal 1:1000 (BD Transduction Laboratories 610154), anti-c-Myc rabbit polyclonal 1:500 (Santa Cruz sc-764), anti-cleaved caspase-3 (Cell Signaling 9661L), anti-lysozyme rabbit polyclonal 1:2000 (Dako A0099), anti-CD44 rat monoclonal 1:500 (AbD Serotec MCA1967) and anti-EphB2 goat polyclonal 1:50 (R&D Systems AF647). Antibody binding was visualised with Powervision (Immunologic) and substrate development was performed using diaminobenzidine (Sigma-Aldrich D5637-10G).
mRNA probe synthesis and in situ hybridisation
For probe synthesis, a total of 1 μg of linearized plasmid DNA was transcribed in vitro using either SP6 or T7 RNA polymerase (Promega) using RNA DIG labeling mix (Roche). The probe was cleaned subsequently through a column (Qiagen RNEasy 74106) and dissolved in diethylpyrocarbonate (DEPC)-treated water containing 50% v/v formamide.40
In situ hybridisation was performed as described previously.41
Organoid culture
Intestinal epithelial organoids were obtained from mice with indicated genotypes. Harvest and expansion of intestinal organoid culture was performed as described previously.4, 42, 43 Recombination of organoids was established by adding 1 μM 4OHT (Sigma-Aldrich, H6278-10MG) to culture medium for 24 h.
Separation of intestinal epithelial cell fractions
Tissue was harvested in PBS, and 2 cm pieces of whole intestine were used for further processing to obtain pure epithelial fractions separate from mesenchyme.44 In short, the pieces of intestine are incubated for 7 min in warm EDTA (30 mM) in HBSS, vortexed and centrifuged after which the supernatant contains the epithelial fraction.
RNA isolation
For gene expression experiments in organoids, mRNA isolation was performed 24 h after treatment with 4OHT using the Bioline ISOLATE II RNA Mini kit (BIO-52073, Bioline) according to manufacturers’ instructions. Organoids were derived from mice bred in our facility, and were tested mycoplasm free. For RNA extraction from mouse intestine, tissue was homogenised (Miccra D-1 homogenizer) in 1 ml Tri-reagent (T9424, Sigma-Aldrich) and RNA extraction was performed according to manufacturer’s protocol.
complementary DNA synthesis and quantative RT-PCR
complementary DNA synthesis was performed using 1 μg of purified RNA using Revertaid reverse transcriptase according to protocol (Fermentas, Vilnius, Lithuania). Quantitative RT-PCR was performed using sensifast SYBR No-ROX Kit (GC-biotech Bio-98020) according to manufacturer’s protocol on a BioRad iCycler. Primers sequences were ordered as found on qPrimerdepot (mouseprimerdepot.nci.nih.gov/). All primersets were intron spanning, primer specificity was tested using melting curve analyses. β-actin was used as reference gene. Relative gene expression was calculated using the 2−delta Ct method.
RNA microarray experiments and GSEA
RNA cleaning was performed using RNeasy columns (Qiagen 74106) according to manufacturer’s protocol and adequate RNA integrity was confirmed on an Agilent 2100 bioanalyzer. All samples had a RNA integrity number of 7, 5 or higher. For microarray experiments, 500 ng RNA was amplified and labelled using Illumina Totalprep RNA amplification kit (Invitrogen AMIL1791) according to manufacturer’s protocol. Of the amplified and biotinylated cRNA 750 ng was hybridised to an Illumina human ref 12BeadChip. The BeadChips were scanned on a BeadArray Reader (Illumina) and processed using Genome Studio software (Illumina). Micro-array results can be found under GEO accession numbers: GSE28467, GSE28466 and GSE83333.
GSEA was performed using software from the Broad Institute. The UPR signature geneset was created by extracting the top 50 upregulated genes (one-way analysis of variance (ANOVA) test, P<0.05) from SubAB treated LS174 cells. The intestinal stem cell signature was previously published.22 The Wnt signature geneset was created by extracting the top 100 upregulated genes (ANOVA test, P<0.05) from small intestinal epithelium of Apc knockout mice.
