Abstract
Chemical modifications of RNA have essential roles in a vast range of cellular processes1,2,3. N6-methyladenosine (m6A) is an abundant internal modification in messenger RNA and long non-coding RNA that can be dynamically added and removed by RNA methyltransferases (MTases) and demethylases, respectively2,3,4,5. An MTase complex comprising methyltransferase-like 3 (METTL3) and methyltransferase-like 14 (METTL14) efficiently catalyses methyl group transfer6,7. In contrast to the well-studied DNA MTase8, the exact roles of these two RNA MTases in the complex remain to be elucidated. Here we report the crystal structures of the METTL3–METTL14 heterodimer with MTase domains in the ligand-free, S-adenosyl methionine (AdoMet)-bound and S-adenosyl homocysteine (AdoHcy)-bound states, with resolutions of 1.9, 1.71 and 1.61 Å, respectively. Both METTL3 and METTL14 adopt a class I MTase fold and they interact with each other via an extensive hydrogen bonding network, generating a positively charged groove. Notably, AdoMet was observed in only the METTL3 pocket and not in METTL14. Combined with biochemical analysis, these results suggest that in the m6A MTase complex, METTL3 primarily functions as the catalytic core, while METTL14 serves as an RNA-binding platform, reminiscent of the target recognition domain of DNA N6-adenine MTase9,10. This structural information provides an important framework for the functional investigation of m6A.
Similar content being viewed by others
Main
N6-methyladenosine is a prevalent RNA modification in species including viruses, bacteria11, yeasts12, plants13 and mammals14,15. It functions in multiple aspects of developmental regulation16, the cell cycle17, fate determination18,19, and the heat shock stress response20 by affecting aspects of RNA metabolism such as pre-mRNA processing21, translation efficiency22,23, transcript stability24 and miRNA biogenesis25. Three distinct classes of protein factor are involved in the function of the m6A modification3,5: ‘writers’ (adenosine MTases)6,7,16,26, ‘erasers’ (m6A-demethylating enzymes)27,28 and ‘readers’ (m6A-binding proteins)24,29. The writers and erasers reversibly install and remove this modification, respectively, thereby generating a dynamic m6A landscape5. The readers, known as the YTH domain family24,29, bind selectively to the m6A-containing sequence and contribute to the determination of RNA fate. Although the erasers and readers have been well characterized, the lack of structures of writers remains a major obstacle to the elucidation of the versatile functions of m6A.
In humans, two MTases, METTL3 (also known as MT-A70) and METTL14 participate in this modification as ‘writers’6,7. Sequence analysis indicates that both proteins belong to the class I MTase family30 (Extended Data Fig. 1) and they form a core catalytic complex that is regulated by an additional subunit, Wilms’ tumour 1-associating protein (WTAP)7,16,26. Individually, METTL3 and METTL14 exhibit comparable weak MTase activity in vitro. However, the METTL3–METTL14 complex has much higher catalytic activity6,7. The mechanism by which the MTases functions synergistically awaits structural investigation.
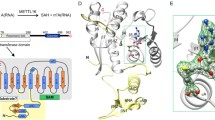
To elucidate the mechanism of m6A methylation by the METTL3–METTL14 complex, we determined the crystal structure of the core METTL3–METTL14 complex comprising the MTase domain (METTL3, residues 369–570; METTL14, residues117–402) (Fig. 1a) in the space group P41212, using bromide-based single-wavelength anomalous diffraction, at a refined resolution of 1.9 Å (Extended Data Table 1). Additionally, residues 200–204 in METTL14 could not be modelled. In the crystal, one METTL3 molecule and one METTL14 molecule form an antiparallel heterodimer in the asymmetric unit, resulting in an overall butterfly appearance with a width of approximately 40 Å and a length of approximately 70 Å (Fig. 1b).
a, Schematic domain structures of METTL3 and METTL14. METTL3 MTase (residues 369–580), METTL14 NHM (residues 116–163), MTase domain (residues 165–378), and CTM (residues 380–402) are magenta, cyan, green, and yellow, respectively. b, Overall structure of the METTL3–METTL14 heterodimer. Residue S399 (red stick) represents the phosphorylation modification. c, The METTL3 (magenta) structure in two perpendicular views. Gate loops 1 and 2 are orange, and the interface loop is blue. METTL14 has been removed for clarity. All structure figures were prepared using PyMOL.
