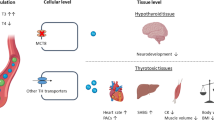
Key Points
-
Many proteins can mediate thyroid hormone transport, but only mutations in genes encoding MCT8, MCT10 and OATP1C1 have pathophysiological effects attributed to this process
-
MCT8 mutations lead to Allan–Herndon–Dudley syndrome, which is characterized by truncal hypotonia and results in spastic quadriplegia, lack of speech, severe intellectual deficit and altered thyroid hormone concentrations
-
MCT8 deficiency impairs the transfer of thyroid hormones across the blood–brain barrier
-
Mct8-deficient mice lack neurological impairment possibly due to the presence of Oatp1c1, a T4 transporter, but levels of OATP1C1 in the primate blood–brain barrier are very low
-
Histopathological studies of patients with mutations in MCT8 support the concept that defective thyroid hormone action in the brain during development leads to the neurological syndrome
Abstract
The cellular influx and efflux of thyroid hormones are facilitated by transmembrane protein transporters. Of these transporters, monocarboxylate transporter 8 (MCT8) is the only one specific for the transport of thyroid hormones and some of their derivatives. Mutations in SLC16A2, the gene that encodes MCT8, lead to an X-linked syndrome with severe neurological impairment and altered concentrations of thyroid hormones. Histopathological analysis of brain tissue from patients who have impaired MCT8 function indicates that brain lesions start prenatally, and are most probably the result of cerebral hypothyroidism. A Slc16a2 knockout mouse model has revealed that Mct8 is an important mediator of thyroid hormone transport, especially T3, through the blood–brain barrier. However, unlike humans with an MCT8 deficiency, these mice do not have neurological impairment. One explanation for this discrepancy could be differences in expression of the T4 transporter OATP1C1 in the blood–brain barrier; OATP1C1 is more abundant in rodents than in primates and permits the passage of T4 in the absence of T3 transport, thus preventing full cerebral hypothyroidism. In this Review, we discuss the relevance of thyroid hormone transporters in health and disease, with a particular focus on the pathophysiology of MCT8 mutations.




Similar content being viewed by others
Change history
07 July 2015
In Table 1 of the originally published article, the Km values for LAT1 and Lat2 were incorrectly labelled and referenced. The Km values for LAT1 shown are for human and not mouse as originally stated. Lat2 was originally labelled as human. These errors have been corrected in the HTML and PDF versions of the article and reference 117 in the original article has been removed.
20 October 2015
In the original published article, reference 93 lists the incorrect article. The correct reference is Arjona, F. J. et al. Identification and functional characterization of zebrafish solute carrier Slc16a2 (Mct8) as a thyroid hormone membrane transporter. Endocrinology 152, 5065–5073 (2011). This error has been corrected in the HTML and PDF versions of the article.
References
Flamant, F. et al. International Union of Pharmacology. LIX. The pharmacology and classification of the nuclear receptor superfamily: thyroid hormone receptors. Pharmacol. Rev. 58, 705–711 (2006).
Chatonnet, F., Flamant, F. & Morte, B. A temporary compendium of thyroid hormone target genes in brain. Biochim. Biophys. Acta 1849, 122–129 (2015).
Kalyanaraman, H. et al. Nongenomic thyroid hormone signaling occurs through a plasma membrane-localized receptor. Sci. Signal. 7, ra48 (2014).
Martin, N. P. et al. A rapid cytoplasmic mechanism for PI3 kinase regulation by the nuclear thyroid hormone receptor, TRβ, and genetic evidence for its role in the maturation of mouse hippocampal synapses in vivo. Endocrinology 155, 3713–3724 (2014).
Davis, P. J., Leonard, J. L. & Davis, F. B. Mechanisms of nongenomic actions of thyroid hormone. Front. Neuroendocrinol. 29, 211–218 (2008).
Cheng, S.-Y., Leonard, J. L. & Davis, P. J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 31, 139–170 (2010).
Visser, T. J. & Peeters, R. P. Metabolism of Thyroid Hormone. Thyroid Disease Manager (ed. DeGroot, L. J.) [online], (2012).
Gereben, B. et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr. Rev. 29, 898–938 (2008).
Larsen, P. R., Davies, T. F. & Hay, I. D. in Williams Text Book of Endocrinology (ed. Wilson, J. D., Foster, D. W., Kronenberg, H. M. & Larsen, P. R.) 389–515 (Saunders Co, 1998).