Statistics
Statistical analysis was performed with GraphPad Prism 5.0 (La Jolla, CA, USA). All values are depicted as the mean±s.e.m. Statistical significance was analysed using Student’s t-test. For multiple comparisons, one-way ANOVA was used followed by a Bonferroni post-test. Organoids experiments are shown as triplicate experiments of recombined organoids compared with non-recombined organoids. For microarray analyses, differentially expressed genes were extracted using ANOVA test and false discovery rate post analysis correction. Differences were considered statistically significant at P<0.05.
References
Huels DJ, Sansom OJ . Stem vs non-stem cell origin of colorectal cancer. Br J Cancer 2015; 113: 1–5.
Kreso A, Dick JE . Evolution of the cancer stem cell model. Cell Stem Cell 2014; 14: 275–291.
Vermeulen L, Snippert HJ . Stem cell dynamics in homeostasis and cancer of the intestine. Nat Rev Cancer 2014; 14: 468–480.
Heijmans J, van Lidth de Jeude JF, Koo BK, Rosekrans SL, Wielenga MC, van de Wetering M et al. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Rep 2013; 3: 1128–1139.
Barker N . Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 2014; 15: 19–33.
van Galen P, Kreso A, Mbong N, Kent DG, Fitzmaurice T, Chambers JE et al. The unfolded protein response governs integrity of the haematopoietic stem-cell pool during stress. Nature 2014; 510: 268–272.
Rosekrans SL, Heijmans J, Büller NVJA, Westerlund J, Lee AS, Muncan V et al. ER stress induces epithelial differentiation in the mouse oesophagus. Gut 2015; 64: 195–202.
Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M et al. Genetic alterations during colorectal-tumor development. N Engl J Med 1988; 319: 525–532.
Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev 2004; 18: 1385–1390.
Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW et al. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J Cell Biol 2001; 152: 87–96.
Paton AW, Beddoe T, Thorpe CM, Whisstock JC, Wilce MCJ, Rossjohn J et al. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature 2006; 443: 548–552.
Wolfson JJ, May KL, Thorpe CM, Jandhyala DM, Paton JC, Paton AW . Subtilase cytotoxin activates PERK, IRE1 and ATF6 endoplasmic reticulum stress-signalling pathways. Cell Microbiol 2008; 10: 1775–1786.
Yang W, Velcich A, Lozonschi I, Liang J, Nicholas C, Zhuang M et al. Inactivation of p21WAF1/cip1 enhances intestinal tumor formation in Muc2−/− mice. Am J Pathol 2005; 166: 1239–1246.
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007; 449: 1003–1007.
van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 2002; 111: 241–250.
Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009; 457: 608–611.
Robine S, Sahuquillo-Merino C, Louvard D, Pringault E . Regulatory sequences on the human villin gene trigger the expression of a reporter gene in a differentiating HT29 intestinal cell line. J Biol Chem 1993; 268: 11426–11434.
Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011; 469: 415–418.
Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, Reed KR et al. Myc deletion rescues Apc deficiency in the small intestine. Nature 2007; 446: 676–679.
van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H . OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology 2009; 137: 15–17.
Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk MEG, Henderson DE et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci USA 2011; 108: 179–184.
Muñoz J, Stange DE, Schepers AG, van de Wetering M, Koo B-K, Itzkovitz S et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent '+4' cell markers. EMBO J 2012; 31: 3079–3091.
Sangiorgi E, Capecchi MR . Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 2008; 40: 915–920.
Hann SR, Eisenman RN . Proteins encoded by the human c-myc oncogene: differential expression in neoplastic cells. Mol Cell Biol 1984; 4: 2486–2497.
Muncan V, Sansom OJ, Tertoolen L, Phesse TJ, Begthel H, Sancho E et al. Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf-4 target gene c-Myc. Mol Cell Biol 2006; 26: 8418–8426.