We traced the METTL3 MTase domain and the METTL14 MTase domain, which is adjacent to the N-terminal α-helical motif (NHM) and to the C-terminal motif (CTM) with an phosphoserine at position 399 (Fig. 1b). The NHM extends across the MTase domain of METTL14 and to the MTase domain of METTL3. The MTase domain of METTL3 adopts a classic α–β–α sandwich fold comprising a mixed eight-stranded β-sheet with a strand order of β1↑, β8↑, β7↑, β2↑, β3↑, β5↓, β4↑ and β6↑ flanked by four α-helices (α1, α2 and α4 on one side, and α3 on the other side) and three 310 helices (Fig. 1c). The overall structure of the METTL3 MTase domain primarily resembles that of the class I DNA N6-adenine MTase8,10,30 (Fig. 1c and Extended Data Fig. 2a). Nevertheless, the MTase domains of METTL3 and METTL14 lack an additional element similar to the target recognition domain (TRD) of DNA N6-adenine MTase, which functions as the substrate-binding scaffold8. Consistent with the 22% sequence identity between the MTase domains of METTL3 and METTL14 (Extended Data Fig. 1), the structures of the two domains were superimposed with a root-mean-squared deviation (r.m.s.d.) of approximately 0.71 Å over 211 Cα atoms, excluding the NHM and CTM domains (Extended Data Fig. 2b). Interestingly, three loops with lower sequence similarities exhibited distinct conformations (Extended Data Figs 1, 2b), suggesting that they have different functions. We refer to these loops as gate loop 1 (residues 396–410 in METTL3), interface loop (residues 462–479 in METTL3) and gate loop 2 (residues 507–515 in METTL3; Fig. 1c).
METTL3 and METTL14 adopt the conformation of a tight asymmetric heterodimer with an interface area of approximately 2,500 Å2 and engage in extensive water-mediated hydrogen-bond interactions with each other. These interactions are mediated by interfaces 1 and 2 (Extended Data Fig. 3a). Interface 1 primarily comprises the interface loop (residues 462–479) of METTL3, NHM, and the long loop connecting β5 and β6 of METTL14 (Extended Data Fig. 3b, left and middle). Additionally, R471 of METTL3 interacts with the C-terminal phosphorylated S399 of METTL14 via a salt bridge, confirming the important regulatory role of phosphorylation. Interface 2 contains helix α2 (residues 438–447) and strand β4 (residues 450–460) of METTL3 and the corresponding helix α2, strand β4 and an interface loop (residues 266–284) from METTL14 (Extended Data Fig. 3b, right). These interfaces allow the two MTases to bind each other tightly, and the extensive interaction networks are difficult to disrupt.
After extensive trials, we determined the structure of METTL3–METTL14 in complex with AdoMet using a soaking approach and refined the structure to 1.71 Å resolution (Extended Data Table 1). In the crystal, one METTL3–METTL14 heterodimer was present in each asymmetric unit (Fig. 2a). Following assignment of most amino acids into the electron density map, electron densities indicative of one AdoMet became clearly visible in METTL3 (Fig. 2b), whereas no additional electron density was observed in METTL14 (Extended Data Fig. 4). The AdoMet molecule is positioned at the end of β1, β7 and β8 (Fig. 2a). The AdoMet-binding site faces the most conserved DPPW motif (residues 395–399) of gate loop 1 (Fig. 2a, b). This orientation suggests a nucleophilic attack methyl-transfer mechanism, reminiscent of the DNA N6-adenine MTase10.
a, Ribbon representation of METTL3–METTL14 in complex with AdoMet. The bound AdoMet is illustrated as a green ball-and-stick representation. The DPPW motif is shown as an orange stick surrounded by a dashed line. b, Close-up view of the AdoMet binding site and DPPW motif of METTL3 showing the electron density (blue) of AdoMet contoured at 1σ. c, Schematic representation of the interactions between METTL3 and AdoMet. Residues are shown with sticks. The side-chain interactions and the main-chain interactions are shown in black and blue letters, respectively. Water is shown as a red ball representation. AdoMet is shown as green sticks. Hydrogen bonds are represented as red dashed lines.
The AdoMet molecule is primarily coordinated by eleven residues of METTL3 via extensive hydrogen bonds (Fig. 2c). The adenine moiety of AdoMet is recognized by the side chain of D377 and the main chain of I378. The hydroxyl groups of ribose are surrounded by Q550, N549 and R536. Several residues, including D395, K513, H538 and N539, contact the methionine moiety of AdoMet directly, while E532 and L533 form water-mediated interactions with AdoMet (Fig. 2c). The importance of these residues in AdoMet coordination is supported by mutational analysis. D377A, D395A, N539A and E532A mutations completely abolished enzyme activity, while substitutions of R536, H538, N549 or Q550 with alanine moderately reduced enzyme activity (Extended Data Fig. 5a). Neither the D377A nor the D395A mutant had detectable AdoMet-binding activity, as measured by isothermal titration calorimetry (ITC), compared to the wild-type complex, which exhibited a dissociation constant of approximately 1.5 μM and binding stoichiometry (N) of approximately 1.15 (Extended Data Fig. 5b, c).
