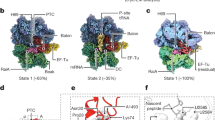
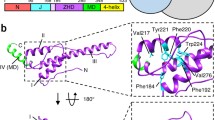
Abstract
Adverse cellular conditions often lead to nonproductive translational stalling and arrest of ribosomes on mRNAs. Here, we used fast kinetics and cryo-EM to characterize Escherichia coli HflX, a GTPase with unknown function. Our data reveal that HflX is a heat shock–induced ribosome-splitting factor capable of dissociating vacant as well as mRNA-associated ribosomes with deacylated tRNA in the peptidyl site. Structural data demonstrate that the N-terminal effector domain of HflX binds to the peptidyl transferase center in a strikingly similar manner as that of the class I release factors and induces dramatic conformational changes in central intersubunit bridges, thus promoting subunit dissociation. Accordingly, loss of HflX results in an increase in stalled ribosomes upon heat shock. These results suggest a primary role of HflX in rescuing translationally arrested ribosomes under stress conditions.








Similar content being viewed by others
References
Verghese, J., Abrams, J., Wang, Y. & Morano, K.A. Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system. Microbiol. Mol. Biol. Rev. 76, 115–158 (2012).
Richter, K., Haslbeck, M. & Buchner, J. The heat shock response: life on the verge of death. Mol. Cell 40, 253–266 (2010).
Yura, T., Nagai, H. & Mori, H. Regulation of the heat-shock response in bacteria. Annu. Rev. Microbiol. 47, 321–350 (1993).
Lindquist, S. Regulation of protein synthesis during heat shock. Nature 293, 311–314 (1981).
Spriggs, K.A., Bushell, M. & Willis, A.E. Translational regulation of gene expression during conditions of cell stress. Mol. Cell 40, 228–237 (2010).
Pechmann, S., Willmund, F. & Frydman, J. The ribosome as a hub for protein quality control. Mol. Cell 49, 411–421 (2013).
Sherman, M.Y. & Qian, S.B. Less is more: improving proteostasis by translation slow down. Trends Biochem. Sci. 38, 585–591 (2013).
Shoemaker, C.J. & Green, R. Translation drives mRNA quality control. Nat. Struct. Mol. Biol. 19, 594–601 (2012).
Kramer, G., Boehringer, D., Ban, N. & Bukau, B. The ribosome as a platform for co-translational processing, folding and targeting of newly synthesized proteins. Nat. Struct. Mol. Biol. 16, 589–597 (2009).
Hartl, F.U. & Hayer-Hartl, M. Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol. 16, 574–581 (2009).
Shalgi, R. et al. Widespread regulation of translation by elongation pausing in heat shock. Mol. Cell 49, 439–452 (2013).
Liu, B., Han, Y. & Qian, S.B. Cotranslational response to proteotoxic stress by elongation pausing of ribosomes. Mol. Cell 49, 453–463 (2013).
Hayes, C.S. & Sauer, R.T. Cleavage of the A site mRNA codon during ribosome pausing provides a mechanism for translational quality control. Mol. Cell 12, 903–911 (2003).
Doma, M.K. & Parker, R. Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature 440, 561–564 (2006).
Subramaniam, A.R., Zid, B.M. & O'Shea, E.K. An integrated approach reveals regulatory controls on bacterial translation elongation. Cell 159, 1200–1211 (2014).
Narberhaus, F. Translational control of bacterial heat shock and virulence genes by temperature-sensing mRNAs. RNA Biol. 7, 84–89 (2010).
Jain, N. et al. E. coli HflX interacts with 50S ribosomal subunits in presence of nucleotides. Biochem. Biophys. Res. Commun. 379, 201–205 (2009).
Shields, M.J., Fischer, J.J. & Wieden, H.J. Toward understanding the function of the universally conserved GTPase HflX from Escherichia coli: a kinetic approach. Biochemistry 48, 10793–10802 (2009).