Wielenga MCB, Colak S, Heijmans J, van Lidth de Jeude JF, Rodermond HM, Paton JC et al. ER-stress-induced differentiation sensitizes colon cancer stem cells to chemotherapy. Cell Rep 2015; 13: 489–494.
Niederreiter L, Fritz TMJ, Adolph TE, Krismer A-M, Offner FA, Tschurtschenthaler M et al. ER stress transcription factor Xbp1 suppresses intestinal tumorigenesis and directs intestinal stem cells. J Exp Med 2013; 210: 2041–2056.
Holik AZ, Krzystyniak J, Young M, Richardson K, Jardé T, Chambon P et al. Brg1 is required for stem cell maintenance in the murine intestinal epithelium in a tissue-specific manner. Stem Cells 2013; 31: 2457–2466.
van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell 2009; 136: 903–912.
Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Göktuna SI, Ziegler PK et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 2013; 152: 25–38.
van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee AN, Gregorieff A et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol 2012; 14: 1099–1104.
Rizvi AZ, Swain JR, Davies PS, Bailey AS, Decker AD, Willenbring H et al. Bone marrow-derived cells fuse with normal and transformed intestinal stem cells. Proc Natl Acad Sci USA 2006; 103: 6321–6325.
Verras M, Papandreou I, Lim AL, Denko NC . Tumor hypoxia blocks Wnt processing and secretion through the induction of endoplasmic reticulum stress. Mol Cell Biol 2008; 28: 7212–7224.
Anastas JN, Moon RT . WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 2013; 13: 11–26.
el Marjou F, Janssen K-P, Chang BH-J, Li M, Hindie V, Chan L et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 2004; 39: 186–193.
Luo S, Mao C, Lee B, Lee AS . GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol 2006; 26: 5688–5697.
Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 2010; 13: 133–140.
Shibata H, Toyama K, Shioya H, Ito M, Hirota M, Hasegawa S et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science 1997; 278: 120–123.
Soriano P . Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 1999; 21: 70–71.
van Dop WA, Heijmans J, Büller NVJA, Snoek SA, Rosekrans SL, Wassenberg EA et al. Loss of Indian Hedgehog activates multiple aspects of a wound healing response in the mouse intestine. Gastroenterology 2010; 139: 1665–1676 1676.e1661-1610.
Heijmans J, Muncan V, Jacobs RJ, de Jonge-Muller ESM, Graven L, Biemond I et al. Intestinal tumorigenesis is not affected by progesterone signaling in rodent models. PLoS One 2011; 6: e22620.
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009; 459: 262–265.
Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 2011; 141: 1762–1772.
Greten FR, Eckmann L, Greten TF, Park JM, Li Z-W, Egan LJ et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004; 118: 285–296.
Acknowledgements
This work was supported by a VENI grant from the Dutch Organisation for Scientific Research (NWO) and by a research stipendium from the Dutch Cancer Foundation (KWF).
Author contributions
JFvLdJ, JH, VM, designed experiments, analysed and discussed data. JFvLdJ, BJM, MCBW, BB, SLR, YHS and SM performed the experiments. ASL provided Grp78fl/fl mice, JCP and AWP provided SubAB and SubAA272BJH, VM and GRvdB supervised the study. JFvLdJ and JH wrote the manuscript with input of all authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
van Lidth de Jeude, J., Meijer, B., Wielenga, M. et al. Induction of endoplasmic reticulum stress by deletion of Grp78 depletes Apc mutant intestinal epithelial stem cells. Oncogene 36, 3397–3405 (2017). https://doi.org/10.1038/onc.2016.326
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.326
- Springer Nature Limited
This article is cited by
-
Endoplasmic reticulum stress regulates the intestinal stem cell state through CtBP2
Scientific Reports (2021)
-
Expression of UPR effector proteins ATF6 and XBP1 reduce colorectal cancer cell proliferation and stemness by activating PERK signaling
Cell Death & Disease (2019)
-
Increased sensitivity to apoptosis upon endoplasmic reticulum stress-induced activation of the unfolded protein response in chemotherapy-resistant malignant pleural mesothelioma
British Journal of Cancer (2018)