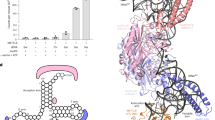
Analysis of the surface electrostatic potential of the AdoMet-bound complex revealed a positively charged groove between METTL3 and METTL14 adjacent to the AdoMet (Fig. 3a). This groove consists of at least ten positively charged residues: R465, R471, H474 and H478 from the interface loop of METTL3 and R245, R249, R254, R255, K297 and R298 from METTL14 (Fig. 3b). We speculated that this groove might be responsible for RNA binding. To test this hypothesis, we first replaced the interface loop (residues 462–479) of METTL3 with six alanine amino acids. Compared to the wild-type complex, this mutant exhibited weaker RNA binding activity and reduced MTase activity, but no effect on AdoMet binding was observed (Fig. 3c and Extended Data Fig. 6a, b). A similar result was obtained when the positively charged residues in METTL14 were mutated (Fig. 3c and Extended Data Fig. 6). These results suggest that the positively charged groove formed by METTL3 and METTL14 contributes to internal RNA binding.
a, Two views of the surface electrostatic potential calculated with PyMOL. The gate loops and the interface loop of METTL3 are highlighted by green dashed ellipses and a magenta dashed ellipse, respectively (left). The potential RNA-binding grove is encircled by a yellow dashed line (right). b, AdoMet in a space-filling representation. Residues from METTL3 and METTL14 are magenta and cyan, respectively. c, Measurement of the MTase activity of mutants of the putative RNA-binding groove of the METTL3–METTL14 complex. The indicated mutations were introduced to METTL3 (Loop to 6A) or METTL14 (R245E, R254E & R255E, K297E & R298E). The error bars represent the s.e.m. of three independent measurements.
The 1.61 Å structure of METTL3–METTL14 in complex with AdoHcy was also determined (Extended Data Table 1). In this structure, one AdoHcy molecule is positioned in the AdoMet-binding pocket of METTL3. AdoHcy adopts a configuration nearly identical to that of AdoMet, except for the ribose (Extended Data Fig. 7a, b). The overall structures of the ligand-free, AdoMet-bound and AdoHcy-bound METTL3–METTL14 complexes are nearly identical, with an r.m.s.d. of 0.24 Å over 438 Cα atoms (ligand-free with AdoMet-bound) and 0.12 Å over 454 Cα atoms (AdoHcy-bound with AdoMet-bound; Fig. 4a). The structural similarities between the ligand-free and ligand-bound METTL3–METTL14 complexes were also confirmed by small-angle X-ray scattering measurements in solution (Extended Data Fig. 7c).
a, Structural superimposition of the ligand-free (orange), AdoMet-bound (green) and AdoHcy-bound (cyan) METTL3–METTL14 complexes shows the conformational changes in gate loops 1 and 2. AdoMet is show as green balls-and-sticks. b, Close-up view of gate loops 1 and 2. The conformational change is highlighted with a blue dashed arrow. c, Proposed working model for m6A modification by the METTL3–METTL14 complex. METTL3 (magenta) primarily functions as a catalytic core, and METTL14 (cyan) serves as an RNA-binding scaffold. The substrate RNA (magenta ribbon) is cooperatively coordinated by METTL3 and METTL14. The adenine base (black) points to the AdoMet binding site in METTL3 surrounded by the two gate loops (green).
Close inspection of the structures revealed that gate loop 1 and gate loop 2, which are adjacent to the AdoMet binding site, displayed large conformational changes upon ligand binding (Fig. 4a, b). Gate loop 1 is flipped outwards in the AdoMet-bound state compared with the AdoHcy-bound state, and the ligand-free state adopts the same fold as the AdoHcy-bound state (Fig. 4b). Likewise, gate loop 2 undergoes a significant conformational rearrangement upon binding of AdoHcy or AdoMet, resulting in closure of the binding pocket (Fig. 4a, b). This rearrangement is reminiscent of the interaction of loops 1 and 2 of M.TaqI with its DNA substrate (Extended Data Fig. 8a) and suggests that gate loops 1 and 2 have important roles in adenosine recognition10.
The METTL3–METTL14 complex displayed much higher catalytic activity than either METTL3 or METTL14 alone (Extended Data Fig. 5a). This result suggests that METTL14 enhances the MTase activity of METTL3 via RNA binding, vice versa, or both. Our structures suggest that the primary function of METTL14 is not to catalyse methyl-group transfer but to offer an RNA-binding scaffold (Fig. 4c), similar to the TRD of DNA MTases8,9,10 (Extended Data Fig. 8a, b). No positively charged area was observed near the potential AdoMet-binding pocket of METTL14 (Extended Data Fig. 8c). Most importantly, although most of the residues involved in AdoMet binding are conserved between METTL3 and METTL14 (Extended Data Figs 1, 8d), mutations of two key residues in METTL14 had little effect on the AdoMet-binding and MTase activities of the binary complex (Extended Data Fig. 8e, f). Consistent with this result, AdoMet was observed in only the METTL3 pocket in the crystal structure (Fig. 2). Nevertheless, we cannot exclude the possibility that METTL14 possesses MTase activity under certain conditions.
