Abstract
Prenatal exposure to infectious or inflammatory insults is increasingly recognized to contribute to the etiology of psychiatric disorders with neurodevelopmental components, including schizophrenia, autism and bipolar disorder. It remains unknown, however, if such immune-mediated brain anomalies can be transmitted to subsequent generations. Using an established mouse model of prenatal immune activation by the viral mimetic poly(I:C), we show that reduced sociability and increased cued fear expression are similarly present in the first- and second-generation offspring of immune-challenged ancestors. We further demonstrate that sensorimotor gating impairments are confined to the direct descendants of infected mothers, whereas increased behavioral despair emerges as a novel phenotype in the second generation. These transgenerational effects are mediated via the paternal lineage and are stable until the third generation, demonstrating transgenerational non-genetic inheritance of pathological traits following in-utero immune activation. Next-generation sequencing further demonstrated unique and overlapping genome-wide transcriptional changes in first- and second-generation offspring of immune-challenged ancestors. These transcriptional effects mirror the transgenerational effects on behavior, showing that prenatal immune activation leads to a transgenerational transmission (presence of similar phenotypes across generations) and modification (presence of distinct phenotypes across generations) of pathological traits. Together, our study demonstrates for, we believe, the first time that prenatal immune activation can negatively affect brain and behavioral functions in multiple generations. These findings thus highlight a novel pathological aspect of this early-life adversity in shaping disease risk across generations.
Similar content being viewed by others
Introduction
Maternal exposure to infectious or inflammatory insults during pregnancy increases the offspring’s risk to develop neuropsychiatric disorders, including schizophrenia,1, 2 autism3, 4 and bipolar disorder.5, 6 This epidemiological association is further supported by preclinical animal models demonstrating abnormal brain development and behavioral dysfunctions following prenatal administration of infectious pathogens or immune activating agents.7, 8, 9, 10, 11 The current consensus is that cytokine-associated inflammatory events, together with downstream pathophysiological effects such as oxidative stress and (temporary) macronutrient and micronutrient deficiency, are critical in mediating the adverse effects of maternal infection on the fetal system.10, 11, 12 These post-acute pathological processes disrupt normal development of the central nervous system,13, 14 change subsequent maturation of central nervous system micro- and macro-structures,15, 16 and cumulate into long-term behavioral and cognitive disturbances.7, 8, 9, 10, 11, 12
In contrast to the widely described disturbances manifest in the direct descendants of gestationally infected mothers, it remains unknown if infection-induced brain anomalies can be transmitted to subsequent generations without any further exposure. The phenomenon of non-genetic transgenerational transmission of behavioral traits has gained increasing recognition in view of its potential importance in the etiology and treatment of multi-factorial disorders.17, 18, 19, 20, 21 Transgenerational transmission of disease susceptibility has been observed following early-life exposure to various environmental adversities, including prenatal or neonatal stress,22, 23, 24, 25, 26 prenatal malnutrition,27, 28, 29 and chronic psychostimulant or alcohol intake.30, 31 It likely involves epigenetic mechanisms, that is, changes in genome activity and expression without altering the DNA sequence.32, 33
To the best of our knowledge, the present study is the first to examine transgenerational effects of prenatal immune activation using a well-established model of maternal viral-like immune activation in mice. The model is based on maternal administration of the viral mimetic poly (I:C;=polyriboinosinic-polyribocytidilic acid), which induces a cytokine-associated viral-like acute phase response in maternal and fetal compartments, including the fetal brain.34, 35 Prenatal poly(I:C) treatment in rodents leads to multiple behavioral and cognitive disturbances in the direct descendants, many of which associated with developmental psychiatric disorders such as schizophrenia and autism.9, 10, 34 The prenatal poly(I:C) exposure model thus offers a unique opportunity to identify possible transgenerational effects following prenatal exposure to an etiologically relevant risk factor. Using this model, we compared behavioral and transcriptomic changes in consecutive generations derived from immune-challenged or control ancestors.
Materials and methods
Animals
C57Bl6/N mice were used throughout the study. Female and male mice were originally obtained from Charles River Laboratories (Sulzfeld, Germany) and kept in our in-house specific-pathogen-free (SPF) facility until breeding began to generate poly(I:C) and control offspring (see below). All animal breeding and holding rooms were temperature- and humidity-controlled (21±1 °C, 55±5%) and kept under a reversed light–dark cycle (lights off: 0700–1900 hours). All animals had ad libitum access to food (Kliba 3436, Kaiseraugst, Switzerland) and water throughout the entire study. All procedures described in the present study had been previously approved by the Cantonal Veterinarian’s Office of Zurich, and all efforts were made to minimize the number of animals used and their suffering.
Maternal immune activation in F0 mothers
To generate the first-generation (F1) offspring of polyI:C-exposed or control mothers, female mice were subjected to a timed-mating procedure as fully described in the Supplementary Information. Pregnant F0 dams on gestation day (GD) 9 were randomly assigned to receiving either a single injection of poly(I:C) (5 mg/kg; potassium salt; Sigma–Aldrich, Buchs, St. Gallen, Switzerland) or vehicle (sterile pyrogen-free 0.9% NaCl) as described in the Supplementary Information. For each experimental series involving F0 exposures, a total of 16 pregnant dams were used, half of which were allocated to the poly(I:C) treatment, and the other half to the vehicle treatment. The selected gestational window (that is, GD 9) in mice corresponds roughly to the middle of the first trimester of human pregnancy with respect to developmental biology and percentage of gestation from mice to humans.34 It was selected based on previous findings showing that poly(I:C) exposure on GD 9 leads to multiple behavioral abnormalities in the adult offspring.34, 35, 36, 37, 38
Allocation of F1 offspring and production of subsequent generations
All F1 offspring were weaned and sexed on postnatal day 21. Littermates of the same sex were caged separately and maintained in groups of 3 to 5 animals per cage. On reaching early adulthood (postnatal day 70 onwards), F1 offspring were either allocated to behavioral testing (see below) or breeding, the later of which served to produce subsequent generations of immune-challenged or control ancestors. Hence, we always used behaviorally naïve littermates as breeding pairs to obtain the F2 and F3 generations, thereby avoiding possible confounds in breeding mice arising from prior behavioral testing. Timed-mating procedures were used to generate F2 and F3 offspring as fully described the Supplementary Information.
