Abstract
Recent concern over global pollinator declines has led to considerable research on the effects of pesticides on bees1,2,3,4,5. Although pesticides are typically not encountered at lethal levels in the field, there is growing evidence indicating that exposure to field-realistic levels can have sublethal effects on bees, affecting their foraging behaviour1,6,7, homing ability8,9 and reproductive success2,5. Bees are essential for the pollination of a wide variety of crops and the majority of wild flowering plants10,11,12, but until now research on pesticide effects has been limited to direct effects on bees themselves and not on the pollination services they provide. Here we show the first evidence to our knowledge that pesticide exposure can reduce the pollination services bumblebees deliver to apples, a crop of global economic importance. Bumblebee colonies exposed to a neonicotinoid pesticide provided lower visitation rates to apple trees and collected pollen less often. Most importantly, these pesticide-exposed colonies produced apples containing fewer seeds, demonstrating a reduced delivery of pollination services. Our results also indicate that reduced pollination service delivery is not due to pesticide-induced changes in individual bee behaviour, but most likely due to effects at the colony level. These findings show that pesticide exposure can impair the ability of bees to provide pollination services, with important implications for both the sustained delivery of stable crop yields and the functioning of natural ecosystems.
Similar content being viewed by others
Main
Biotic pollination is required by a large proportion of crops worldwide10, disproportionately including those with economically high values and nutritional content13. The contribution of pollination services to global agriculture has been steadily increasing and was estimated at US$361 billion in 2009 (ref. 14). In addition, animal-vectored pollination is required by an estimated 87.5% of all angiosperms to reproduce11, making this process fundamental to the functioning of natural ecosystems. Therefore, any threats to the delivery of pollination services could have serious consequences for both food security and wider ecosystem function. Neonicotinoid pesticides, the most widely used group of insecticides worldwide15, are implicated as one of the contributing factors in the global declines of bee pollinators3,16. Although previous work has shown that bumblebee foraging activity, colony growth and reproduction can be altered by sublethal exposure to neonicotinoid pesticides1,2,5,6,7, all research on pesticide effects has focused on bees as the service providers, but has not assessed the pollination service itself. Therefore it is unknown whether pesticide exposure actually results in changes to the delivery of pollination services to crops and wild plants (for a discussion of potential mechanisms see ref. 17). This information is essential to assess the severity of pesticide effects on ecosystem services, and to inform actions to mitigate negative effects.
Apples are an important global crop, with 75 million tonnes harvested from 95 countries in 2012 and an estimated export value of US$71 billion (Food and Agriculture Organisation statistics, http://faostat3.fao.org). Apple crops benefit from insect pollination with seed number, fruit set, fruit size and shape all improved with increased pollination services18. Bumblebees are major pollinators of apples19 and many other crops across the world12, and are exposed to low levels of pesticides when foraging in agricultural areas. Here we investigated how exposure to low, field-realistic levels of a widely used neonicotinoid insecticide (thiamethoxam) could affect the ability of bumblebees to pollinate apple trees. We pre-exposed colonies to 2.4 parts per billion (ppb) thiamethoxam, 10 ppb thiamethoxam or control solutions (containing no pesticide; rationale for selecting pesticide concentrations and relevance of results are outlined in Methods and Supplementary Information) in their nectar source (artificial sugar water) for a period of 13 days (8 colonies per treatment, that is, 24 colonies in total). Subsequently, colonies were brought to the field and allowed access to virgin apple trees of a dessert (Scrumptious) variety, along with trees of a polliniser (Everest) variety, in pollinator exclusion cages in which we observed both individual- and colony-level behaviour. At the end of the season, apples from tested trees were collected to assess pollination service delivery in terms of fruit and seed set.