A recent study identified a METTL3 protein interaction network comprising additional components such as METTL14, WTAP and KIAA1429 (refs 7, 16, 26). Perturbation of these factors alters global m6A levels, resulting in epitranscriptomic changes4,5. Additionally, the complex exhibited a substrate sequence preference (Extended Data Fig. 9). Further biochemical and structural characterization of the m6A writer complex containing regulatory factors, substrate RNA or both is required to completely elucidate the molecular basis of m6A modification. The structures reported here provide unprecedented mechanistic insight into m6A RNA methylation and new opportunities for the development of therapeutic agents, and serve as an important foundation for understanding m6A epitranscriptomics.
Methods
No statistical methods were used to predetermine sample size. The experiments were not randomized and the investigators were not blinded to allocation during experiments and outcome assessment.
METTL3 and METTL14 construction, expression and purification
The METTL3 and METTL14 genes were amplified from a Homo sapiens cDNA library using the following primer pairs: METTL3-M1-F (5′-ATGTCGGACACGTGGAGCT-3′) and M3ETTL3-L580-R (5′-CTATAAATTCTTAGGTTTAGAGATGATAC-3′); and METTL14-M1-F (5′-GATAGCCGCTTGCAGGAGATCCG-3′) and METTL14-R456-R (5′-TCGAGGTGGAAAGCCACCTCTG-3′), and cloned into the T-vector. Both gene strands were verified by sequencing. The full length of genes METTL3 and METTL14 was subcloned into a modified pFastBac1 vector with a His10 affinity tag fused to the N terminus. Bacmids were generated in DH10Bac cells following the instructions for the Bac-to-Bac baculovirus expression system (Invitrogen), and baculoviruses were generated and amplified in Sf-9 insect cells. For protein expression and purification, High Five (Trichoplusia ni) insect cells were grown in SIM HF medium (Sino Biological Inc.) supplemented with L-glutamine. The METTL3–METTL14 complex was co-expressed in High Five insect cells at 27 °C for 72 h using the METTL3 and METTL14 viruses. All complex mutants were co-expressed using a mutant virus and a wild-type partner virus. Cells were harvested by centrifugation at 2,000g for 15 min and homogenized in ice-cold lysis buffer containing 25 mM Tris-HCl, pH 8.0, 150 mM NaCl and 0.5 mM phenylmethanesulfonyl-fluoride (PMSF). The cells were disrupted using a cell homogenizer. The insoluble fraction was precipitated by ultracentrifugation (20,000g) for 1 h at 4 °C. The supernatant was loaded onto a Ni-NTA superflow affinity column (Qiagen) and washed three times with lysis buffer plus 10 mM imidazole. Elution was performed in buffer containing 25 mM Tris-HCl, pH 8.0, and 250 mM imidazole. The protein was further purified using anion-exchange chromatography (Source 15Q, GE Healthcare). The purified complex was concentrated to approximately 20 mg ml−1 (Amicon 30-kDa cutoff, Millipore), and if for crystallization, it was digested with chymotrypsin (0.5 mg ml−1) at room temperature for 30 min. The undigested or digested protein was subjected to size-exclusion chromatography (Superdex-200 Increase 10/300, GE Healthcare). The buffer used for size-exclusion chromatography contained 25 mM Tris-HCl, pH 8.0, 150 mM NaCl and 5 mM dithiothreitol (DTT). The peak fractions of the METTL3–METTL14 complex were pooled and immediately used for crystallization.
Crystallization
Crystallization experiments were performed using the sitting-drop vapour diffusion method at 18 °C by mixing equal volumes (1 μl) of protein (15 mg ml−1) and reservoir solution. After several rounds of optimization, good-quality crystals appeared after several days and grew as a thin diamond to full size within 15 days in drops containing 18% (v/v) PEG 8000 (Sigma) and 0.1 M sodium citrate, pH 5.7. The crystals were flash-frozen in liquid nitrogen and cryoprotected by adding ethylene glycol to a final concentration of 20%. The crystals were diffracted to 1.9 Å at the Shanghai Synchrotron Radiation Facility on beamlines BL17U and BL19U. To obtain phase information, high-quality crystals were immersed in cryoprotectant solution plus 0.3 M NaBr for 10 min. Before the crystals were harvested, 1 μl of a solution containing 25 mM Tris, pH 8.0, 150 mM NaCl, 20% (v/v) ethylene glycol and 0.1 M NaBr was added. The crystals were immediately transferred to a new solution containing 25 mM Tris, pH 8.0, 150 mM NaCl, ~18% (v/v) ethylene glycol and 0.3 M NaBr. Finally, the bromide-soaked crystals were diffracted to 2.6 Å resolution.
To obtain the AdoMet-bound and AdoHcy-bound structures, we performed extensive trials. We initially failed to obtain diffracting crystals by co-crystallization of METTL3–METTL14 with ligand. We then systematically soaked high-quality diffracting crystals of METTL3–METTL14 with AdoMet or AdoHcy (Sigma). Native crystals of METTL3–METTL14 were obtained after at least 15 days of incubation at 18 °C. The crystals were soaked with a series of ligand concentrations in the presence of 3 mM ATP as an additive. The final concentrations of ligand used were 0.5, 1, 2 and 5 mM. The crystals were soaked for 30 min to 72 h, depending on their survival in the soaking solution. The crystals were examined under a microscope every 30 min. If the crystals appeared damaged, they were transferred to a cryoprotectant solution containing 25 mM Tris pH 8.0, 150 mM NaCl and 18% (v/v) ethylene glycol. The crystals were collected and immediately flash-frozen in liquid nitrogen.