In a first series of experiments (Figure 1a), F1 males born to poly(I:C)-exposed mothers were mated with F1 females born to poly(I:C)-exposed mothers (N=6 litters); and F1 males born to control mothers were mated with F1 females born to control mothers (N=8 litters). In a second series of experiments (Figure 2a), we dissected the maternal (ML) and paternal (PL) lineages of F1 poly(I:C) offspring for the subsequent generation of F2 offspring. To obtain F2 poly(I:C) offspring via the ML, we crossed female F1 poly(I:C) offspring with male F1 control offspring (N=6 litters); and to generate F2 poly(I:C) offspring via the PL, we mated male F1 poly(I:C) offspring with female F1 control offspring (N=7 litters). F1 control males and F1 control females were crossed to obtain the F2 control lineage. A third series of experiments was performed to generate F3 offspring with poly(I:C)-exposed or control ancestors, thereby focusing on the PL (Figure 3a). To this end, F1 males born to poly(I:C)-exposed mothers were mated with F1 control females to generate PL-derived F2 poly(I:C) offspring. The latter were then mated with F2 control offspring to obtain PL-derived F3 poly(I:C) offspring (N=9 litters). Control F3 offspring were generated by crossing F2 control males and F2 control females (N=8 litters).
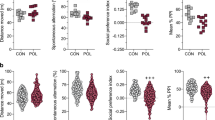
Behavioral phenotypes in F1 and F2 offspring derived from immune-challenged and control ancestors. (a) Breeding scheme used to generate F1 and F2 offspring with control and poly(I:C)-exposed ancestors. Pregnant F0 mice were treated with control (CON) or poly(I:C) (POL) solution to obtain F1 CON and F1 POL offspring, which in turn were either assigned to behavioral testing or used as breeders to obtain the F2 generation. F1 CON males and females were mated to generate F2 CON offspring, and F1 POL males and females were crossed to generate F2 POL offspring. (b) Social interaction test. The bar plots show the percent time spent with an unfamiliar mouse and total distance moved during the test. (c) Prepulse inhibition (PPI) of the acoustic startle reflex. The line plot shows percent PPI as a function of different pulse intensities (P-100, P-110 and P-120, corresponding to 110, 110 and 120 dBA) and prepulse intensities (+6, +12 and +18 dBA above background of 65 dBA); the bar plot depicts the mean PPI scores for each of the three pulse conditions. (d) Cued Pavlovian fear conditioning. The line plot shows the fear response (indexed by the percent time freezing) to successive CS(tone)-US(foot shock) presentations, and the bar plot depicts the conditioned fear response during the subsequent test phase when the CS(tone) was no longer followed by the US(foot shock). (e) Behavioral despair test. The bar plots shows the percent time floating in the forced swim test. All values are means + s.e.m.; for each test, *P<0.05, P<0.01 and ***P<0.001, based on analysis of variance (ANOVA). N(F1 CON)=26 (14m, 12f), N(F1 POL)=21 (10m, 11f), N(F2 CON)=18 (9m, 9f), N(F2 POL) = 18 (9m, 9f).
F2 phenotypes separated by maternal (ML) and paternal (PL) lineages. (a) Breeding scheme used to generate F1 and F2 generations with control and immune-challenged ancestors. Pregnant F0 mice were treated with control (CON) or poly(I:C) (POL) solution to obtain F1 CON and F1 POL offspring. To obtain F2 POL offspring via the ML (F2 POL/ML offspring), female F1 POL offspring were crossed with male F1 CON offspring; and to generate F2 POL offspring via the PL (F2 POL/PL offspring), male F1 POL offspring were mated with female F1 CON offspring. F1 CON males and females were crossed to obtain the F2 control lineage (F2 CON). (b) Social interaction test. The bar plots show the percent time spent with an unfamiliar mouse and total distance moved during the test. (c) Prepulse inhibition (PPI) of the acoustic startle reflex. The line plot shows percent PPI as a function of different pulse intensities (P-100, P-110 and P-120, corresponding to 110, 110 and 120 dBA) and prepulse intensities (+6, +12 and +18 dBA above background of 65 dBA); the bar plot depicts the mean PPI scores for each of the three pulse conditions. (d) Cued Pavlovian fear conditioning. The line plot shows the fear response (indexed by the percent time freezing) to successive CS(tone)-US(foot shock) presentations, and the bar plot depicts the conditioned fear response during the subsequent test phase when the CS(tone) was no longer followed by the US(foot shock). (e) Behavioral despair test. The bar plots shows the percent time floating in the forced swim test. All values are means + s.e.m.; for each test, *P<0.05 and P<0.01, based on Fisher's least significant difference post hoc test. N(F2 CON)=10m, N(F2 POL/ML)=10m, N(F2 POL/PL)=10m.
Persistence of behavioral phenotypes in F3 offspring derived from immune-challenged ancestors (a) Breeding scheme used to obtain the F3 generation with control and immune-challenged ancestors deriving from the paternal lineage (PL). Pregnant F0 mice were treated with control (CON) or poly(I:C) (POL) solution, and F1 POL males were mated with F1 CON females to generate F2 POL/PL offspring. F2 POL/PL males were then mated with F2 CON females to obtain F3 POL/PL offspring. CON F3 offspring were generated by crossing F2 CON males and females. (b) Social interaction test. The bar plots show the percent time spent with an unfamiliar mouse and total distance moved during the test. (c) Cued Pavlovian fear conditioning. The line plot shows the fear response (indexed by the percent time freezing) to successive CS(tone)-US(foot shock) presentations, and the bar plot depicts the conditioned fear response during the subsequent test phase when the CS(tone) was no longer followed by the US(foot shock). (d) Prepulse inhibition (PPI) of the acoustic startle reflex. The line plot shows percent PPI as a function of different pulse intensities (P-100, P-110 and P-120, corresponding to 110, 110 and 120 dBA) and prepulse intensities (+6, +12 and +18 dBA above background of 65 dBA); the bar plot depicts the mean PPI scores for each of the three pulse conditions. (e) Behavioral despair test. The bar plots shows the percent time floating in the forced swim test. All values are means + s.e.m.; for each test, *P<0.05, based on analysis of variance (ANOVA). N(F3 CON)=10m, N(F3 POL/PL)=10m.
Behavioral testing of F1, F2 and F3 offspring
For each generation, behavioral testing started when the offspring reached postnatal day 70 and included tests assessing social interaction, cued Pavlovian fear conditioning, prepulse inhibition (PPI) of the acoustic startle reflex and behavioral despair in the forced swim test. These tests were selected based on their relevance to neuropsychiatric disorders with prenatal infectious etiologies, including schizophrenia, autism and bipolar disorder.34, 36, 39, 40 Furthermore, prenatal immune activation has previously been shown to cause deficits in social interaction, learned fear, PPI and behavioral despair.36, 37, 41, 42, 43, 44, 45 A detailed description of the apparatuses and procedures is provided in the Supplementary Information. For each generation, 1–2 offspring per sex and litter were randomly selected and behaviorally tested to minimize possible confounds arising from litter effects.46 Both male and female offspring were used in the first experimental series (Figure 1a). Given that the first experimental series did not reveal sex-dependent effects in the F1 and F2 poly(I:C) offspring, all subsequent experimental series were conducted using male offspring only to minimize the number of animals. The sample sizes ranged from 9 to 14 offspring per group and sex based on our previous studies.13, 35, 36 The offspring in each generation were behaviorally tested in the following order: (1) social interaction test, (2) cued Pavlovian fear conditioning, (3) PPI test and (4) forced swim test. A test-free resting period of 5–7 days was imposed between individual tests.