When whole colonies were given access to apple trees we found an effect of insecticide treatment on visitation rates to apple flowers (F2,86 = 3.1, P = 0.05); colonies exposed to 10 ppb pesticide provided lower visitation rates to apple flowers than controls (Fig. 1a; Extended Data Table 1). We also found an effect of treatment on the number of foraging trips from which bees returned carrying pollen (χ2 = 9.65, degrees of freedom (df) = 2, P = 0.008), with fewer bees from colonies exposed to 10 ppb pesticide returning with pollen than workers from control colonies (Fig. 1b). Apple abortion rate was affected by treatment (χ2 = 5.94, df = 2, P = 0.05), with trees pollinated by 2.4 ppb pesticide-exposed colonies aborting more fruit than controls (Fig. 2a), although overall levels of fruit set did not differ (χ2 = 4.1, df = 2, P = 0.13) and there was no difference in the proportion of trees that produced fruit among treatments (χ2 = 1.2, df = 2, P = 0.55). However, we found a significant effect of treatment on the number of seeds produced per apple, an indicator of fruit quality, (χ2 = 8.27, df = 2, P = 0.02); flowers pollinated by colonies exposed to 10 ppb pesticide produced significantly fewer seeds than those pollinated by 2.4 ppb colonies (Fig. 2b). These results show that colonies exposed to pesticide can deliver reduced pollination services to apple crops.
a, b, Visitation rates provided by colonies to Scrumptious apple flowers (number of visits per flower per minute) (a) and number of foraging trips from which bees returned carrying pollen (b), from colonies exposed to different pesticide treatments. Eight colonies were observed per treatment group, and means ± s.e.m. are shown, *P < 0.05. NS, not significant. Results from statistical models are given in Extended Data Table 1.
a, b, The change in proportion of fruit set for trees (48 trees in total, 16 per treatment) pollinated by colonies exposed to different pesticide treatments measured early (May) and late (September), which represents fruit abortion level (a), and number of seeds produced per apple (134 apples in total; 53 in control, 46 in 2.4 ppb and 35 in 10 ppb pesticide treatments) pollinated by colonies exposed to different pesticide treatments (b). Eight colonies were observed per treatment group, and means ± s.e.m. are shown, *P < 0.05, † indicates a difference of P = 0.06 between control and 10 ppb. NS, not significant. Results from statistical models are given in Extended Data Table 1.
These colony-level effects could be explained by several mechanisms, including individual behavioural changes. Individual bees exposed to 10 ppb pesticide spent longer foraging (F2,57 = 3.72, P = 0.03; Fig. 3a), visited more Scrumptious flowers (χ2 = 12.79, df = 2, P = 0.002) and switched more frequently between varieties during each trip (χ2 = 11.32, df = 2, P = 0.003: Fig. 3b; Extended Data Table 2), which suggests a modification of their floral preferences7. Neonicotinoids target neurotransmitter receptors in insects and, as well as causing neuronal inactivation20, some have been shown to be partial neuronal agonists21; therefore increases in individual foraging activity may be explained by acute increases in neuronal activity causing hormesis (a biphasic response in which low levels of an otherwise toxic compound can result in stimulation of a biological process22). However, we found no effect of treatment on whether flowers visited by these individual bees produced apples (χ2 = 0.88, df = 2, P = 0.64), showed higher rates of fruit abortion (χ2 = 0.42, df = 2, P = 0.81) or different levels of seed set (χ2 = 0.11, df = 2, P = 0.95). This suggests that bees exposed to pesticide must somehow be behaving differently on flowers, in a way that was not readily observable in our experiment (for example, changes in stigmatic contact23), such that increased visit frequency did not result in better pollination service delivery at the individual level.
a, b, Time spent foraging per foraging trip (seconds; n = 68 bees) (a) and number of switches between Scrumptious and Everest apple varieties (n = 93 bees) (b) for individual bees exposed to different pesticide treatments. Means ± s.e.m. are shown, *P < 0.05, † indicates a difference of P = 0.06 between control and 2.4 ppb. NS, not significant. Results from statistical models are given in Extended Data Table 2.