Data collection and structure determination
All diffraction data were collected at the Shanghai Synchrotron Radiation Facility (SSRF) on beamlines BL17U or BL19U using a CCD detector cooled to 100 K. The data from the METTL3–METTL14 crystals were processed with the HKL2000 program suite and XDS packages31. Further processing was performed using the programs from the CCP4 suite32. The ligand-free METTL3–METTL14 structure was solved via single anomalous diffraction (SAD) of bromide using the ShelxC/D/E program33. Then, a crude model was manually built in the Coot program34. The P41212 crystal forms of the AdoMet-bound and AdoHcy-bound complexes were solved by molecular replacement with PHASER using the structure of the ligand-free METTL3–METTL14 complex as the initial searching model35. All four crystal structures were built using Coot and refined using the Phenix program36. The data collection and structure refinement statistics are summarized in Extended Data Table 1. All figures representing structures were prepared with PyMOL37.
m6A methylation assay
A 5′-GGACUGGACUGGACUGGACU-3′ RNA probe containing four repeats of the canonical RRACH sequence was synthesized in vitro (Takara). Before reaction, the proteins were subjected to size-exclusion chromatography (Superdex-200 Increase 10/300, GE Healthcare) and the running buffer contains 15 mM HEPES pH 7.3, 150 mM NaCl, 5 mM MgCl2, and 5 mM dithiothreitol (DTT). The 50-μl reaction mixture contained 15 mM HEPES pH 7.3, 50 mM KCl, 50 mM NaCl, 1 mM MgCl2, 1 mM dithiothreitol, 4% glycerol, 0.04 μCi [methyl-3H]AdoMet (PerkinElmer), 2 nM RNA probe, and 250 ng purified protein. The solution was incubated at 30 °C for 1 h. A 5′-GGGCUGGGCUGGGCUGGGCU-3′ RNA probe without adenine was used as a negative control. This reaction was quenched with 500 μl of 1:1 (v/v) Tris-phenol (pH 8.0):chloroform, followed by the addition of 450 μl double-distilled (dd)H2O. Then, the solution was centrifuged at 20,000g for 15 min. The supernatant was removed to a new tube and precipitated using an equal volume of isopropanol and 50 μg yeast tRNA at −20 °C for 1 h. The precipitated RNA was dissolved in 100 μl ddH2O. The products were confirmed by immunoblotting using the commercial m6A antibody (Synaptic Systems, catalogue number 202 003, 1:3,000). The counts per minute (c.p.m.) of the RNA was measured in a scintillation counter (1450 MicroBeta Trilux, PerkinElmer). All experiments were repeated three times for each measurement. The average (±s.e.m.) c.p.m. was determined from three independent experiments.
Isothermal titration calorimetry (ITC) assays
ITC experiments for the binding of AdoMet to the METTL3–METTL14 complex were performed at 25 °C using Auto-iTC100 titration calorimetry (MicroCal). AdoMet (200 μM) was dissolved in reaction buffer containing 20 mM Tris-HCl, pH 8.0, and 150 mM NaCl (40 μl) and titrated against 20 μM wild-type or mutant METTL3–METTL14 complex (200 μl) in the same buffer. The first injection (0.5 μl) was followed by 19 injections of 2 μl. The heat of dilution values for AdoMet were measured by injecting AdoMet into buffer alone. The values were subtracted from the experimental curves before data analysis. The stirring rate was 750 r.p.m. The MicroCal ORIGIN software supplied with the instrument was used to determine the site-binding model that produced a good fit (low × 2 value) for the resulting data.
Electrophoretic mobility shift assay (EMSA)
The ssRNA oligonucleotide 5′-GGACUGGACUGGACUGGACU-3′ was radiolabelled at the 5′ end with [γ-32P]ATP (PerkinElmer), catalysed by T4 polynucleotide kinase (Takara). In addition, the RNA was purified by centrifugation through a 2-cm bed of G-25 size exclusion resin packed in a mini-spin column (GE Healthcare) and centrifuged at 750g for 2 min. For EMSA, proteins were incubated with approximately 10 nM 32P-labelled probe in final binding reactions containing 20 mM HEPES, pH 7.0, 5 mM MgCl2, 40 mM NaCl, 1.5 μM yeast tRNA and 10% glycerol for 20 min at 25 °C. The reactions were then resolved on 6% native acrylamide gels (37.5:1 acrylamide:bis-acrylamide) in 0.5 × Tris-glycine buffer under an electric field of 15 V cm−1 for 1 h. Gels were visualized on a phosphor screen (Amersham Biosciences) using a Typhoon Trio Imager (Amersham Biosciences).