Next-generation mRNA sequencing
We performed next-generation messenger RNA (mRNA) sequencing to compare genome-wide transcriptional changes in F1 and F2 offspring of immune-challenged mothers relative to F1 and F2 control offspring. Behaviorally naïve mice were killed by decapitation at the age of 12 weeks. mRNA was extracted from discrete brain regions and quantified using the Illumina TruSeq stranded mRNA preparation protocol and the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) system as described in the Supplementary Information. Differential expression was computed by an experimenter who was blind to the treatments for pairwise comparisons using the Bioconductor package Deseq2 with false discovery rate-corrected P-values (P<0.05) set to a 10% threshold (q<0.1).47 The FASTQ files were deposited at the European Nucleotide Archive (study accession number is: PRJEB12581). Functional network prediction was generated through the use of QIAGEN’s Ingenuity Pathway Analysis48, 49 (IPA; QIAGEN, Redwood City, CA, USA) as described in the Supplementary Information. In the present study, we focused on mRNA expression in the amygdalar complex (bregma: −1.0 to −2.0 mm) based on the results obtained in the behavioral tests.
Statistical analyses
All behavioral data met the assumptions of normal distribution and equality of variance and were analyzed using analysis of variance to identify the main effects of prenatal treatment and sex, as well as their interaction. Exclusion of animals was not applied. The individual analysis of variances used for each behavioral test are outlined in Supplementary Tables 1–4, which also summarize the statistical outcomes obtained by analysis of variance. Whenever appropriate, Fisher's least significant difference post hoc tests were used to test group differences in the behavioral analyses. All statistical analyses for the behavioral tests were performed using the statistical software SPSS (version 13.0; IBM, New York, NY, USA), and statistical significance was set at P<0.05. Differential gene expression was analyzed using the Bioconductor package Deseq2 as described in the Supplementary Information.
Results
Behavioral phenotypes in first- and second-generation offspring of immune-challenged mothers
To identify possible transgenerational effects of prenatal viral immune activation, we first compared the behavioral phenotypes emerging in F1 and F2 offspring of poly(I:C)-exposed mothers relative with corresponding control offspring. The F1 and F2 generations were generated as illustrated in Figure 1a.
Consistent with previous studies,41, 42, 45 we found that F1 poly(I:C)-exposed offspring displayed impaired sociability in a social interaction test, in which they were allowed to concomitantly explore an inanimate dummy object and an unfamiliar live mouse. Whereas F1 control offspring showed a strong preference for the live mouse versus the inanimate dummy object, F1 poly(I:C) offspring did not display such a preference (Figure 1b). These effects were not confounded by changes in general locomotor activity (Figure 1b). Moreover, F1 control and poly(I:C) offspring explored the dummy object to a similar extent, whereas the exploration times for the live mouse markedly differed between the two groups (Supplementary Figure 1). Hence, the poly(I:C)-induced social interaction deficits is not attributable to a general deficiency in exploration but rather reflects a genuine and selective impairment in social approach behavior. F2 offspring of poly(I:C)-exposed ancestors showed a similar deficit in sociability without concomitant changes in general locomotor activity (Figure 1b) or dummy object exploration (Supplementary Figure 1).
F1 poly(I:C) offspring also displayed increased fear expression in a cued Pavlovian fear conditioning test, which involved a tone as the conditioned stimulus (CS) and electric foot shock as the unconditioned stimulus (US). Whereas they did not differ from F1 controls during the initial acquisition of the conditioned fear response to successive CS(tone)-US(foot shock) presentations, F1 poly(I:C) offspring showed increased conditioned fear when the CS was no longer followed by the US (Figure 1d). Similar fear-related abnormalities were also present F2 offspring of poly(I:C)-exposed ancestors: Although the acquisition of the fear response during initial tone-shock conditioning was not different between F2 control and F2 poly(I:C) offspring, the latter displayed increased cued fear expression in the subsequent test phase (Figure 1d).
We further assessed sensorimotor gating in the F1 and F2 generations using the paradigm of PPI of the acoustic startle reflex, which indexes pre-attentive information processing and gating. Consistent with numerous previous studies in mice,9, 13, 36, 38 F1 poly(I:C) offspring displayed a robust reduction in PPI scores when 110 and 120 dBA stimuli served as pulses (Figure 1c). These effects were not associated with changes in the responses to prepulse-alone or pulse-alone trials (data not shown), suggesting that prepulse detection and startle reactivity per se were not affected by prenatal immune activation. F2 offspring derived from poly(I:C)-exposed or control ancestors showed comparable PPI (Figure 1c) and responses to prepulse- and pulse-alone trials (data not shown). Hence, despite the robust PPI deficits in the F1 generation of immune-challenged mothers, the F2 generation did not inherit the sensorimotor gating deficit.
We also examined affective behaviors in F1- and F2-generation offspring of immune-challenged mothers. To this end, we used the forced swim test, which assesses behavioral despair associated with depressive-like behaviors in rodents. The critical readout in this test is the relative time an animal is immobile (floating) when confined to a cylinder filled with water, from which it cannot escape.50 F1 poly(I:C) offspring did not differ from F1 control offspring with regards to the time spent floating (Figure 1e). Despite the absence of behavioral despair in the F1 generation, however, F2 offspring of poly(I:C)-exposed ancestors spent significantly more time floating than F2 control offspring (Figure 1e). Hence, F2 but not F1 offspring derived from immune-challenged mothers develop signs of behavioral despair.
Together, our data demonstrate that prenatal viral-like immune activation leads to a transmission of behavioral phenotypes across generations. Whereas social interaction deficits and increased cued fear expression are similarly present in the F1 and F2 offspring of immune-challenged mothers, increased behavioral despair emerges as a novel phenotype in the second generation. Our findings further show that sensorimotor gating impairments are confined to the direct descendants of infected mothers and are not transmitted across generations. The lack of significant interactions between treatment and sex (Supplementary Tables 1 and 2) further suggests that these transgenerational effects emerge in both male and female offspring and are, therefore, independent of the offspring’s sex.
Second-generation phenotypes separated by maternal and PLs
We next aimed to dissect the relative contributions of the ML and PL in the transgenerational transmission of behavioral phenotypes following prenatal immune activation. ML- and PL-derived F2 poly(I:C) offspring and corresponding controls were generated as illustrated in Figure 2a.