Our results suggest that effects on pollination service delivery are not due to individual behavioural modification, but instead are most likely due to changes in colony activity levels as evidenced by reduced floral visitation rates and pollen collection. Bees collecting pollen may be more effective pollinators as they can deposit more pollen on plant stigmas24; therefore if pesticide-exposed colonies are collecting less pollen they are also likely to be depositing less on stigmas than bees from control colonies. While individual bees exposed to pesticides visited more flowers, overall pesticide-exposed colonies provided lower visitation rates and collected less pollen, thus explaining why reduced pollination services were delivered. Gill & Raine7 found that control (untreated) bees improved their pollen foraging performance over time, whereas imidacloprid-treated bees became less successful foragers; foragers in our colony-level experiment may have carried out multiple trips and become more experienced foragers, potentially explaining why we find effects on pollen collection here but not in the individual-level experiment. Interestingly, for almost all parameters measured in this study we found significant effects on both individual behaviour and colony-level function following 10 ppb thiamethoxam exposure, but not at the 2.4 ppb level. This suggests that there are dose-dependent effects that lie between these two exposure levels. Both these exposure levels are highly relevant as they are within the range measured in the field, but further work is necessary to elucidate the lowest level at which these effects become significant (for further discussion of rationale for exposure and relevance of results, see Methods and Supplementary Information).
A 36% reduction in the number of seeds produced in apples pollinated by colonies exposed to 10 ppb pesticide in comparison to control colonies has important agronomic implications for crop production. The number of seeds in apples is closely linked to fruit crop quality in most, but not all, varieties18,25 and the enhancement of fruit quality, particularly the proportion of Class 1 fruit, underpins the economic value of UK orchards26: growers must typically thin out their apple crops making the quality of each fruit very important. Therefore impacts on seed set and fruit quality have direct implications for apple production value, and as seed set and fruit set are positively linked in many varieties, reduced seed set can have direct negative implications for fruit set and total crop yield26,27. As certain apple varieties in the UK currently experience pollination deficits19,26, mitigating the effects of pesticides on bumblebee pollinators could improve pollination service delivery. Apple crops are visited by a wide variety of pollinator groups, and neonicotinoid pesticides differentially affect insect taxa4,28. Apart from bumblebees, one of the other main pollinator groups that visit apple flowers are solitary bees19, and it has been suggested that pesticide sensitivity of solitary bees is likely to be higher than for larger, social species like bumblebees4,5,17,29. Therefore, apple pollination in a field setting could be more vulnerable to pesticide exposure than measured here.
Bumblebees are essential pollinators of many important crops other than apples, including field beans, berries, tomatoes and oilseed rape12,26. If exposure to pesticides alters pollination services to apple crops, it is likely that these other bee-pollinated crops would also be affected. Most importantly, the majority of wild plant species benefit from insect pollination services11. Therefore reduced pollination by pesticide-affected colonies, as evidenced by reduced seed set, also has significant implications for pollination in wild systems. Many wild plant species are both self-incompatible and pollen limited30, so any reduction in the delivery of pollination services could have substantial effects on wild plant communities and therefore wider ecosystem function.
Concerns over global bee declines are strongly driven by the need for the essential pollination services they provide to both crops and wild plants. The use of neonicotinoid pesticides presents a potential threat to bee health and, although the evidence base reporting sublethal (behavioural) effects of pesticides on bees is mounting3, we have shown for the first time that there is also an important effect of pesticide exposure on the pollination services bees provide. This information provides a new perspective when trying to fully understand the trade-offs involved when using insecticides, showing that both the potential benefits and the true costs of pest control options need to be considered.
Methods
Pesticide preparation
A stock pesticide solution was made by dissolving 100 mg thiamethoxam (PESTANAL, Analytical Standard, Sigma Aldrich) in 100 ml acetone (1 mg ml−1). Aliquots of stock solution were added to 40% sucrose to create treatment solutions of 10 μg l−1 (10 ppb) and 2.4 μg l−1 (2.4 ppb) thiamethoxam. These concentrations were chosen as field-realistic; the lower concentration (2.4 ppb) was based on thiamethoxam concentrations found in nectar pots of bumblebee colonies foraging in agricultural areas in the UK31 and in pollen collected by honeybees32, and the higher concentration (10 ppb) is within the range measured in pollen and nectar and of a variety of treated crops33,34,35 and contaminated wild flowers35,36,37, and has been used in previous studies examining effects of another neonicotinoid (imidacloprid) on bumblebee behaviour1,7. A control solution was also made by repeating the process outlined above but using an aliquot of 10 ppb acetone only (that is, no pesticide).