Small-angle X-ray scattering (SAXS) measurement
Solution SAXS data were collected at the National Center for Protein Science Shanghai using the BL19U2 beamline at 18 °C. The complex proteins for SAXS measurement were prepared at 30 μM in buffer containing 25 mM HEPES, pH 7.0, 150 mM NaCl without ligand or in the presence of equimolar AdoMe or AdoHcy. For each measurement, 20 consecutive frames of 1-s exposure time were recorded and were averaged after checking there was no difference between the first and last frames of the SAXS data. Similarly, the background data were recorded using the sample buffer and were subtracted from the protein patterns.
Accession codes
References
Lee, M., Kim, B. & Kim, V. N. Emerging roles of RNA modification: m6A and U-tail. Cell 158, 980–987 (2014)
Meyer, K. D. & Jaffrey, S. R. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nature Rev. Mol. Cell Biol. 15, 313–326 (2014)
Fu, Y., Dominissini, D., Rechavi, G. & He, C. Gene expression regulation mediated through reversible m6A RNA methylation. Nature Rev. Genet. 15, 293–306 (2014)
Schwartz, S. Cracking the epitranscriptome. RNA 22, 169–174 (2016)
Liu, N. & Pan, T. N-methyladenosine-encoded epitranscriptomics. Nature Struct. Mol. Biol. 23, 98–102 (2016)
Wang, Y. et al. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nature Cell Biol. 16, 191–198 (2014)
Liu, J. et al. A METTL3–METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nature Chem. Biol. 10, 93–95 (2014)
Malone, T., Blumenthal, R. M. & Cheng, X. Structure-guided analysis reveals nine sequence motifs conserved among DNA amino-methyltransferases, and suggests a catalytic mechanism for these enzymes. J. Mol. Biol. 253, 618–632 (1995)
Gupta, Y. K., Chan, S. H., Xu, S. Y. & Aggarwal, A. K. Structural basis of asymmetric DNA methylation and ATP-triggered long-range diffusion by EcoP15I. Nat. Commun. 6, 7363 (2015)
Goedecke, K., Pignot, M., Goody, R. S., Scheidig, A. J. & Weinhold, E. Structure of the N6-adenine DNA methyltransferase M.TaqI in complex with DNA and a cofactor analog. Nature Struct. Biol. 8, 121–125 (2001)
Deng, X. et al. Widespread occurrence of N6-methyladenosine in bacterial mRNA. Nucleic Acids Res. 43, 6557–6567 (2015)
Schwartz, S. et al. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell 155, 1409–1421 (2013)
Luo, G. Z. et al. Unique features of the m6A methylome in Arabidopsis thaliana . Nat. Commun. 5, 5630 (2014)
Meyer, K. D. et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149, 1635–1646 (2012)
Dominissini, D. et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206 (2012)
Ping, X. L. et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 24, 177–189 (2014)
Fustin, J. M. et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell 155, 793–806 (2013)
Geula, S. et al. Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science 347, 1002–1006 (2015)
Chen, T. et al. m6A RNA methylation is regulated by microRNAs and promotes reprogramming to pluripotency. Cell Stem Cell 16, 289–301 (2015)
Zhou, J. et al. Dynamic m6A mRNA methylation directs translational control of heat shock response. Nature 526, 591–594 (2015)
Liu, N. et al. N6-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 518, 560–564 (2015)
Wang, X. et al. N6-methyladenosine modulates messenger RNA translation efficiency. Cell 161, 1388–1399 (2015)
Choi, J. et al. N6-methyladenosine in mRNA disrupts tRNA selection and translation-elongation dynamics. Nature Struct. Mol. Biol. 23, 110–115 (2016)
Wang, X. et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120 (2014)
Alarcón, C. R., Lee, H., Goodarzi, H., Halberg, N. & Tavazoie, S. F. N6-methyladenosine marks primary microRNAs for processing. Nature 519, 482–485 (2015)
Schwartz, S. et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5′ sites. Cell Rep. 8, 284–296 (2014)
Jia, G. et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nature Chem. Biol. 7, 885–887 (2011)
Zheng, G. et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 49, 18–29 (2013)
Xiao, W. et al. Nuclear m6A reader YTHDC1 regulates mRNA splicing. Mol. Cell 61, 507–519 (2016)
Iyer, L. M., Zhang, D. & Aravind, L. Adenine methylation in eukaryotes: Apprehending the complex evolutionary history and functional potential of an epigenetic modification. Bioessays 38, 27–40 (2016)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Collaborative Computational Project The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Schneider, T. R. & Sheldrick, G. M. Substructure solution with SHELXD. Acta Crystallogr. D 58, 1772–1779 (2002)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007)
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D 58, 1948–1954 (2002)
DeLano, W. L. The PyMOL molecular graphics system. http://www.pymol.org (2002)
Acknowledgements
We thank B. Sun (SSRF beamline BL17U), R. Zhang (BL19U1), and N. Li (BL19U2) for on-site assistance; S. Fan for data collection support; and research associates at the Center for Protein Research, Huazhong Agricultural University, for technical support. This work was supported by funds from the Ministry of Science and Technology (grants 2015CB910900 and 2013CB910200), Fok Ying-Tong Education Foundation (grant 151021), the Fundamental Research Funds for the Central Universities (Program No. 2014PY026, No. 2015PY219, and No. 2014JQ001), and Huazhong Agricultural University Scientific & Technological Self-innovation Foundation (Program No. 2013RC013).