Verstraeten, N., Fauvart, M., Versees, W. & Michiels, J. The universally conserved prokaryotic GTPases. Microbiol. Mol. Biol. Rev. 75, 507–542 (2011).
Morimoto, T. et al. Six GTP-binding proteins of the Era/Obg family are essential for cell growth in Bacillus subtilis. Microbiology 148, 3539–3552 (2002).
Dutta, D., Bandyopadhyay, K., Datta, A.B., Sardesai, A.A. & Parrack, P. Properties of HflX, an enigmatic protein from Escherichia coli. J. Bacteriol. 191, 2307–2314 (2009).
Gerdes, S.Y. et al. Experimental determination and system level analysis of essential genes in Escherichia coli MG1655. J. Bacteriol. 185, 5673–5684 (2003).
Engels, S. et al. The transcriptional activator ClgR controls transcription of genes involved in proteolysis and DNA repair in Corynebacterium glutamicum. Mol. Microbiol. 57, 576–591 (2005).
Chuang, S.E. & Blattner, F.R. Characterization of twenty-six new heat shock genes of Escherichia coli. J. Bacteriol. 175, 5242–5252 (1993).
Richmond, C.S., Glasner, J.D., Mau, R., Jin, H. & Blattner, F.R. Genome-wide expression profiling in Escherichia coli K-12. Nucleic Acids Res. 27, 3821–3835 (1999).
Carruthers, M.D. & Minion, C. Transcriptome analysis of Escherichia coli O157:H7 EDL933 during heat shock. FEMS Microbiol. Lett. 295, 96–102 (2009).
Fischer, J.J. et al. The ribosome modulates the structural dynamics of the conserved GTPase HflX and triggers tight nucleotide binding. Biochimie 94, 1647–1659 (2012).
Blombach, F. et al. An HflX-type GTPase from Sulfolobus solfataricus binds to the 50S ribosomal subunit in all nucleotide-bound states. J. Bacteriol. 193, 2861–2867 (2011).
Polkinghorne, A. et al. Chlamydophila pneumoniae HflX belongs to an uncharacterized family of conserved GTPases and associates with the Escherichia coli 50S large ribosomal subunit. Microbiology 154, 3537–3546 (2008).
Antoun, A., Pavlov, M.Y., Tenson, T. & Ehrenberg, M.M. Ribosome formation from subunits studied by stopped-flow and Rayleigh light scattering. Biol. Proced. Online 6, 35–54 (2004).
Wilson, D.N. The A-Z of bacterial translation inhibitors. Crit. Rev. Biochem. Mol. Biol. 44, 393–433 (2009).
Pavlov, M.Y., Antoun, A., Lovmar, M. & Ehrenberg, M. Complementary roles of initiation factor 1 and ribosome recycling factor in 70S ribosome splitting. EMBO J. 27, 1706–1717 (2008).
Gao, N. et al. Mechanism for the disassembly of the posttermination complex inferred from cryo-EM studies. Mol. Cell 18, 663–674 (2005).
Weixlbaumer, A. et al. Crystal structure of the ribosome recycling factor bound to the ribosome. Nat. Struct. Mol. Biol. 14, 733–737 (2007).
Borovinskaya, M.A. et al. Structural basis for aminoglycoside inhibition of bacterial ribosome recycling. Nat. Struct. Mol. Biol. 14, 727–732 (2007).
Nakamura, Y. & Ito, K. tRNA mimicry in translation termination and beyond. Wiley Interdiscip. Rev. RNA 2, 647–668 (2011).
Valle, M. et al. Locking and unlocking of ribosomal motions. Cell 114, 123–134 (2003).
Frank, J. & Agrawal, R.K. A ratchet-like inter-subunit reorganization of the ribosome during translocation. Nature 406, 318–322 (2000).
Shoemaker, C.J. & Green, R. Kinetic analysis reveals the ordered coupling of translation termination and ribosome recycling in yeast. Proc. Natl. Acad. Sci. USA 108, E1392–E1398 (2011).