We found that PL-derived F2 poly(I:C) offspring showed deficits in social interaction (Figure 2b, Supplementary Figure 1), increased fear expression (Figure 2d) and behavioral despair (Figure 2e), but not sensorimotor gating (Figure 2c). The former behavioral abnormalities were not associated with changes in basal locomotor activity (Figure 2b) or altered acquisition of the fear response during initial tone-shock conditioning (Figure 2d). Interestingly, F2 poly(I:C) offspring did not display overt behavioral impairments when they were derived from the ML ancestor lineage (Figures 2b). These findings thus demonstrate that the transgenerational transmission of behavioral deficits following prenatal immune activation is mediated via the PL but not ML.
Persistence of behavioral phenotypes in third-generation offspring with immune-challenged ancestors
We further examined whether abnormal behavioral phenotypes induced by prenatal immune activation persist in the third generation. For this purpose, we generated PL-derived F3 offspring with poly(I:C)-exposed or control ancestors as illustrated in Figure 3a.
Consistent with PL-derived F2 poly(I:C) offspring (Figure 2), PL-derived F3 poly(I:C) offspring displayed deficits in social interaction (Figure 3b, Supplementary Figure 1) and increased cued fear expression (Figure 3c). Again, these abnormalities were not associated with changes in basal locomotor activity (Figure 3b) or altered acquisition of the fear response during initial tone-shock conditioning (Figure 3c). PL-derived F3 poly(I:C) offspring also showed increased behavioral despair (Figure 3e) but normal sensorimotor gating (Figure 3d). These data demonstrate that abnormal behavioral phenotypes induced by prenatal immune activation persist in the third generation of offspring.
Transcriptional changes in first- and second-generation offspring of immune-challenged mothers
To identify possible transgenerational effects of prenatal immune activation in the central nervous system, we used next-generation mRNA sequencing to compare genome-wide transcriptional changes in the amygdalar complex of F1 and F2 offspring of poly(I:C)-exposed and control offspring. We included behaviorally naïve F1 poly(I:C) and F1 control offspring (generated as shown in Figure 1a), as well as PL-derived F2 poly(I:C) offspring and F2 control offspring (generated as shown in Figure 2a). We focused on transcriptional changes in the amygdalar complex based on its critical involvement in social behavior,51 learned fear52 and behavioral despair.53, 54
We revealed 2217 differentially expressed genes (DEGs) in F1 poly(I:C) offspring relative to F1 controls (Figures 4a, 5a), and 4015 DEGs in PL-derived F2 poly(I:C) offspring relative to F2 controls (Figures 4c, 5a). A summary of the top 10 canonical pathways predicted by IPA to be significantly affected in F1 poly(I:C) offspring (relative to F1 control offspring) and in PL-derived F2 poly(I:C) offspring (relative to F2 control offspring) is provided in Supplementary Table 5. Interestingly, G-protein-coupled receptor signaling was one of the top canonical pathways affected specifically in the F1 generation (Figure 4b). Various genes deregulated in this pathway have previously been associated with schizophrenia, bipolar disorder and/or autism, including regulator of G-protein signaling 4,55, 56 cannabinoid receptor 1,57 γ-aminobutyric acid (GABA) B1 and B2 receptors (GABBR1 and GABBR2),58 adenosine A2A receptor (ADORA2A),57 and calcium/calmodulin-dependent protein kinase 2b (CaMK2b)57 (for a full list of genes included in this pathway, see Supplementary Table 6). In addition to those involved in G-protein-coupled receptor signaling, a number of other genes were differentially expressed in F1 poly(I:C) offspring, many of which have been associated with schizophrenia, bipolar disorder and/or autism (Supplementary Table 7). These included reelin (RELN),59 forkhead box protein 1 and 2 (FOXP1 and FOXP2),60 serpin peptidase inhibitor A3 (SERPINA3),61, 62 ankyrin 3 (ANK3)57 and neurexin 3 (NRXN3)57 (Supplementary Table 7). Moreover, transcriptional changes in numerous other signaling pathways, including tight junction signaling, Sertoli cell junction signaling and axonal guidance signaling, were affected in F1 offspring of poly(I:C)-exposed mothers (Supplementary Table 5). These findings thus show that the transcriptomic effects of prenatal immune activation are not restricted to neuronal signaling but further extend to various other biological functions (Supplementary Table 5).
Transcriptomic changes in F1 and F2 offspring derived from immune-challenged and control ancestors. Next-generation mRNA sequencing was used to assess genome-wide transcriptomic changes in the amygdala. (a) Heat map of all differentially expressed genes (DEGs) in F1 offspring born to poly(I:C)-exposed mothers (F1 POL) relative to F1 control (F1 CON) offspring. The color-coded key denotes upregulation (purple) and downregulation (yellow) in terms of log2 ratios. (b) Graphical illustration of the G-protein-coupled receptor signaling pathway, which is deregulated in F1 POL relative to F1 CON offspring. Colored elements in the pathway denote significant expression changes. (c) Heat map of all DEGs in F2 offspring derived from immune-challenged ancestors (F2 POL) relative to F2 CON offspring. The color-coded key denotes upregulation (purple) and downregulation (yellow) in terms of log2 ratios. (d) Graphical illustration of the glutamatergic signaling pathway, which is deregulated in F2 POL relative to F2 CON offspring. Colored elements in the pathway denote significant expression changes. All pathways were generated using Ingenuity Pathway Analysis. N(F1 CON)=6m, N(F1 POL)=5m, N(F2 CON)=5m and N(F2 POL)=4m.
Unique and mutual transcriptomic changes in the amygdala of F1 and F2 offspring derived from immune-challenged and control ancestors. (a) The Venn diagram depicts the number of genes with differential expression only in F1 offspring born to poly(I:C)-exposed mothers (F1 POL), differential expression only in F2 offspring with poly(I:C)-exposed ancestors (F2 POL), and differential expression in both generations. (b) Graphical illustration of the dopamine and cAMP-regulated phophoprotein 32 (DARPP-32) signaling pathway, which is deregulated in both F1 POL and F2 POL offspring. Colored elements in the pathway denote significant transcriptional changes with the functional nodes. Orange and blue color symbolizes changes that are specific to F1 POL and F2 POL offspring, whereas green color symbolizes common changes in both generations. The direction of transcriptional changes of specific genes that are encompassed in the illustrated nodes is summarized in Supplementary Table 10. The pathway was generated using Ingenuity Pathway Analysis.