Hennemann, G. et al. Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr. Rev. 22, 451–476 (2001).
Stein, W. D. & Litman, T. Channels, Carriers, and Pumps. (Elsevier, 2014).
Dumitrescu, A. M., Liao, X.-H., Best, T. B., Brockmann, K. & Refetoff, S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am. J. Hum. Genet. 74, 168–175 (2004).
Friesema, E. C. H. et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364, 1435–1437 (2004).
Lafrenière, R. G., Carrel, L. & Willard, H. F. A novel transmembrane transporter encoded by the XPCT gene in Xq13.2. Hum. Mol. Genet. 3, 1133–1139 (1994).
Friesema, E. C. H. et al. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J. Biol. Chem. 278, 40128–40135 (2003).
Friesema, E. C. H., Jansen, J., Milici, C. & Visser, T. J. Thyroid hormone transporters. Vitam. Horm. 70, 137–167 (2005).
Escobar-Morreale, H. F., Obregon, M. J., Escobar del Rey, F. & Morreale de Escobar, G. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J. Clin. Invest. 96, 2828–2838 (1995).
Oppenheimer, J. H. & Schwartz, H. L. Stereospecific transport of triiodothyronine from plasma to cytosol and from cytosol to nucleus in rat liver, kidney, brain, and heart. J. Clin. Invest. 75, 147–154 (1985).
Spencer, C. A. Thyroid function tests: Assay of thyroid hormones and related substances. Thyroid Disease Manager (ed. DeGroot, L. J.) [online], (2013).
Kinne, A., Schülein, R. & Krause, G. Primary and secondary thyroid hormone transporters. Thyroid Res. 4 (Suppl. 1), S7 (2011).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Halestrap, A. P. The monocarboxylate transporter family--structure and functional characterization. IUBMB Life 64, 1–9 (2012).
Friesema, E. C. H. et al. Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10. Mol. Endocrinol. 22, 1357–1369 (2008).
Heuer, H. et al. The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone-sensitive neuron populations. Endocrinology 146, 1701–1706 (2005).
Wirth, E. K. et al. Neuronal 3′,3,5-triiodothyronine (T3) uptake and behavioral phenotype of mice deficient in Mct8, the neuronal T3 transporter mutated in Allan–Herndon–Dudley syndrome. J. Neurosci. 29, 9439–9449 (2009).
Roberts, L. M. et al. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology 149, 6251–6261 (2008).
Braun, D. et al. Developmental and cell type-specific expression of thyroid hormone transporters in the mouse brain and in primary brain cells. Glia 59, 463–471 (2011).
Müller, J. & Heuer, H. Expression pattern of thyroid hormone transporters in the postnatal mouse brain. Front. Endocrinol. (Lausanne) 5, 92 (2014).
Anwer, M. S. & Stieger, B. Sodium-dependent bile salt transporters of the SLC10A transporter family: more than solute transporters. Pflugers Arch. 466, 77–89 (2014).
Visser, W. E. et al. Study of the transport of thyroid hormone by transporters of the SLC10 family. Mol. Cell. Endocrinol. 315, 138–145 (2010).
Ritchie, J. W., Peter, G. J., Shi, Y.-B. & Taylor, P. M. Thyroid hormone transport by 4F2hc–IU12 heterodimers expressed in Xenopus oocytes. J. Endocrinol. 163, R5–R9 (1999).
Friesema, E. C. et al. Thyroid hormone transport by the heterodimeric human system L amino acid transporter. Endocrinology 142, 4339–4348 (2001).
Morimoto, E. et al. Establishment and characterization of mammalian cell lines stably expressing human L-type amino acid transporters. J. Pharmacol. Sci. 108, 505–516 (2008).
Boado, R. J., Li, J. Y., Nagaya, M., Zhang, C. & Pardridge, W. M. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc. Natl Acad. Sci. USA 96, 12079–12084 (1999).
Núñez, B. et al. Cerebral cortex hyperthyroidism of newborn Mct8-deficient mice transiently suppressed by Lat2 inactivation. PLoS ONE 9, e96915 (2014).
Hagenbuch, B. & Meier, P. J. The superfamily of organic anion transporting polypeptides. Biochim. Biophys. Acta 1609, 1–18 (2003).