Pisareva, V.P., Skabkin, M.A., Hellen, C.U., Pestova, T.V. & Pisarev, A.V. Dissociation by Pelota, Hbs1 and ABCE1 of mammalian vacant 80S ribosomes and stalled elongation complexes. EMBO J. 30, 1804–1817 (2011).
Shoemaker, C.J., Eyler, D.E. & Green, R. Dom34:Hbs1 promotes subunit dissociation and peptidyl-tRNA drop-off to initiate no-go decay. Science 330, 369–372 (2010).
Tsuboi, T. et al. Dom34:hbs1 plays a general role in quality-control systems by dissociation of a stalled ribosome at the 3′ end of aberrant mRNA. Mol. Cell 46, 518–529 (2012).
Guydosh, N.R. & Green, R. Dom34 rescues ribosomes in 3′ untranslated regions. Cell 156, 950–962 (2014).
van den Elzen, A.M., Schuller, A., Green, R. & Seraphin, B. Dom34-Hbs1 mediated dissociation of inactive 80S ribosomes promotes restart of translation after stress. EMBO J. 33, 265–276 (2014).
Zavialov, A.V., Hauryliuk, V.V. & Ehrenberg, M. Splitting of the posttermination ribosome into subunits by the concerted action of RRF and EF-G. Mol. Cell 18, 675–686 (2005).
Hirokawa, G. et al. Post-termination complex disassembly by ribosome recycling factor, a functional tRNA mimic. EMBO J. 21, 2272–2281 (2002).
Shimizu, Y. Biochemical aspects of bacterial strategies for handling the incomplete translation processes. Front. Microbiol. 5, 170 (2014).
Chadani, Y., Ono, K., Kutsukake, K. & Abo, T. Escherichia coli YaeJ protein mediates a novel ribosome-rescue pathway distinct from SsrA- and ArfA-mediated pathways. Mol. Microbiol. 80, 772–785 (2011).
Handa, Y., Inaho, N. & Nameki, N. YaeJ is a novel ribosome-associated protein in Escherichia coli that can hydrolyze peptidyl-tRNA on stalled ribosomes. Nucleic Acids Res. 39, 1739–1748 (2011).
Connolly, L., De Las Penas, A., Alba, B.M. & Gross, C.A. The response to extracytoplasmic stress in Escherichia coli is controlled by partially overlapping pathways. Genes Dev. 11, 2012–2021 (1997).
Wang, Q.P. & Kaguni, J.M. A novel sigma factor is involved in expression of the rpoH gene of Escherichia coli. J. Bacteriol. 171, 4248–4253 (1989).
Wesolowska, M.T., Richter-Dennerlein, R., Lightowlers, R.N. & Chrzanowska-Lightowlers, Z.M. Overcoming stalled translation in human mitochondria. Front. Microbiol. 5, 374 (2014).
Gianfrancesco, F. et al. A novel pseudoautosomal gene encoding a putative GTP-binding protein resides in the vicinity of the Xp/Yp telomere. Hum. Mol. Genet. 7, 407–414 (1998).
Dunkle, J.A. et al. Structures of the bacterial ribosome in classical and hybrid states of tRNA binding. Science 332, 981–984 (2011).
Schuwirth, B.S. et al. Structures of the bacterial ribosome at 3.5 A resolution. Science 310, 827–834 (2005).
Voorhees, R.M., Weixlbaumer, A., Loakes, D., Kelley, A.C. & Ramakrishnan, V. Insights into substrate stabilization from snapshots of the peptidyl transferase center of the intact 70S ribosome. Nat. Struct. Mol. Biol. 16, 528–533 (2009).
Korostelev, A. et al. Crystal structure of a translation termination complex formed with release factor RF2. Proc. Natl. Acad. Sci. USA 105, 19684–19689 (2008).
Schmittgen, T.D. & Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 (2008).
Ron, E.Z., Kohler, R.E. & Davis, B.D. Polysomes extracted from Escherichia coli by freeze-thaw-lysozyme lysis. Science 153, 1119–1120 (1966).