Experimental setup
Twenty-four commercially reared Bombus terrestris audax colonies were obtained from Biobest (Westerlo, Belgium) at the start of April 2014, each containing a queen and an average of 99 workers (range 57–133). Colonies were weighed on arrival to estimate the overall colony size, and each assigned sequentially to one of three treatment groups (2.4 ppb thiamethoxam, 10 ppb thiamethoxam and control) based on decreasing mass (but randomly assigned within block). Each day, three colonies (one from each treatment) were assigned to treatment groups, until after 7 days all colonies were receiving treated sucrose (16 colonies exposed to thiamethoxam and 8 to control solution). We chose this sequential exposure regime to mimic subsequent field testing and ensure all colonies had comparable durations of exposure to their treatment. Colonies were fed treated sucrose solution from a gravity feeder inserted at the base of the nest box. Feeders were initially refilled every 2–3 days, and then every 1–2 days when the colonies had grown significantly. Untreated, defrosted honeybee-collected pollen was provided to colonies every 2–3 days. Colonies were exposed to treatments for an average of 13 days (range 12–15) before field testing. Before being moved to the field, colonies had access to a feeder containing sucrose (40%) in a laboratory flight arena for 48 h to become accustomed to leaving the nest to forage. There was no difference in colony weights at the start (ANOVA: F2,21 = 0.091, P = 0.91) or end (ANOVA: F2,21 = 0.88, P = 0.43) of the experimental period, indicating no treatment effect on colony size.
Field testing
Cage experiments were carried out at Sonning Farm, University of Reading, UK. 100 apple trees of a commercial dessert apple (Scrumptious variety) were moved into holding pollinator exclusion cages in mid-March 2014 before flowering to prevent insect visitation. Field experiments began when trees were entering full flower in mid-April. Each day, one colony from each treatment was taken from the laboratory, placed individually in one of the three test cages and observed simultaneously (with one observer per cage) in a randomized block design (see below for details of observations). Each day a different treatment was assigned to each observer. Cages were 4.8 × 2.1 × 2.1 m frames covered in polyethylene mesh (gauge size = 1.33 mm, Extended Data Fig. 1). Observations were carried out on 8 dry, bright days from 16–26 April 2014 spanning the peak flowering of apples (daily means: maximum temperature 16 °C, rainfall 2.5 mm). This flowering period limited the number of days on which testing could be carried out, and therefore the number of colonies that could be tested; as a result no statistical methods were used to predetermine sample size.
The investigators were not blinded to allocation during experiments and outcome assessment.
Individual-level measurements
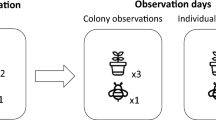
Each morning, three cages were populated with two virgin Scrumptious trees each from the holding cages (mean ± s.e.m. = 130 ± 8.5 flowers per tree) as well as two polliniser trees (Everest variety, mean ± s.e.m. = 305 ± 15 flowers per tree, Extended Data Fig. 1). The number of flowers of each variety was standardized across cages to ensure equal floral density each day, and 40 open and receptive flowers were marked with cable ties on each Scrumptious tree for subsequent estimation of pollination services (fewer flowers were marked on the last day of observations as there were no longer 40 full-bloom flowers—flower numbers on these days were noted). The nest boxes in each cage were then opened to allow a single worker to exit. This bee was observed for the duration of its foraging trip (until it attempted to return to the nest), or until 60 min had elapsed (Extended Data Fig. 2). The duration of the foraging trip, the number of flowers of each apple variety visited, and the handling time for each flower visit was recorded using Etholog software (EthoLog: Behavioural observation transcription tool, University of Sao Paulo, Brazil, 2011). If the individual bee did not visit any flowers within the first 20 min, it was assumed not to be a forager and was captured, returned to the colony and another bee released. All bees that foraged were paint-marked before they were returned to the colony to ensure the same individuals were not observed twice. This process was repeated until all cages had the same number of active foragers recorded (3–5 bees per colony each day). Individual level observations took place between 10:00 and 16:30.
Colony-level measurements
After individual-level observations, the two focal Scrumptious trees in each cage were removed and replaced with two new virgin trees. Again we standardized the number of flowers of each variety across cages with 40 open and receptive flowers on each tree marked with cable ties. Colony boxes were opened to allow free entry and exit to all active bees for a period of 60 min. This time period was chosen to avoid over-pollination of test flowers based on pilot observations. Colony activity was monitored at the nest entrance using video cameras. After an initial 10-min period to allow the bees to become accustomed to the setup, four 10-min focal observations were carried out on separate patches of Scrumptious flowers in each cage to estimate visitation rates. At the end of the 60-min period, the Scrumptious trees were immediately removed to prevent further visitation. Colony level observations were carried out between 14:30 and 18:30.