Hagenbuch, B. & Meier, P. J. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 447, 653–665 (2004).
Obaidat, A., Roth, M. & Hagenbuch, B. The expression and function of organic anion transporting polypeptides in normal tissues and in cancer. Annu. Rev. Pharmacol. Toxicol. 52, 135–151 (2012).
Hagenbuch, B. Cellular entry of thyroid hormones by organic anion transporting polypeptides. Best Pract. Res. Clin. Endocrinol. Metab. 21, 209–221 (2007).
Visser, W. E., Friesema, E. C. H. & Visser, T. J. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol. Endocrinol. 25, 1–14 (2011).
Abe, T. et al. Molecular characterization and tissue distribution of a new organic anion transporter subtype (Oatp3) that transports thyroid hormones and taurocholate and comparison with Oatp2. J. Biol. Chem. 273, 22395–22401 (1998).
Pizzagalli, F. et al. Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Mol. Endocrinol. 16, 2283–2296 (2002).
Sugiyama, D. et al. Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood-brain barrier: high affinity transporter for thyroxine. J. Biol. Chem. 278, 43489–43495 (2003).
Huber, R. D. et al. Characterization of two splice variants of human organic anion transporting polypeptide 3A1 isolated from human brain. Am. J. Physiol. Cell Physiol. 292, C795–C806 (2007).
Leuthold, S. et al. Mechanisms of pH-gradient driven transport mediated by organic anion polypeptide transporters. Am. J. Physiol. Cell Physiol. 296, C570–C582 (2009).
Allan, W., Herndon, C. N. & Dudley, F. L. Some examples of the inheritance of mental deficiency: apparently sex-linked iodicy and microcephaly. Am. J. Ment. Defic. 48, 325–334 (1944).
Holden, K. R. et al. X-linked MCT8 gene mutations: characterization of the pediatric neurologic phenotype. J. Child Neurol. 20, 852–857 (2005).
Schwartz, C. E. et al. Allan–Herndon–Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am. J. Hum. Genet. 77, 41–53 (2005).
Schwartz, C. E. & Stevenson, R. E. The MCT8 thyroid hormone transporter and Allan–Herndon–Dudley syndrome. Best Pract. Res. Clin. Endocrinol. Metab. 21, 307–321 (2007).
Papadimitriou, A. et al. A novel monocarboxylate transporter 8 gene mutation as a cause of severe neonatal hypotonia and developmental delay. Pediatrics 121, e199–e202 (2008).
Biebermann, H. et al. Extended clinical phenotype, endocrine investigations and functional studies of a loss-of-function mutation A150V in the thyroid hormone specific transporter MCT8. Eur. J. Endocrinol. 153, 359–366 (2005).
Boccone, L., Dessì, V., Meloni, A. & Loudianos, G. Allan–Herndon–Dudley syndrome (AHDS) in two consecutive generations caused by a missense MCT8 gene mutation. Phenotypic variability with the presence of normal serum T3 levels. Eur. J. Med. Genet. 56, 207–210 (2013).
Herzovich, V. et al. Unexpected peripheral markers of thyroid function in a patient with a novel mutation of the MCT8 thyroid hormone transporter gene. Horm. Res. 67, 1–6 (2007).
Kakinuma, H., Itoh, M. & Takahashi, H. A novel mutation in the monocarboxylate transporter 8 gene in a boy with putamen lesions and low free T4 levels in cerebrospinal fluid. J. Pediatr. 147, 552–554 (2005).
Namba, N. et al. Clinical phenotype and endocrinological investigations in a patient with a mutation in the MCT8 thyroid hormone transporter. Eur. J. Pediatr. 167, 785–791 (2008).
Dumitrescu, A. M., Liao, X.-H., Weiss, R. E., Millen, K. & Refetoff, S. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (Mct) 8-deficient mice. Endocrinology 147, 4036–4043 (2006).
Trajkovic-Arsic, M. et al. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J. Clin. Invest. 117, 627–635 (2007).
Liao, X.-H. et al. Distinct roles of deiodinases on the phenotype of Mct8 defect: a comparison of eight different mouse genotypes. Endocrinology 152, 1180–1191 (2011).
Di Cosmo, C. et al. Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. J. Clin. Invest. 120, 3377–3388 (2010).
Trajkovic-Arsic, M. et al. Impact of monocarboxylate transporter-8 deficiency on the hypothalamus-pituitary-thyroid axis in mice. Endocrinology 151, 5053–5062 (2010).