Feng, B. et al. Structural and functional insights into the mode of action of a universally conserved Obg GTPase. PLoS Biol. 12, e1001866 (2014).
Rao, A.R. & Varshney, U. Specific interaction between the ribosome recycling factor and the elongation factor G from Mycobacterium tuberculosis mediates peptidyl-tRNA release and ribosome recycling in Escherichia coli. EMBO J. 20, 2977–2986 (2001).
Hirashima, A. & Kaji, A. Role of elongation factor G and a protein factor on the release of ribosomes from messenger ribonucleic acid. J. Biol. Chem. 248, 7580–7587 (1973).
Huang, C., Mandava, C.S. & Sanyal, S. The ribosomal stalk plays a key role in IF2-mediated association of the ribosomal subunits. J. Mol. Biol. 399, 145–153 (2010).
Mandava, C.S. et al. Bacterial ribosome requires multiple L12 dimers for efficient initiation and elongation of protein synthesis involving IF2 and EF-G. Nucleic Acids Res. 40, 2054–2064 (2012).
Koripella, R.K. et al. Mechanism of elongation factor-G-mediated fusidic acid resistance and fitness compensation in staphylococcus aureus. J. Biol. Chem. 287, 30257–30267 (2012).
Goodrich, J.A. & Kugel, J.F. Binding and Kinetics for Molecular Biologists (CSHL press, New York, 2007).
Lei, J. & Frank, J. Automated acquisition of cryo-electron micrographs for single particle reconstruction on an FEI Tecnai electron microscope. J. Struct. Biol. 150, 69–80 (2005).
Shaikh, T.R. et al. SPIDER image processing for single-particle reconstruction of biological macromolecules from electron micrographs. Nat. Protoc. 3, 1941–1974 (2008).
Rath, B.K. & Frank, J. Fast automatic particle picking from cryo-electron micrographs using a locally normalized cross-correlation function: a case study. J. Struct. Biol. 145, 84–90 (2004).
Shaikh, T.R., Trujillo, R., LeBarron, J.S., Baxter, W.T. & Frank, J. Particle-verification for single-particle, reference-based reconstruction using multivariate data analysis and classification. J. Struct. Biol. 164, 41–48 (2008).
Scheres, S.H. A Bayesian view on cryo-EM structure determination. J. Mol. Biol. 415, 406–418 (2012).
Kucukelbir, A., Sigworth, F.J. & Tagare, H.D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Huang, B. et al. Functional study on GTP hydrolysis by the GTP-binding protein from Sulfolobus solfataricus, a member of the HflX family. J. Biochem. 148, 103–113 (2010).
Sali, A. & Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993).
Roy, A., Kucukural, A. & Zhang, Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5, 725–738 (2010).
Ng, C.L. et al. Conformational flexibility and molecular interactions of an archaeal homologue of the Shwachman-Bodian-Diamond syndrome protein. BMC Struct. Biol. 9, 32 (2009).
Ash, M.R., Maher, M.J., Guss, J.M. & Jormakka, M. A suite of Switch I and Switch II mutant structures from the G-protein domain of FeoB. Acta Crystallogr. D Biol. Crystallogr. 67, 973–980 (2011).
Villa, E. et al. Ribosome-induced changes in elongation factor Tu conformation control GTP hydrolysis. Proc. Natl. Acad. Sci. USA 106, 1063–1068 (2009).
Trabuco, L.G., Villa, E., Mitra, K., Frank, J. & Schulten, K. Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics. Structure 16, 673–683 (2008).
Pettersen, E.F. et al. UCSF Chimera: a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Acknowledgements
This work was funded by grants from the National Natural Science Foundation of China (31170677 and 31422016 to N.G.) and the Ministry of Science and Technology of China (2013CB910404 to N.G.). S.S. acknowledges research funding from the Swedish Research Council (2010-2619 (M), 2011-6088 (NT), 2014-4423 (NT) and 2008-6593 (Linnaeus grant to Uppsala RNA Research Center); and the Knut and Alice Wallenberg Foundation (KAW 2011.0081 to RiboCORE platform)). We thank the National BioResource Project of Japan for providing E. coli strains BW25113 and JW4131. We also thank the China National Center for Protein Sciences (Beijing) and Tsinghua National Laboratory for Information Science and Technology ('Explorer 100' cluster system) for providing computational resources.