Likewise, F2 offspring of poly(I:C)-exposed ancestors showed transcriptional changes in several canonical pathways that are associated with diverse biological functions (Supplementary Table 5). Glutamatergic signaling was one of the top canonical pathways specifically affected in this generation (Figure 4d; Supplementary Table 8). This pathway included numerous genes encoding for subunits of the N-methyl-d-aspartate receptors receptor, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors and metabotropic glutamate receptors (Figure 4a, Supplementary Table 8), many of which have been implicated in schizophrenia, bipolar disorder and/or autism.57, 63, 64 Transcriptional changes that relate to altered oxidative phosphorylation and mitochondrial dysfunctions were also among the top canonical pathways affected in the F2 generation of poly(I:C)-exposed ancestors (Supplementary Table 8; Supplementary Figure 2). The presence of altered oxidative phosphorylation appears particularly relevant in view of previous findings demonstrating abnormal oxidative and metabolic processing in models of prenatal immune activation and other neurodevelopmental disruption models relevant to schizophrenia and related disorders.65, 66, 67
Besides the unique association with either F1 or F2 poly(I:C) offspring (Figure 4; Supplementary Tables 5–8), a remarkable number of genes (1132) were differentially expressed in both generations (Figure 5a). IPA revealed that the signaling pathway involving dopamine and cAMP-regulated phosphoprotein 32 (DARPP-32; also known as PPP1R1B) is affected both in F1 and F2 poly(I:C) offspring (Figure 5b). This pathway provides a mechanism for integrating neuronal information in multiple brain regions and via a variety of neurotransmitters, including dopamine and glutamate.68 Alterations in the DARPP-32 pathway, or in the expression of DARPP-32 itself, has previously been associated with neuropsychiatric disorders, including schizophrenia, bipolar disorder and major depression.68, 69 This pathway has also been widely implicated in fear-related behavior,70 which in turn is disrupted in multiple generations with immune-challenged ancestors (Figures 1d, 2d and 3c). Interestingly, some transcriptional changes in the DARPP-32 pathway showed the same direction in F1 or F2 poly(I:C) offspring, whereas others showed opposite directions of changes in the two generations (Supplementary Table 10). Examples of the former include calcineurin subunits (PPP3CB and PPP3R1), the protein kinase A subunit PRKAG2, protein kinase D1, adenylate cyclase 6 and potassium channel inwardly rectifying subfamily members (KCNJ6 and KCNJ12). Examples showing an opposite direction of transcriptional changes in the F1 and F2 generations involve DARPP-32 (PPP1R1B) itself, dopamine receptor D2, protein kinase C family members (PRKCH and PRKCZ), cGMP-dependent protein kinase type I and inositol 1,4,5-trisphosphate receptor type 1. Despite the opposite transcriptional changes of some genes, pathway activity analysis by IPA predicted an overall inhibition state of the DARPP-32 pathway in both generations. Taken together, we identified gene sets that are uniquely changed in F1 and F2 offspring of immune-challenged mothers, and at the same time, we revealed genes sets commonly affected in both generations. These transcriptional effects thus mirror the transgenerational effects on behavior, showing that prenatal immune activation leads to a transgenerational transmission (presence of similar phenotypes across generations) and modification (presence of distinct phenotypes across generations) of pathological traits.
Discussion
Prenatal exposure to infection and/or inflammation is increasingly recognized to contribute to the etiology of psychiatric disorders with neurodevelopmental components, including schizophrenia,1, 2, 7 autism3, 4 and bipolar disorder.5, 6 Using a well-established mouse model of prenatal immune activation,8, 9, 10, 11 our findings suggest for the first time that this environmental risk factor can lead to pathological effects on brain and behavior in multiple generations. Hence, our study identifies a novel role of immunological early-life adversities in shaping the risk of brain and behavioral abnormalities across generations. These findings add to the growing evidence that transgenerational transmission of disease susceptibility can occur following early-life exposures to various environmental challenges, including prenatal and neonatal stress,22, 23, 24, 25, 26 prenatal malnutrition27, 28, 29 and prenatal alcohol intake.31
The pathological effects induced by in-utero exposure to environmental insults such as infection may be restricted to the direct descendants or transmitted across generations depending on whether the exposure affects the offspring’s somatic and/or germ lines.18, 21, 32 If the exposure affects somatic cells but spares primordial germ cells, it likely changes the offspring’s developmental trajectories and leads to a specific phenotype in later life, but without transmitting any effects across generations.18, 21, 32 The PPI phenotype, which was only present in the direct descendants of immune-challenged mothers but not transmitted across generations, seems to involve such effects. However, our observations showing transgenerational transmission of deficits in social interaction and learned fear down to the grand-offspring (F3 generation) strongly suggest that in-utero exposure to immune activation altered the programming of germ cells. According to this scenario, the phenotypes were transmitted to subsequent generations because of molecular changes in primordial germ cells.18, 21, 32 The presence of behavioral abnormalities in the grand-offspring (F3 generation) further supports the involvement of transgenerational non-genetic inheritance.18, 21, 32 Prenatal immune activation thus likely altered epigenetic marks in the germ line of the F1 offspring, which resisted erasure and epigenetic reestablishment during germ cell development.32
Our data further suggest that the transgenerational effects of prenatal immune activation occur via the PL. This emphasizes a critical role of the male germ cells as possible mediators of transgenerational non-genetic inheritance following prenatal immune activation. Indeed, male ancestor mice were only present during mating but were not involved in the rearing of the pups, leaving sperm cells as the only direct biological link between father and offspring. This paternal mode of transgenerational transmission is in line with other models of early-life adversities, including models of prenatal or neonatal stress22, 23, 24, 25, 26 and prenatal malnutrition,27, 28 which similarly emphasize a crucial role of the PL in the transgenerational inheritance of environmentally acquired brain pathology. Based on our findings, it is tempting to speculate that prenatal immune activation may induce transgenerational effects via epigenetic modifications in male gametes.23, 25, 27 Interestingly, rodent models of prenatal viral-like infection have already identified several epigenetic alterations in the offspring’s central nervous system, including altered DNA methylation,71, 72 histone modifications73 and micro RNA expression.74 The future exploration of possible epigenetic changes in male gametes may thus offer important insights into the molecular processes underlying the transgenerational inheritance of brain pathology following prenatal immune activation.
It should be pointed out, however, that milder transgenerational effects tended to emerge via the ML. Even though these effects were far from significant, these findings suggest the maternal line may mediate subthreshold levels or latent forms of behavioral anomalies, which in turn may be unmasked when the offspring are exposed to additional environmental stressors.75 Furthermore, whilst our data clearly underscore the importance of the PL for the transgenerational transmission of social and fear-related behavioral dysfunctions, our study does not exclude the possibility that deficits in other behavioral and cognitive domains could be transmitted from one generation to the next via the ML.