Estimation of pollination services
At the end of both the individual and colony observation periods, all test trees were returned to holding cages in which they were not visited by any other insects until apples were harvested at the end of the season. An initial assessment of fruit set from marked flowers (indicating flowers open during cage tests) was made at the end of May for all test Scrumptious trees to assess how many flowers were proceeding to fruit set stage (and how many aborted, Fig. 2a). Marked apples were collected on 27 August, and a final assessment made of the proportion of marked flowers that had produced mature fruit (Extended Data Fig. 2). In the lab, seed number was counted per apple for all collected fruit (274 apples from 96 trees across both experiments). Details of all data analyses carried out are given in the Supplementary Information.
Data analysis
Individual level. Measures of the number of flowers visited, numbers of switches between apple varieties, duration of total time in cage (from when the bee left the colony box until it returned/end of 60 min period) and time taken to visit the first flower (latency) were recorded for all individual bees. For 68 of 93 bees observed (evenly distributed across cages and treatments) a number of additional response variables were also recorded including mean duration of the first 5 flower visits, number of inter flower intervals longer than 60 s, mean duration of flower visits, mean period of time between flower visits, length of time spent foraging (time between first and last flower visit) and total time spent on flowers (sum of durations for all individual flower visits). We tested for differences in these measures among treatments by constructing mixed-effects models with pesticide treatment as a fixed effect. As several variables differed among days, including weather, floral abundance and the identity of colonies used, day of testing was included as a random blocking factor in all models. Data were analysed in R version 3.1.0 (ref. 38), using either linear mixed effects (LME) models with the lmer function in the nlme package for continuous data39, generalized mixed effects (GLMM) models with Poisson distribution used for response variables that were counts using the glmer function in the lme4 package40, or the glmmPQL function in the MASS package41 when data were overdispersed. Models were validated by plotting standardized residuals versus fitted values, normal qq-plots and histograms of residuals, and continuous response variables were logarithmically transformed (log (X + 1)) if necessary to improve residual fit. If treatment was significant, Tukey’s post hoc tests were performed using the glht function in the multcomp package42.
To assess differences in apple production on trees visited by pesticide exposed and control bees, we examined a number of variables including the number of fruits produced at the start of the season (May) and at the end (September; Fig. 2a), the change in proportion of apples forming from marked flowers per tree between the start and end measures (fruit abortion levels) and number of seeds per apple (measured in early September; Fig. 2b). Models were run as described previously with treatment as a fixed effect, although the tree on which fruits were produced, the number of bees released and date of testing were included as random effects. As a number of trees produced no fruit, seed set data were analysed in two steps. First, we tested whether there was a treatment difference in the number of trees that produced any fruit. Second, we tested for treatment differences in seeds per apple (a measure that only included trees that had produced some fruit).
Colony level. We tested for differences in colony activity levels (the combined number of entries and exits by workers to the colony box) and the number of bees carrying pollen among treatments using GLMM models in the MASS package41, with Poisson distribution for count data. Treatment differences in flower visitation rate to Scrumptious trees were tested using LME models39. Date of testing was used as a random effect in all models (and patch included as a random effect in the flower visitation rate model), and models were validated as described above. Fruit abortion and seed set variables were analysed as described for the individual level experiment, using tree and date of testing as random effects.