Author information
Authors and Affiliations
Contributions
J.L., S.S. and N.G. designed experiments. Yanqing Zhang performed quantitative PCR, western blotting, cell growth experiments (with X.L. and K.M.), WT and mutant protein preparation (with X.Z.), ribosome purification, SDGC-based experiments, polysome profile analysis (with D.Z. and Y.Q.), cryo-EM data collection (with J.L.) and image processing (with W.C., N.L., Yixiao Zhang and N.G.). C.S.M. performed kinetics experiments. Y.Z., C.S.M., S.S. and N.G. prepared the manuscript; all authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Growth curves of wild-type and hflX-knockout strains.
Growth curves of WT (wild type) and ∆hflX (KO) cells cultured at 30 °C (a) or 45 °C (b). Data shown are means and s.d. (n = 3 cell-culture replicates).
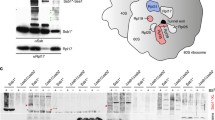
Supplementary Figure 2 An excess of HflX splits 70S ribosomes in vitro and in vivo.
(a-b) The splitting of purified 70S (0.3 μM) (a) or crude 70S ribosome in cell lysate (freshly prepared by ultra-sonication) (b), with 20-fold excess of HflX in the absence (Apo) or presence of different nucleotides (GMPPNP, GTP, and GDP, 1 mM) was checked by SDGC. (c) Overexpression of HflX from the hflX-pBAD plasmid with 1% L-arabinose resulted in slower growth rate of the bacterial host E. coli BW25113 (WT). The vector alone was treated similarly as a control. Data shown are means and s.d. (n= 3 cell-culture replicates). (d) Polysome profile of the mid-log phase E. coli BW25113 cells without (0%) and with induction of HflX by adding 1 % or 2 % (w/v) L-arabinose (L-ara). While HflX overexpression leads to the increase in the 50S fraction. (e) SDS-PAGE and western blotting analysis (anti-his) to check the expression level of recombinant HflX in the above cells. P, empty pBAD vector; H, pBADhflX plasmid. The bands corresponding to HflX are indicated as asterisks.
Supplementary Figure 3 GTP hydrolysis is essential for release of HflX from the 50S subunit.
(a) Formation of 70S ribosome by association of vacant 30S (0.25 µM) with 50S (0.25 µM) in the absence (brown right triangle), or presence of HflX–GTP (black square) and HflX–GMPPNP (olive green down triangle), followed by increase in Rayleigh light scattering with time in a stopped flow apparatus. (b) The effect of HflX–GTP (black square) and HflX–GMPPNP (olive green down triangle) on the formation of 70S initiation complex. The experimental setup and data fitting were same as in a, except that an mRNA programmed 30S pre–IC was used instead of vacant 30S. As a control, the association reaction was also run without HflX (brown right triangle).
Supplementary Figure 4 Resolution of the cryo-EM density map and conformational changes of the 50S subunit upon HflX binding.
(a) Gold-standard Fourier Shell Correlation (FSC) curve. When FSC is 0.143, resolution of cryo-EM density map of the 50S–HflX–GMPPNP complex is 4.5 Å. (b) Local-resolution map of the cryo-EM map. (c) Histogram of local resolution in terms of individual voxels. (d) The atomic model of the 50S–HflX–GMPPNP complex is displayed in cartoon representation (rRNA colored marine and r-proteins colored green as above), superimposed with the model of the 50S subunit from a crystal structure of empty 70S ribosome (PDB id: 2AWB) (rRNA colored red and r-proteins colored yellow)1. (e-j) Close-up views of the local conformational changes on respective rRNA helices as boxed in d. The displacements of the helices upon HflX binding are marked with arrows.