Author information
Authors and Affiliations
Contributions
X.W., T.Z. and P.Y. designed all experiments. X.W., J.F. and Y.X. performed protein purification and crystallization. Z.Gu. determined all of the structures. X.W., Z.L., Z.Go., Q.W., D.Z., J.H., C.T., T.Z. and P.Y. performed the biochemical assays. All authors analysed the data and contributed to manuscript preparation. X.W., T.Z. and P.Y. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks M. Helm, W. Versées and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Sequence alignment of human METTL3 and METTL14.
Sequence alignment of Homo sapiens METTL3 (UniProt accession Q86U44) and METTL14 (UniProt accession Q9HCE5). The alignment was generated using the MultAlin and ENDscript programs. Secondary structural elements are shown above. Sequence identity is shown in white letters with a red background, and sequence similarity is shown in red letters. The coloured dots highlight functionally important positions. Residues of METTL3 and METTL14 that are involved in protein interactions are indicated by magenta and green dots, respectively. Cyan dots indicate residues that interact with AdoMet that were analysed by mutagenesis in this study. Blue dots represent residues that compose the RNA binding groove. The dots at the top and bottom of the sequences indicate residues from METTL3 and METTL14, respectively. Phosphoserine is highlighted by a red arrow.
Extended Data Figure 2 The MTase domains of METTL3 and METTL14 adopt the class I MTase fold.
a, Diagram of the METTL3–METTL14 secondary structure profiles. METTL3 (magenta) and METTL14 (green) are boxed with a light teal background and a wheat background, respectively. The MTase domain contains an eight-stranded β-sheet (triangles) flanked by four α-helices and three 310-helices (circles). Structural elements are numbered by their linear order in the sequence. The loops in the front are indicated by black lines, and loops in the back are indicated by black dashed lines. b, Structural comparison of METTL3 and METTL14. Two perpendicular views of superimposed METTL3 and METTL14 coloured magenta and green, respectively. The NHM and CTM of METTL14 are coloured cyan and yellow, respectively. The main differences between the MTase domains of METTL3 and METTL14 are the two gate loops (orange) and the interface loop (blue).
Extended Data Figure 3 Extensive hydrogen network between METTL3 and METTL14.
a, The main interface of the METTL3–METTL14 heterodimer comprises interface 1 (boxed with orange and green rectangles) and interface 2 (boxed with a cyan rectangle), which generate an extensive water-mediated hydrogen network. METTL3 and METTL14 are coloured wheat and silver, respectively. The interface loop of METTL3 (blue) primarily contributes to the heterodimer interaction. b, Details of interfaces 1 and 2. Water is shown as a red ball. Hydrogen bonds are represented by red dashed lines. Residues from METTL3 (magenta) and METTL14 (green) that are involved in interactions are shown as sticks.
Extended Data Figure 4 One AdoMet was located at the AdoMet binding site of METTL3.
a, Lattice packing of AdoMet-bound complex. One AdoMet (green sphere) was coordinated by METTL3 (purple) but not METTL14 (green). The arrow shows the putative AdoMet-binding pocket. b, Stereo views of electron density map of AdoMet binding site of METTL3. 2Fo − Fc electron density (grey) of AdoMet binding site in METTL3, contoured at 1.0σ. AdoMet is show as green balls-and-sticks and surrounding residues in magenta with the DPPW motif (orange). c, Representative 2Fo − Fc electron density (grey) of AdoMet binding site in METTL14, contoured at 1.0σ. The electron density of METTL14 (grey) is clearly visible and the EPPL motif is coloured orange. No additional apparent electron density was observed in the putative AdoMet binding site of METTL14.
Extended Data Figure 5 Mutagenesis analysis of the METTL3–METTL14–AdoMet interaction.
a, Characterization of METTL3–METTL14 mutations affecting MTase activity. The indicated point mutations were introduced into METTL3. Each METTL3 mutant was co-expressed and purified with wild-type METTL14 as a binary complex and used for the MTase and ITC assays. Methylation yields were calculated based on the c.p.m. of the extracted tritium-labelled RNA probe. The c.p.m. of the extracted RNA was measured in a scintillation counter. The data are shown as mean ± s.e.m. from experiments that were independently repeated at least three times. All alanine substitutions resulted in remarkable decreases in activity. b, c, Measurement of the binding affinity between AdoMet and the METTL3–METTL14 complex (wild-type and D377A for METTL3 and D395A for METTL3) using ITC. Individual peaks from titrations were integrated and presented in a Wiseman plot. The first dot was removed from the analysis. The dissociation constant (Kd) and the binding stoichiometry (N) of the wild type were approximately 1.5 μM and 1.15, respectively. The mutants exhibited undetectable AdoMet binding activity.