References
Gill, R. J., Ramos-Rodriguez, O. & Raine, N. E. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 491, 105–108 (2012)
Whitehorn, P. R., O’Connor, S., Wäckers, F. L. & Goulson, D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336, 351–352 (2012)
Godfray, H. C. J. et al. A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. R. Soc. Lond. B 281, 20140558 (2014)
Arena, M. & Sgolastra, F. A meta-analysis comparing the sensitivity of bees to pesticides. Ecotoxicology 23, 324–334 (2014)
Rundlöf, M. et al. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521, 77–80 (2015)
Feltham, H., Park, K. & Goulson, D. Field realistic doses of pesticide imidacloprid reduce bumblebee pollen foraging efficiency. Ecotoxicology 23, 317–323 (2014)
Gill, R. J. & Raine, N. E. Chronic impairment of bumblebee natural foraging behaviour induced by sublethal pesticide exposure. Funct. Ecol. 28, 1459–1471 (2014)
Henry, M. et al. A common pesticide decreases foraging success and survival in honey bees. Science 336, 348–350 (2012)
Fischer, J. et al. Neonicotinoids interfere with specific components of navigation in honeybees. PLoS One 9, e91364 (2014)
Klein, A. M. et al. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. Lond. B 274, 303–313 (2007)
Ollerton, J., Winfree, R. & Tarrant, S. How many flowering plants are pollinated by animals? Oikos 120, 321–326 (2011)
Kleijn, D. et al. Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat. Commun. 6, 7414 (2015)
Eilers, E. J., Kremen, C., Greenleaf, S. S., Garber, A. K. & Klein, A. M. Contribution of pollinator-mediated crops to nutrients in the human food supply. PLoS One 6, e21363 (2011)
Lautenbach, S., Seppelt, R., Liebscher, J. & Dormann, C. F. Spatial and temporal trends of global pollination benefit. PLoS One 7, e35954 (2012)
Goulson, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 50, 977–987 (2013)
Goulson, D., Nicholls, E., Botías, C. & Rotheray, E. L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957 (2015)
Brittain, C. & Potts, S. G. The potential impacts of insecticides on the life-history traits of bees and the consequences for pollination. Basic Appl. Ecol. 12, 321–331 (2011)
Sheffield, C. S. Pollination, seed set and fruit quality in apple: studies with Osmia lignaria (Hymenoptera: Megachilidae) in the Annapolis Valley, Nova Scotia, Canada. J. Pollinat. Ecol . 12, 120–128 (2014)
Garratt, M. P. D. et al. Pollination deficits in UK apple orchards. J. Pollinat. Ecol. 12, 9–14 (2014)
Palmer, M. J. et al. Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nat. Commun. 4, 1634 (2013)
Déglise, P., Grünewald, B. & Gauthier, M. The insecticide imidacloprid is a partial agonist of the nicotinic receptor of honeybee Kenyon cells. Neurosci. Lett. 321, 13–16 (2002)
Cutler, G. C. & Rix, R. R. Can poisons stimulate bees? Appreciating the potential of hormesis in bee-pesticide research. Pest Manag. Sci. 71, 1368–1370 (2015)
Sakamoto, R. L., Morinaga, S.-I., Ito, M. & Kawakubo, N. Fine-scale flower-visiting behavior revealed by using a high-speed camera. Behav. Ecol. Sociobiol. 66, 669–674 (2012)
Castro, S., Loureiro, J., Ferrero, V., Silveira, P. & Navarro, L. So many visitors and so few pollinators: variation in insect frequency and effectiveness governs the reproductive success of an endemic milkwort. Plant Ecol. 214, 1233–1245 (2013)
Buccheri, M. & Di Vaio, C. Relationship among seed number, quality, and calcium content in apple fruits. J. Plant Nutr. 27, 1735–1746 (2004)
Garratt, M. P. D. et al. Avoiding a bad apple: Insect pollination enhances fruit quality and economic value. Agric. Ecosyst. Environ. 184, 34–40 (2014)
Volz, R. K., Tustin, D. S. & Ferguson, I. B. Pollination effects on fruit mineral composition, seeds and cropping characteristics of ‘Braeburn’ apple trees. Sci. Hortic. (Amsterdam) 66, 169–180 (1996)
Biddinger, D. J. et al. Comparative toxicities and synergism of apple orchard pesticides to Apis mellifera (L.) and Osmia cornifrons (Radoszkowski). PLoS One 8, e72587 (2013)
Decourtye, A., Henry, M. & Desneux, N. Environment: overhaul pesticide testing on bees. Nature 497, 188 (2013)
Burd, M. Bateman’s principle and plant reproduction: the role of pollen limitation in fruit and seed set. Bot. Rev. 60, 83–139 (1994)
Thompson, H. et al. Effects of neonicotinoid seed treatments on bumble bee colonies under field conditions . (Food and Environment Research Agency (FERA), 2013)