Another intriguing aspect of our findings relates to the induction of novel phenotypes across generations. We show that increased behavioral despair emerges in the F2 and F3 offspring of immune-challenged ancestors, but not in the direct descendants (F1) born to infected mothers. These data suggest that maternal immune activation during pregnancy can induce latent behavioral symptoms that are passed on to and become manifest only in subsequent generations. Hence, certain abnormalities induced by in-utero immune activation can skip generations. Similar transgenerational patterns of inheritance involving a ‘silent carrier’ have been reported in response to chronic stress exposure.32 A possible molecular explanation for this scenario is that the exposure does not cause overt effects on phenotype-relevant somatic tissues in F1 offspring, but is still able to reprogram primordial germ cells. The latter may then affect developmental processes following fertilization of the oocyte and precipitate the appearance of a certain phenotype in subsequent generations, even if it was not present in the F1 generation.32
Similar to the behavioral alterations, prenatal immune activation also caused a transgenerational transmission of transcriptional changes. Although some gene expression abnormalities were common to both generations, others emerged specifically in either F1 or F2 offspring of immune-challenged mothers. Some of the transcriptional changes revealed in F1 poly(I:C) offspring are consistent with previous studies using the poly(I:C)-based maternal immune activation model. For example, a previous study found increased protein expression of the α2 subunit of the γ-aminobutyric acid-A receptor in the amygdala of prenatally immune-challenged mice,76 which is consistent with the present findings of increased GABRA2 expression in F1 poly(I:C) offspring. Furthermore, the decrease in RELN expression found in the F1 poly(I:C) offspring is in line with previous studies showing reduced RELN immunohistochemistry following prenatal immune activation in mice.35, 77 It would be interesting to further examine the transcriptome in other brain regions such as the prefrontal or striatal areas to assess the similarities or differences with the amygdala. Such additional investigations would also allow a more detailed comparison with previous transcriptomic or proteomic studies using maternal immune activation models, which mostly focused on prefrontal or striatal areas thus far.16, 73, 78
It should also be emphasized that most transcripts in F1 and F2 poly(I:C) offspring showed relatively modest changes from corresponding F1 and F2 controls: even though they withstood correction by false discovery rate, the majority of transcripts showed fold changes ranging between 1.5 and 2 (corresponding to log2 ratios between 0.58 and 1). These effect sizes are comparable to those occurring in cortical areas of adult mice exposed to viral-like immune activation73 or influenza infection78 during early gestation. Such modest effect sizes are not unprecedented given the early prenatal timing of the environmental insult, which typically leads to pathological changes in brain and behavior that are widespread but often relatively mild in terms of effect size.37 Moreover, some of the transcriptional changes present in the direct descendants of immune-challenged mothers can be transmitted to the next generation and may contribute to the transgenerational transmission of behavioral abnormalities.
One example of such transgenerational effects on the amygdalar transcriptome involves genes that are part of the DARPP-32 signaling pathway. We found that some transcriptional changes in the DARPP-32 pathway showed the same direction in F1 or F2 poly(I:C) offspring, which paralleled the transgenerational effects of social and fear-related behaviors. On speculative grounds, these findings indicate that transcriptomic changes in the DARPP-32 signaling pathway may, at least in part, underlie the modulation of these behavioral traits in prenatally poly(I:C)-exposed offspring and their subsequent generations. In agreement with this hypothesis, genetic and pharmacological studies in rodent models have repeatedly implicated DARPP-32 signaling in emotional behavior, especially fear conditioning.70 For example, genetic or pharmacological inhibition of protein kinase A activity leads to impaired expression of conditioned fear,79, 80, 81 whereas inhibition of calcineurin activity results in the opposite effects.82, 83, 84 Here, we found that the transcripts of distinct protein kinase A subunits were upregulated in F1 and F2 offspring of poly(I:C)-exposed mothers, whereas the expression of calcineurin subunits was decreased in both generations. These transcriptional changes are consistent with, and may even contribute to, the emergence of increased fear expression in offspring with immune-challenged ancestors.79, 80, 81, 82, 83, 84 Future studies will be needed, however, to ascertain the functional contribution of these molecular changes to the social and fear-related phenotypes in prenatally poly(I:C)-exposed offspring and their subsequent generations.
Another aspect that warrants future investigations relates to the potential influence of the timing of prenatal immune activation. Numerous previous findings in rodent and monkey models of prenatal immune activation suggest that the precise timing of prenatal immune challenge critically determines the specificity of inflammation-mediated brain and behavioral pathology.35, 45, 73, 85, 86, 87, 88 In the mouse, the early/middle (GD 6–3) and late (GD 17 and beyond) gestational windows clearly differ in terms of infection-mediated neurodevelopmental anomalies, so that the developmental vulnerability of specific forms of postnatal brain dysfunctions varies across different gestational stages.37, 89 Against this background, it appears essential to further explore whether immune activation at distinct gestational time points induces similar or differential transgenerational effects. We believe that the latter possibility is more likely because epigenetic processes such as DNA de- and remethylation follow timed developmental patterns during embryogenesis and fetal development.90, 91 Prenatal environmental insults such as maternal infection might therefore induce differential epigenetic modifications depending on the precise prenatal timing, which in turn might shape the nature of transgenerational effects on behavior.
In conclusion, the transgenerational transmission and modification of brain pathology following early prenatal immune activation highlights a novel pathological aspect of this early-life adversity in shaping disease risk across generations. Prenatal poly(I:C) administration in mice is currently one of the most widely used models in developmental brain research and has applicability to various neurodevelopmental disorders, especially to those with inflammatory etiologies.10, 11, 12, 13 Therefore, our findings appear relevant to developmental brain disorders independently of existing diagnostic classifications and may help identifying complex patterns of transgenerational disease transmission beyond genetic inheritance.
References
Brown AS, Derkits EJ . Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 2010; 167: 261–280.
Miller BJ, Culpepper N, Rapaport MH, Buckley P . Prenatal inflammation and neurodevelopment in schizophrenia: a review of human studies. Prog Neuropsychopharmacol Biol Psychiatry 2013; 42: 92–100.
Atladóttir HÓ, Henriksen TB, Schendel DE, Parner ET . Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics 2012; 130: e1447–e1454.
Brown AS, Sourander A, Hinkka-Yli-Salomäki S, McKeague IW, Sundvall J, Surcel HM . Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatry 2014; 19: 259–264.
Canetta SE, Bao Y, Co MD, Ennis FA, Cruz J, Terajima M et al. Serological documentation of maternal influenza exposure and bipolar disorder in adult offspring. Am J Psychiatry 2014; 171: 557–563.
Parboosing R, Bao Y, Shen L, Schaefer CA, Brown AS . Gestational influenza and bipolar disorder in adult offspring. JAMA Psychiatry 2013; 70: 677–685.
Kneeland RE, Fatemi SH . Viral infection, inflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2013; 42: 35–48.
Harvey L, Boksa P . Prenatal and postnatal animal models of immune activation: relevance to a range of neurodevelopmental disorders. Dev Neurobiol 2012; 72: 1335–1348.
Meyer U . Prenatal poly(I:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry 2014; 75: 307–315.
Meyer U, Feldon J . Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol 2010; 90: 285–326.