Wirth, E. K. et al. Monocarboxylate transporter 8 deficiency: altered thyroid morphology and persistent high triiodothyronine/thyroxine ratio after thyroidectomy. Eur. J. Endocrinol. 165, 555–561 (2011).
Lavado-Autric, R., Calvo, R.-M., de Mena, R. M., de Escobar, G. M. & Obregón, M. J. Deiodinase activities in thyroids and tissues of iodine-deficient female rats. Endocrinology 154, 529–536 (2013).
Müller, J. et al. Tissue-specific alterations in thyroid hormone homeostasis in combined Mct10 and Mct8 deficiency. Endocrinology 155, 315–325 (2014).
Darras, V. M., Hume, R. & Visser, T. J. Regulation of thyroid hormone metabolism during fetal development. Mol. Cell. Endocrinol. 151, 37–47 (1999).
Trajkovic-Arsic, M. et al. Consequences of monocarboxylate transporter 8 deficiency for renal transport and metabolism of thyroid hormones in mice. Endocrinology 151, 802–809 (2010).
Di Cosmo, C. et al. Mct8-deficient mice have increased energy expenditure and reduced fat mass that is abrogated by normalization of serum T3 levels. Endocrinology 154, 4885–4895 (2013).
Abe, S., Abe, M., Fujiwara, M., Aikawa, T. & Kogo, M. Monocarboxylate transporter 10 functions as a thyroid hormone transporter in chondrocytes. Endocrinology 153, 4049–4058 (2012).
Ferrara, A. M. et al. Changes in thyroid status during perinatal development of Mct8-deficient male mice. Endocrinology 154, 2533–2541 (2013).
Hernandez, A., Martinez, M. E., Fiering, S., Galton, V. A. & St Germain, D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J. Clin. Invest. 116, 476–484 (2006).
Refetoff, S. & Dumitrescu, A. M. Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Pract. Res. Clin. Endocrinol. Metab. 21, 277–305 (2007).
Boccone, L. et al. Allan–Herndon–Dudley syndrome (AHDS) caused by a novel SLC16A2 gene mutation showing severe neurologic features and unexpectedly low TRH-stimulated serum TSH. Eur. J. Med. Genet. 53, 392–395 (2010).
Di Cosmo, C., Liao, X.-H., Dumitrescu, A. M., Weiss, R. E. & Refetoff, S. A thyroid hormone analog with reduced dependence on the monocarboxylate transporter 8 for tissue transport. Endocrinology 150, 4450–4458 (2009).
Fekete, C. & Lechan, R. M. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr. Rev. 35, 159–194 (2014).
Alkemade, A. et al. Novel neuroanatomical pathways for thyroid hormone action in the human anterior pituitary. Eur. J. Endocrinol. 154, 491–500 (2006).
Pardridge, W. M. Carrier-mediated transport of thyroid hormones through the rat blood–brain barrier: primary role of albumin-bound hormone. Endocrinology 105, 605–612 (1979).
Ceballos, A. et al. Importance of monocarboxylate transporter 8 for the blood–brain barrier-dependent availability of 3,5,3′-triiodo-L-thyronine. Endocrinology 150, 2491–2496 (2009).
Sandler, B. et al. Thyroxine-thyroid hormone receptor interactions. J. Biol. Chem. 279, 55801–55808 (2004).
Guadaño-Ferraz, A., Obregon, M. J., St Germain, D. L. & Bernal, J. The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proc. Natl Acad. Sci. USA 94, 10391–10396 (1997).
Morte, B. et al. Thyroid hormone-regulated mouse cerebral cortex genes are differentially dependent on the source of the hormone: a study in monocarboxylate transporter-8- and deiodinase-2-deficient mice. Endocrinology 151, 2381–2387 (2010).
Mayerl, S. et al. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J. Clin. Invest. 124, 1987–1999 (2014).
Mayerl, S., Visser, T. J., Darras, V. M., Horn, S. & Heuer, H. Impact of Oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain. Endocrinology 153, 1528–1537 (2012).
Ito, K. et al. Quantitative membrane protein expression at the blood-brain barrier of adult and younger cynomolgus monkeys. J. Pharm. Sci. 100, 3939–3950 (2011).
Roberts, L. M. et al. Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience 155, 423–438 (2008).