Pilling, E., Campbell, P., Coulson, M., Ruddle, N. & Tornier, I. A four-year field program investigating long-term effects of repeated exposure of honey bee colonies to flowering crops treated with thiamethoxam. PLoS One 8, e77193 (2013)
Castle, S. J., Byrne, F. J., Bi, J. L. & Toscano, N. C. Spatial and temporal distribution of imidacloprid and thiamethoxam in citrus and impact on Homalodisca coagulata populations. Pest Manag. Sci. 61, 75–84 (2005)
Dively, G. P. & Kamel, A. Insecticide residues in pollen and nectar of a cucurbit crop and their potential exposure to pollinators. J. Agric. Food Chem. 60, 4449–4456 (2012)
Botías, C. et al. Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ. Sci. Technol. 9, 12731–12740 (2015)
Krupke, C. H., Hunt, G. J., Eitzer, B. D., Andino, G. & Given, K. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS One 7, e29268 (2012)
Stewart, S. D. et al. Potential exposure of pollinators to neonicotinoid insecticides from the use of insecticide seed treatments in the mid-southern United States. Environ. Sci. Technol. 48, 9762–9769 (2014)
R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org (2011)
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & R Development Core Team. Package “nlme”: Linear and nonlinear mixed effects models. R package version 3.1-104 (2012)
Bates, D., Maechler, M., Bolker, B. & Walker, S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7 http://CRAN.R-project.org/package=lme4 (2014)
Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S. 4th edn (Springer, 2002)
Hothorn, T., Bretz, F. & Westfall, P. Simultaneous inference in general parametric models. Biom. J. 50, 346–363 (2008)
Acknowledgements
We thank technicians at the University of Reading for assistance in apple collection and seed counting, and E. van Leeuwen and colleagues at Royal Holloway University of London for useful discussions. This study was supported by UK Insect Pollinators Initiative grants BB/I000178/1 awarded to N.E.R. and BB/1000348/1 awarded to S.G.P. (funded jointly by the Living with Environmental Change programme, Biotechnology and Biological Sciences Research Council (BBSRC), Wellcome Trust, Scottish Government, Department for Environment, Food and Rural Affairs (Defra) and Natural Environment Research Council (NERC)). N.E.R. is supported as the Rebanks Family Chair in Pollinator Conservation by The W. Garfield Weston Foundation.
Author information
Authors and Affiliations
Contributions
D.A.S. and N.E.R. conceived the project, D.A.S., N.E.R. and M.P.D.G. designed the research, D.A.S., J.B.W and V.J.W. carried out the research, D.A.S., N.E.R., M.P.D.G. and S.G.P. contributed equipment for the research, D.A.S. analysed the data, all authors were involved in writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 An example of the experimental setup at the Sonning Farm field site.
Experimental pollinator exclusion cages containing a bumblebee colony (located in the corner of the cage) and potted experimental apple trees are shown. Photos: D.A.S.
Extended Data Figure 2 An experimental bumblebee (Bombus terrestris) worker visiting an apple flower (left), and an example of an apple produced from a marked (yellow cable tie) apple flower (right; Scrumptious variety).
Photos: D.A.S. and C. L. Truslove.
Supplementary information
Supplementary Information
This file contains Supplementary text and references. (PDF 127 kb)
Rights and permissions
About this article
Cite this article
Stanley, D., Garratt, M., Wickens, J. et al. Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature 528, 548–550 (2015). https://doi.org/10.1038/nature16167
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature16167
- Springer Nature Limited
This article is cited by
-
The impact of chronic exposure to field-level thiamethoxam on sunflower visitation and yield for Apis cerana
Apidologie (2024)
-
Pesticide use negatively affects bumble bees across European landscapes
Nature (2024)
-
Low concentrations of acetamiprid, deltamethrin, and sulfoxaflor, three commonly used insecticides, adversely affect ant queen survival and egg laying
Scientific Reports (2023)
-
Floral resources,energetic value and pesticide residues in provisions collected by Osmia bicornis along a gradient of oilseed rape coverage
Scientific Reports (2023)
-
Ecological traits interact with landscape context to determine bees’ pesticide risk
Nature Ecology & Evolution (2023)