Extended Data Figure 6 Biochemical analysis of the role of the potential RNA binding groove.
a, RNA binding activity of the METTL3–METTL14 complex revealed by EMSA. The final concentrations of proteins in each set of five lanes (1–5, 6–10, 11–15, 16–20 and 21–25) were 0, 0.19, 0.56, 1.67 and 5 μM, respectively. ‘Well’ indicates the top of native gel. The RNA-bound complex is highlighted by a black asterisk. The wild-type complex binds to the substrate RNA probe weakly (the dissociation constant is approximately 10 μM). All of the mutants showed moderately reduced RNA binding activity. These results suggested that the positively charged groove is involved in RNA interactions. For uncropped gels, see Supplementary Fig. 1. b, Measurement of the binding affinity between AdoMet and the METTL3–METTL14 complex mutants using ITC. These mutations in METTL3 or METTL14 had little effect on AdoMet binding activity.
Extended Data Figure 7 There is little conformational change in overall structure between the AdoMe-bound and AdoHcy-bound states.
a, Electron density maps of AdoHcy showing 2Fo − Fc electron density (red) of AdoHcy adjacent to the DPPW motif (orange) contoured at 1.0σ. The DPPW motif is shown as sticks. AdoHcy is shown as cyan sticks. b, Structural comparison of AdoHcy (cyan) and AdoMet (green); the electron densities are shown as red and blue meshes, respectively. AdoHcy and AdoMet exhibited nearly identical configurations except for ribose. c, SAXS measurements reveal little structural difference among the ligand-free, AdoMe-bound and AdoHcy-bound states. Superposition of the SAXS curves of ligand-free protein complex (black), and in the presence of AdoMet (red) or AdoHcy (blue).
Extended Data Figure 8 Potential role of METTL14.
a, Structural comparison with the DNA-free (PDB: 2ADM) and DNA-bound (PDB: 1G38) states of M.TaqI. M.TaqI contains the target recognition domain (TRD, green), DNA (orange) and MTase domain (slate). The TRD functions as a scaffold for substrate DNA recognition, and the MTase domain functions as an enzyme. Adenine (magenta) is flipped out and points to the ligand-binding pocket. Black arrows highlight the loop conformational changes, which are similar to those of gate loops 1 and 2 in the METTL3–METTL14 complex. b, Ribbon representation of the DNA-bound state of EcoP15I (PDB: 4ZCF). The TRD (green) of ModA recognizes DNA, while the MTase (slate) of ModB methylates the target adenine. c, The putative AdoMet-binding site of METTL14 (green) is highlighted by a red dashed ellipse. AdoMet coordinated by METTL3 (magenta) is shown as a space-filling representation. The surface electrostatic potential around the putative AdoMet-binding site of METTL14 revealed a negative charge (black dashed ellipse) and suggests a dispensable role for this region in RNA binding. d, Most of the putative AdoMet-binding site residues were conserved between METTL3 (cyan) and METTL14 (yellow). e, Each complex containing alanine substitution mutants of residues in METTL14 (D173 and E192) that correspond to critical residues in METTL3 (D377 and D395) displayed similar methylation activity to the wild type. The average (± s.e.m.) c.p.m. was determined from three independent experiments. f, The complex mutants exhibited similar AdoMet-binding activities to the wild-type complex.
Extended Data Figure 9 Substrate sequence preference of the METTL3–METTL14 complex.
The 20-nucleotide RNA substrate contains four repeats of the consensus sequence GGACU. Each site was substituted by the other three kinds of nucleotide. The average (± s.e.m.) c.p.m. was determined from three independent experiments.
Supplementary information
Supplementary Figure
This file contains Supplementary Figure 1, the uncropped gel images for Extended Data Figure 6a. (PDF 1200 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Feng, J., Xue, Y. et al. Structural basis of N6-adenosine methylation by the METTL3–METTL14 complex. Nature 534, 575–578 (2016). https://doi.org/10.1038/nature18298
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature18298
- Springer Nature Limited
This article is cited by
-
Intrafamily heterooligomerization as an emerging mechanism of methyltransferase regulation
Epigenetics & Chromatin (2024)
-
The m6A methyltransferase METTL3 drives thyroid cancer progression and lymph node metastasis by targeting LINC00894
Cancer Cell International (2024)
-
RNA m6A methylation and regulatory proteins in pulmonary arterial hypertension
Hypertension Research (2024)
-
Epitranscriptomic modifications in mesenchymal stem cell differentiation: advances, mechanistic insights, and beyond
Cell Death & Differentiation (2024)
-
Advances in brain epitranscriptomics research and translational opportunities
Molecular Psychiatry (2024)