Braun, D., Wohlgemuth, F., Reix, N., Köhrle, J. & Schweizer, U. Aminoaciduria, but normal thyroid hormone levels and signalling, in mice lacking the amino acid and thyroid hormone transporter Slc7a8. Biochem. J. 439, 249–255 (2011).
Grijota-Martínez, C., Díez, D., Morreale de Escobar, G., Bernal, J. & Morte, B. Lack of action of exogenously administered T3 on the fetal rat brain despite expression of the monocarboxylate transporter 8. Endocrinology 152, 1713–1721 (2011).
Van Herck, S. L. J., Geysens, S., Delbaere, J., Tylzanowski, P. & Darras, V. M. Expression profile and thyroid hormone responsiveness of transporters and deiodinases in early embryonic chicken brain development. Mol. Cell. Endocrinol. 349, 289–297 (2012).
Dratman, M. B., Crutchfield, F. L. & Schoenhoff, M. B. Transport of iodothyronines from bloodstream to brain: contributions by blood:brain and choroid plexus:cerebrospinal fluid barriers. Brain Res. 554, 229–236 (1991).
Dziegielewska, K. M., Ek, J., Habgood, M. D. & Saunders, N. R. Development of the choroid plexus. Microsc. Res. Tech. 52, 5–20 (2001).
Nishikawa, M. et al. 3,3′5′-triiodothyronine (reverse T3) in human cerebrospinal fluid. J. Clin. Endocrinol. Metab. 53, 1030–1035 (1981).
Gika, A. D. et al. White matter abnormalities and dystonic motor disorder associated with mutations in the SLC16A2 gene. Dev. Med. Child. Neurol. 52, 475–482 (2010).
Brockmann, K., Dumitrescu, A. M., Best, T. T., Hanefeld, F. & Refetoff, S. X-linked paroxysmal dyskinesia and severe global retardation caused by defective MCT8 gene. J. Neurol. 252, 663–666 (2005).
Rodrigues, T. B. et al. Increased oxidative metabolism and neurotransmitter cycling in the brain of mice lacking the thyroid hormone transporter SLC16A2 (MCT8). PLoS ONE 8, e74621 (2013).
Arjona, F. J. et al. Identification and functional characterization of zebrafish solute carrier Slc16a2 (Mct8) as a thyroid hormone membrane transporter. Endocrinology 152, 5065–5073 (2011).
Vatine, G. D. et al. Zebrafish as a model for monocarboxyl transporter 8-deficiency. J. Biol. Chem. 288, 169–180 (2013).
Zada, D., Tovin, A., Lerer-Goldshtein, T., Vatine, G. D. & Appelbaum, L. Altered behavioral performance and live imaging of circuit-specific neural deficiencies in a zebrafish model for psychomotor retardation. PLoS Genet. 10, e1004615 (2014).
de Vrieze, E. et al. Knockdown of monocarboxylate transporter 8 (mct8) disturbs brain development and locomotion in zebrafish. Endocrinology 155, 2320–2330 (2014).
López-Espíndola, D. et al. Mutations of the thyroid hormone transporter MCT8 cause prenatal brain damage and persistent hypomyelination. J. Clin. Endocrinol. Metab. 99, E2799–E2804 (2014).
Berbel, P., Marco, P., Cerezo, J. R. & DeFelipe, J. Distribution of parvalbumin immunoreactivity in the neocortex of hypothyroid adult rats. Neurosci. Lett. 204, 65–68 (1996).
Guadaño-Ferraz, A., Escobar del Rey, F., Morreale de Escobar, G., Innocenti, G. M. & Berbel, P. The development of the anterior commissure in normal and hypothyroid rats. Brain. Res. Dev. Brain. Res. 81, 293–308 (1994).
Bernal, J. & Guadaño-Ferraz, A. Analysis of thyroid hormone-dependent genes in the brain by in situ hybridization. Methods Mol. Biol. 202, 71–90 (2002).
Sijens, P. E., Rödiger, L. A., Meiners, L. C. & Lunsing, R. J. 1H magnetic resonance spectroscopy in monocarboxylate transporter 8 gene deficiency. J. Clin. Endocrinol. Metab. 93, 1854–1859 (2008).
Vaurs-Barriere, C. et al. Pelizaeus-Merzbacher-Like disease presentation of MCT8 mutated male subjects. Ann. Neurol. 65, 114–118 (2009).