Labouesse MA, Langhans W, Meyer U . Long-term pathological consequences of prenatal infection: beyond brain disorders. Am J Physiol Regul Integr Comp Physiol 2015; 309: R1–R12.
Meyer U . Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2013; 42: 20–34.
Vuillermot S, Weber L, Feldon J, Meyer U . A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. J Neurosci 2010; 30: 1270–1287.
Stolp HB, Turnquist C, Dziegielewska KM, Saunders NR, Anthony DC, Molnár Z . Reduced ventricular proliferation in the foetal cortex following maternal inflammation in the mouse. Brain 2011; 134: 3236–3248.
Piontkewitz Y, Arad M, Weiner I . Abnormal trajectories of neurodevelopment and behavior following in utero insult in the rat. Biol Psychiatry 2011; 70: 842–851.
Richetto J, Calabrese F, Riva MA, Meyer U . Prenatal immune activation induces maturation-dependent alterations in the prefrontal GABAergic transcriptome. Schizophr Bull 2014; 40: 351–361.
Bohacek J, Mansuy IM . Epigenetic inheritance of disease and disease risk. Neuropsychopharmacology 2013; 38: 220–236.
Gapp K, von Ziegler L, Tweedie-Cullen RY, Mansuy IM . Early life epigenetic programming and transmission of stress-induced traits in mammals: how and when can environmental factors influence traits and their transgenerational inheritance? Bioessays 2014; 36: 491–502.
Grossniklaus U, Kelly WG, Ferguson-Smith AC, Pembrey M, Lindquist S . Transgenerational epigenetic inheritance: how important is it? Nat Rev Genet 2013; 14: 228–235.
Nilsson EE, Skinner MK . Environmentally induced epigenetic transgenerational inheritance of disease susceptibility. Transl Res 2014; 165: 12–16.
Bale TL . Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci 2015; 16: 332–344.
Franklin TB, Russig H, Weiss IC, Gräff J, Linder N, Michalon A et al. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry 2010; 68: 408–415.
Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci 2014; 17: 667–669.
Bohacek J, Farinelli M, Mirante O, Steiner G, Gapp K, Coiret G et al. Pathological brain plasticity and cognition in the offspring of males subjected to postnatal traumatic stress. Mol Psychiatry 2015; 20: 621–631.
Rodgers AB, Morgan CP, Leu NA, Bale TL . Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci USA 2015; 112: 13699–13704.
Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL . Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 2013; 33: 9003–9012.
Veenendaal MV, Painter RC, de Rooij SR, Bossuyt PM, van der Post JA, Gluckman PD et al. Transgenerational effects of prenatal exposure to the 1944-45 Dutch famine. BJOG 2013; 120: 548–553.
Dunn GA, Bale TL . Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology 2011; 152: 2228–2236.
Rechavi O, Houri-Ze'evi L, Anava S, Goh WS, Kerk SY, Hannon GJ et al. Starvation-induced transgenerational inheritance of small RNAs in C. elegans. Cell 2014; 158: 277–287.
Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC . Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci 2013; 16: 42–47.
Govorko D, Bekdash RA, Zhang C, Sarkar DK . Male germline transmits fetal alcohol adverse effect on hypothalamic proopiomelanocortin gene across generations. Biol Psychiatry 2012; 72: 378–388.
Bohacek J, Mansuy IM . Molecular insights into transgenerational non-genetic inheritance of acquired behaviours. Nat Rev Genet 2015; 16: 641–652.
Vassoler FM, Sadri-Vakili G . Mechanisms of transgenerational inheritance of addictive-like behaviors. Neuroscience 2014; 264: 198–206.
Meyer U, Feldon J, Fatemi SH . In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev 2009; 33: 1061–1079.
Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci 2006; 26: 4752–4762.
Meyer U, Feldon J, Schedlowski M, Yee BK . Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev 2005; 29: 913–947.
Meyer U, Yee BK, Feldon J . The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist 2007; 13: 241–256.
Lipina TV, Zai C, Hlousek D, Roder JC, Wong AH . Maternal immune activation during gestation interacts with Disc1 point mutation to exacerbate schizophrenia-related behaviors in mice. J Neurosci 2013; 33: 7654–7666.
Crawley JN . Translational animal models of autism and neurodevelopmental disorders. Dialogues Clin Neurosci 2012; 14: 293–305.
Kato T, Kasahara T, Kubota-Sakashita M, Kato TM, Nakajima K . Animal models of recurrent or bipolar depression. Neuroscience 2015 ; in press (epub ahead of print [PMID: 26265551])..
Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH . Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun 2012; 26: 607–616.
Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, Ashwood P . Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl Psychiatry 2013; 3: e240.
Vorhees CV, Graham DL, Braun AA, Schaefer TL, Skelton MR, Richtand NM et al. Prenatal immune challenge in rats: effects of polyinosinic-polycytidylic acid on spatial learning, prepulse inhibition, conditioned fear, and responses to MK-801 and amphetamine. Neurotoxicol Teratol 2015; 47: 54–65.
Zhang Z, van Praag H . Maternal immune activation differentially impacts mature and adult-born hippocampal neurons in male mice. Brain Behav Immun 2015; 45: 60–70.
Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U . Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology 2010; 35: 2462–2478.
Zorrilla EP . Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol 1997; 30: 141–150.
Love MI, Huber W, Anders S . Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014; 15: 550.
Thomas S, Bonchev D . A survey of current software for network analysis in molecular biology. Hum Genomics 2010; 4: 353–360.
Young MD, Wakefield MJ, Smyth GK, Oshlack A . Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol 2010; 11: R14.
Cryan JF, Holmes A . The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 2005; 4: 775–790.
Fox AS, Kalin NH . A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. Am J Psychiatry 2014; 171: 1162–1173.
Maren S, Phan KL, Liberzon I . The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 2013; 14: 417–428.
Del Boca C, Lutz PE, Le Merrer J, Koebel P, Kieffer BL . Cholecystokinin knock-down in the basolateral amygdala has anxiolytic and antidepressant-like effects in mice. Neuroscience 2012; 218: 185–195.
Chang CH, Grace AA . Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol Psychiatry 2014; 76: 223–230.
Mirnics K, Middleton FA, Stanwood GD, Lewis DA, Levitt P . Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol Psychiatry 2001; 6: 293–301.
Volk DW, Eggan SM, Lewis DA . Alterations in metabotropic glutamate receptor 1α and regulator of G protein signaling 4 in the prefrontal cortex in schizophrenia. Am J Psychiatry 2010; 167: 1489–1498.
Butler MG, McGuire AB, Masoud H, Manzardo AM . Currently recognized genes for schizophrenia: high-resolution chromosome ideogram representation. Am J Med Genet B Neuropsychiatr Genet 2016; 171: 181–202.