Supplementary Figure 5 Comparison of HflX with translation factors on the 50S subunit.
(a) A thumbnail of the 50S–HflX–GMPPNP structure in the top left corner and superimposition of HflX with canonical translation factors on the 50S subunit. HflX is colored magenta and other translation factors are colored blue. IF2 (PDB id:1ZO1)2, EF-Tu (PDB id: 2XQD)3, EF-G (PDB id: 2WRI)4, LepA (PDB id: 4W2E)5, RF1 (PDB id: 3D5A)6, RF2 (PDB id: 3F1G)7, RF3 (PDB id: 3SFS)8, RRF (PDB id: 4GD1)9, and YaeJ (PDB id: 4DH9)10. (b) Relative orientation of the GTPase domain (yellow) of HflX and known translation factors to the SRL of the 23S rRNA. For clarification, only the GTPase domains of translation factors are shown. PDB codes of the GTPases shown are same as in a.
Supplementary Figure 6 Point and truncation mutations of HflX affect the 70S-spliting activity of HflX.
(a) The splitting of vacant 70S ribosomes, with two truncation mutations of HflX (in the presence of GMPPNP) determined by SDGC, with a comparison to wild type HflX. (b) HflX double point mutations (R49A-K50A, K55A-K62A, R164A-R165A, R168A-R170A, R185A-R189A, R192A-K194A) significantly impairs the 70S-splitting activity. (c) Binding of mutant forms of HflX to the 50S subunits in the presence of GMPPNP examined using co-sedimentation assay. (d) Control experiments for (c) run without 50S subunits. Bands of HflX are indicated by asterisks.
Supplementary Figure 7 Overexpressing the RRF-encoding gene partially restores the growth of ∆hflX cells under heat-stress conditions.
(a-b) Spotting assay of E. coli strains (WT-pBAD, ∆hflX-pBAD, ∆hflX-pBADfrr, ∆hflX-pBADhflX) without (a) or with heat treatment at 50°C for 30 min (b). Spotting assay data in a and b are representative of three individual experiments. (c) Thermo-killing curves of bacteria incubated at 50°C and detected at different time points. Data shown are means and s.d. (n= 3 cell-culture replicates). WT, E. coli BW25113.
Supplementary Figure 8 Distribution of HflX in ribosomal fractions at normal or high-temperature conditions.
Polysome profile analysis of wild type cells cultured at 30°C (a) or 45°C (b). Distribution of HflX in sedimentation fractions was detected using Western blotting.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Table 1 and Supplementary Note (PDF 1563 kb)
Supplementary Data Set 1
Original blots for Figure 1b (PDF 134 kb)
Cryo-EM density map of the 50S-HflX-GMP-PNP complex (4.5 Å)
The movie shows surface presentation of the cryo-EM density map of the 50S-HflX- GMP-PNP complex (4.5 Å), with atomic model superimposed. Also, a close-up view of interactions between HflX and PTC region of the 50S subunit is shown. (MP4 45791 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Mandava, C., Cao, W. et al. HflX is a ribosome-splitting factor rescuing stalled ribosomes under stress conditions. Nat Struct Mol Biol 22, 906–913 (2015). https://doi.org/10.1038/nsmb.3103
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3103
- Springer Nature America, Inc.
This article is cited by
-
Structural basis of GTPase-mediated mitochondrial ribosome biogenesis and recycling
Nature Communications (2021)
-
Mechanisms and regulation of protein synthesis in mitochondria
Nature Reviews Molecular Cell Biology (2021)
-
The stringent response and physiological roles of (pp)pGpp in bacteria
Nature Reviews Microbiology (2021)
-
In vitro synthesis of 32 translation-factor proteins from a single template reveals impaired ribosomal processivity
Scientific Reports (2021)
-
The nucleotide pGpp acts as a third alarmone in Bacillus, with functions distinct from those of (p)ppGpp
Nature Communications (2020)