Azzolini, S. et al. Delayed myelination is not a constant feature of Allan-Herndon-Dudley syndrome: report of a new case and review of the literature. Brain Dev. 36, 716–720 (2014).
Friesema, E. C., Kuiper, G. G., Jansen, J., Visser, T. J. & Kester, M. H. Thyroid hormone transport by the human monocarboxylate transporter 8 and its rate-limiting role in intracellular metabolism. Mol. Endocrinol. 20, 2761–2772 (2006).
Jansen, J., Kester, M. H., Schwartz, C. E. & Visser, T. J. Genotype-phenotype relationship in patients with mutations in thyroid hormone transporter MCT8. Endocrinology 149, 2184–2190 (2008).
Visser, W. E. et al. Novel pathogenic mechanism suggested by ex vivo analysis of MCT8 (SLC16A2) mutations. Hum. Mutat. 30, 29–38 (2009).
Visser, W. E. et al. Identification, functional analysis, prevalence and treatment of monocarboxylate transporter 8 (MCT8) mutations in a cohort of adult patients with mental retardation. Clin. Endocrinol. (Oxf) 78, 310–315 (2013).
Kersseboom, S. et al. Mutations in MCT8 in patients with Allan–Herndon–Dudley-syndrome affecting its cellular distribution. Mol. Endocrinol. 27, 801–813 (2013).
Kinne, A. et al. Surface translocation and tri-iodothyronine uptake of mutant MCT8 proteins are cell type-dependent. J. Mol. Endocrinol. 43, 263–271 (2009).
Smith, V. E. et al. PTTG-binding factor (PBF) is a novel regulator of the thyroid hormone transporter MCT8. Endocrinology 153, 3526–3536 (2012).
DeLong, G. R., Stanbury, J. B. & Fierro-Benitez, R. Neurological signs in congenital iodine-deficiency disorder (endemic cretinism). Dev. Med. Child. Neurol. 27, 317–324 (1985).
Ma, T., Lian, Z. C., Qi, S. P., Heinz, E. R. & DeLong, G. R. Magnetic resonance imaging of brain and the neuromotor disorder in endemic cretinism. Ann. Neurol. 34, 91–94 (1993).
Halpern, J. P. et al. The neurology of endemic cretinism. A study of two endemias. Brain 114, 825–841 (1991).
Bernal, J. & Pekonen, F. Ontogenesis of the nuclear 3,5,3′-triiodothyronine receptor in the human fetal brain. Endocrinology 114, 677–679 (1984).
Tohyama, K., Kusuhara, H. & Sugiyama, Y. Involvement of multispecific organic anion transporter, Oatp14 (Slc21a14), in the transport of thyroxine across the blood–brain barrier. Endocrinology 145, 4384–4391 (2004).
Fujiwara, K. et al. Identification of thyroid hormone transporters in humans: different molecules are involved in a tissue-specific manner. Endocrinology 142, 2005–2012 (2001).
Acknowledgements
The authors would like to acknowledge funding support from the Spanish Ministry of Economy (Plan Nacional de I+D+I, SAF2011-25608), the Centre for Biomedical Research on Rare Diseases, Instituto de Salud Carlos III, Madrid, Spain, and under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases, and the Ramon Areces Foundation (CIVP16A1805). We also thank J. Perez for help with the initial artwork.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, discussed the content, and reviewed and edited the manuscript before submission. J.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bernal, J., Guadaño-Ferraz, A. & Morte, B. Thyroid hormone transporters—functions and clinical implications. Nat Rev Endocrinol 11, 406–417 (2015). https://doi.org/10.1038/nrendo.2015.66
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2015.66
- Springer Nature Limited
This article is cited by
-
Genetics of ischemic stroke functional outcome
Journal of Neurology (2024)
-
Neurovascular unit disruption and blood–brain barrier leakage in MCT8 deficiency
Fluids and Barriers of the CNS (2023)
-
Gene polymorphisms and thyroid hormone signaling: implication for the treatment of hypothyroidism
Endocrine (2023)
-
Defining diurnal fluctuations in mouse choroid plexus and CSF at high molecular, spatial, and temporal resolution
Nature Communications (2023)
-
Transcriptional profiling of transport mechanisms and regulatory pathways in rat choroid plexus
Fluids and Barriers of the CNS (2022)