Fatemi SH, Folsom TD, Thuras PD . Deficits in GABA(B) receptor system in schizophrenia and mood disorders: a postmortem study. Schizophr Res 2011; 128: 37–43.
Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP et al. Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology 2011; 60: 1007–1016.
Ingason A, Giegling I, Hartmann AM, Genius J, Konte B, Friedl M et al. Expression analysis in a rat psychosis model identifies novel candidate genes validated in a large case-control sample of schizophrenia. Transl Psychiatry 2015; 5: e656.
Arion D, Unger T, Lewis DA, Levitt P, Mirnics K . Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry 2007; 62: 711–721.
Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry 2013; 18: 206–214.
Ginsberg SD, Hemby SE, Smiley JF . Expression profiling in neuropsychiatric disorders: emphasis on glutamate receptors in bipolar disorder. Pharmacol Biochem Behav 2012; 100: 705–711.
Rojas DC . The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. J Neural Transm 2014; 121: 891–905.
Lanté F, Meunier J, Guiramand J, Maurice T, Cavalier M, de Jesus Ferreira MC . Neurodevelopmental damage after prenatal infection: role of oxidative stress in the fetal brain. Free Radic Biol Med 2007; 42: 1231–1245.
Oskvig DB, Elkahloun AG, Johnson KR, Phillips TM, Herkenham M . Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav Immun 2012; 26: 623–634.
Cabungcal JH, Counotte DS, Lewis EM, Tejeda HA, Piantadosi P, Pollock C et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron 2014; 83: 1073–1084.
Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P . DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol 2004; 44: 269–296.
Kunii Y, Hyde TM, Ye T, Li C, Kolachana B, Dickinson D et al. Revisiting DARPP-32 in postmortem human brain: changes in schizophrenia and bipolar disorder and genetic associations with t-DARPP-32 expression. Mol Psychiatry 2014; 19: 192–199.
Selcher JC, Weeber EJ, Varga AW, Sweatt JD, Swank M . Protein kinase signal transduction cascades in mammalian associative conditioning. Neuroscientist 2002; 8: 122–131.
Basil P, Li Q, Dempster EL, Mill J, Sham PC, Wong CC et al. Prenatal maternal immune activation causes epigenetic differences in adolescent mouse brain. Transl Psychiatry 2014; 4: e434.
Labouesse MA, Dong E, Grayson D, Guidotti A, Meyer U . Maternal immune activation induces GAD1 and GAD2 promoter remodeling in the offspring prefrontal cortex. Epigenetics 2015; 10: 1143–1155.
Connor CM, Dincer A, Straubhaar J, Galler JR, Houston IB, Akbarian S . Maternal immune activation alters behavior in adult offspring, with subtle changes in the cortical transcriptome and epigenome. Schizophr Res 2012; 140: 175–184.
Hollins SL, Zavitsanou K, Walker FR, Cairns MJ . Alteration of imprinted Dlk1-Dio3 miRNA cluster expression in the entorhinal cortex induced by maternal immune activation and adolescent cannabinoid exposure. Transl Psychiatry 2014; 4: e452.
Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 2013; 339: 1095–1099.
Nyffeler M, Meyer U, Yee BK, Feldon J, Knuesel I . Maternal immune activation during pregnancy increases limbic GABAA receptor immunoreactivity in the adult offspring: implications for schizophrenia. Neuroscience 2006; 143: 51–62.
Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P et al. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol Psychiatry 1999; 4: 145–154.
Fatemi SH, Folsom TD, Rooney RJ, Mori S, Kornfield TE, Reutiman TJ . The viral theory of schizophrenia revisited: abnormal placental gene expression and structural changes with lack of evidence for H1N1 viral presence in placentae of infected mice or brains of exposed offspring. Neuropharmacology 2012; 62: 1290–1298.
Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R . Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 1997; 88: 615–626.
Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER . Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem 1998; 5: 365–374.
Schafe GE, LeDoux JE . Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci 2000; 20: RC96.
Ikegami S, Inokuchi K . Antisense DNA against calcineurin facilitates memory in contextual fear conditioning by lowering the threshold for hippocampal long-term potentiation induction. Neuroscience 2000; 98: 637–646.
Havekes R, Nijholt IM, Visser AK, Eisel UL, Van der Zee EA . Transgenic inhibition of neuronal calcineurin activity in the forebrain facilitates fear conditioning, but inhibits the extinction of contextual fear memories. Neurobiol Learn Mem 2008; 89: 595–598.
Almeida-Corrêa S, Moulin TC, Carneiro CF, Gonçalves MM, Junqueira LS, Amaral OB . Calcineurin inhibition blocks within-, but not between-session fear extinction in mice. Learn Mem 2015; 22: 159–169.
Meyer U, Feldon J, Schedlowski M, Yee BK . Immunological stress at the maternal-foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav Immun 2006; 20: 378–388.
Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J . Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun 2008; 22: 469–486.
Fortier ME, Luheshi GN, Boksa P . Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res 2007; 181: 270–277.
Bauman MD, Iosif AM, Smith SE, Bregere C, Amaral DG, Patterson PH . Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol Psychiatry 2014; 75: 332–341.
Meyer U, Feldon J, Yee BK . A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull 2009; 35: 959–972.
Kohli RM, Zhang Y . TET enzymes, TDG and the dynamics of DNA demethylation. Nature 2013; 502: 472–479.
Seisenberger S, Peat JR, Hore TA, Santos F, Dean W, Reik W . Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers. Philos Trans R Soc Lond B Biol Sci 2013; 368: 20110330.
Acknowledgements
We thank Weihong Qi and Anna Bratus from the Functional Genomics Center Zurich, Switzerland, for their technical assistance in RNA sequencing. This work was supported by the Swiss National Science Foundation (Grant No. 310030_146217) and by the European Union Seventh Framework Program (FP7/2007–2011; Grant Agreement No. 259679) awarded to UM.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website .
Supplementary information
Rights and permissions
About this article
Cite this article
Weber-Stadlbauer, U., Richetto, J., Labouesse, M. et al. Transgenerational transmission and modification of pathological traits induced by prenatal immune activation. Mol Psychiatry 22, 102–112 (2017). https://doi.org/10.1038/mp.2016.41
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.41
- Springer Nature Limited
This article is cited by
-
Prenatal immune activation in mice induces long-term alterations in brain mitochondrial function
Translational Psychiatry (2024)
-
Gestational immune activation disrupts hypothalamic neurocircuits of maternal care behavior
Molecular Psychiatry (2024)
-
Differential effects of purified low molecular weight Poly(I:C) in the maternal immune activation model depend on the laboratory environment
Translational Psychiatry (2024)
-
Transcriptomic profiling of the developing brain revealed cell-type and brain-region specificity in a mouse model of prenatal stress
BMC Genomics (2023)
-
Interaction of the pre- and postnatal environment in the maternal immune activation model
Discover Mental Health (2023)