Abstract
Two major types of leukemogenic BCR-ABL fusion proteins are p190BCR-ABLand p210BCR-ABL. Although the two fusion proteins are closely related, they can lead to different clinical outcomes. A thorough understanding of the signaling programs employed by these two fusion proteins is necessary to explain these clinical differences. We took an integrated approach by coupling protein–protein interaction analysis using biotinylation identification with global phosphorylation analysis to investigate the differences in signaling between these two fusion proteins. Our findings suggest that p190BCR-ABL and p210BCR-ABL differentially activate important signaling pathways, such as JAK-STAT, and engage with molecules that indicate interaction with different subcellular compartments. In the case of p210BCR-ABL, we observed an increased engagement of molecules active proximal to the membrane and in the case of p190BCR-ABL, an engagement of molecules of the cytoskeleton. These differences in signaling could underlie the distinct leukemogenic process induced by these two protein variants.
Similar content being viewed by others
Introduction
Chromosomal translocations involving the breakpoint cluster region (BCR) and Abelson murine leukemia viral oncogene homolog 1 (ABL) genes generate BCR-ABL, a potent oncogene that drives leukemia. Depending upon the exact breakpoint of translocation within the BCR gene, distinct fusion genes leading to different fusion proteins are generated. The most common translocations generate fusion protein products with molecular weights of 190 and 210 kD.1 These variants, designated as p190 and p210 (hereafter referred to as p190BCR-ABL and p210BCR-ABL), are associated with distinct clinical features.2 Generally, p190BCR-ABLleads to acute lymphoblastic leukemia (ALL), whereas the p210BCR-ABL variant leads to chronic myelogenous leukemia (CML). The two types of leukemia driven by these variants exhibit different treatment response to chemotherapy including tyrosine kinase inhibitors leading to distinct clinical outcomes.3, 4 The reasons for this discrepancy are still not fully understood.
BCR-ABL fusion genes code for proteins with constitutively active tyrosine kinase activity that mediate transformation by inducing signaling pathways that enhance cell growth and evade normal regulation. Despite numerous studies into the mechanisms underlying p190BCR-ABL and p210BCR-ABL induced leukemia, the fundamental questions of whether and how these proteins engage different signaling programs remain unanswered. The major structural difference between the two variants is the presence two domains commonly found in guanine nucleotide exchange factors (GEFs); a Dbl homology (DH) domain followed by a Pleckstrin homology domain (PH) in p210BCR-ABL. DH domains have catalytic GEF activity and PH domains facilitate intercellular targeting including plasma membrane binding. Ablation of the DH domain’s GEF activity or deletion of the PH domain results in phenotypes similar to p190BCR-ABL, suggesting that both domains may be essential for sustaining p210BCR-ABL-specific phenotypes.2, 5 Direct comparison of p190BCR-ABL and p210BCR-ABLin in vitro kinase activity assays has shown p190BCR-ABL to exhibit higher auto-phosphorylation, leading to the conclusion that p190BCR-ABL is likely a more active kinase.6, 7 Several studies have analyzed signaling pathways induced by BCR-ABL (reviewed in Hantschel,8 Cilloni and Saglio9) although, studies directly comparing signaling differences between p190BCR-ABL and p210BCR-ABL are more limited.2, 10, 11 A few studies have examined global phosphorylation events induced by either p190BCR-ABL or p210BCR-ABL,12, 13, 14 but to date no study has directly compared the differential signaling patterns induced by these two variants in the same cellular environment. Similarly, very little is known about the potential protein–protein interaction differences that mediate p190BCR-ABL and p210BCR-ABL-specific signaling. The p210BCR-ABL 'core' interactome has been previously cataloged15 but, to date, there are no reports of direct comparisons between p190BCR-ABL and p210BCR-ABL interactomes.
In order to test the hypothesis that p190BCR-ABL and p210BCR-ABL leverage different protein partners and induce different signaling programs, we decided to express these proteins in the same cell type. We chose an early hematopoietic progenitor model, the well-studied Ba/F3 cell line, to directly compare the differential signaling and protein–protein interactome of the two variants. We used the proximity dependent biotin identification (BioID) system to identify protein–protein interactions and phosphotyrosine (pTyr) peptide enrichment to identify site-specific tyrosine phosphorylation events on target proteins. An integrative analysis of the interactome and phosphorylation data allowed us to discover overlapping and differential signaling pathways. Specifically, we found an increased interaction of p210BCR-ABL with membrane proximal signaling proteins including ubiquitin-associated and SH3 domain-containing protein B (Ubash3b or Sts-1) and phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 1 (Inpp5d or SHIP1), with both proteins displaying increased phosphorylation in p210BCR-ABL-harboring cells. p190BCR-ABL, on the other hand, displayed increased interaction and phosphorylation of many cytoskeletal proteins, including members of the Wiskott–Aldrich syndrome protein family. In agreement with previous studies, we also observed differential p190BCR-ABL and p210BCR-ABL phosphorylation signatures of signal transducer and activator of transcription (Stat) proteins. In addition, our study provides evidence for differential phosphorylation of Stat proteins at the level of individual tyrosine phosphorylation sites, which could be mediated by differential interactions. Our findings reveal that p190BCR-ABL and p210BCR-ABL have both overlapping and different specificities for substrates, potentially interact with different subcellular compartments and mediate differential downstream signaling events that ultimately lead to different clinical outcomes.
Materials and Methods
Plasmids, cloning, antibodies and reagents
The mouse retroviral vector pMSCV modified with an IRES-GFP containing either the p190BCR-ABL or the p210BCR-ABL form of BCR-ABL was provided by Saradhi Mallampati and Xiaoping Sun. The plasmid pcDNA3.1 myc-BioID containing the mutant BirA-R118G was purchased through Addgene (Cambridge, MA, USA) (Plasmid #35700). BirA was cloned in-frame on the N-terminus of both p190BCR-ABL and p210BCR-ABL to generate BirA-R118G-p190BCR-ABL -IRES-GFP-pMSCV and BirA-R118G-p210BCR-ABL -IRES-GFP-pMSCV. A detailed description of the cloning procedure is provided in Supplementary Methods. SILAC amino acids were purchased from Cambridge Isotopes; heavy (lysine-13C6, 15N2 #CNLM-291, arginine-13C6, 15N4 #CNLM-539) and medium (lysine-13C4 #CLM-2247, arginine-13C6 #CLM-2265) labels were dissolved in arginine- and lysine-deficient RPMI 1640 medium, then sterile filtered. A complete list of antibodies used is provided in Supplementary Methods.
Peptide preparation and phosphopeptide enrichment
Complete description provided in Supplementary Methods.
BioID; biotinylated protein capture, on bead digestion, and Western blotting validation
Methods used for execution of the BioID experiment were an amalgamation of protocols from the Roux and Gingras groups16, 17 with slight modifications to both. Complete description provided in Supplementary Methods.
Liquid chromatography and tandem mass spectrometry analysis
Peptides from on-bead digests of streptavidin-captured biotinylated proteins, pTyr immunoprecipitation and total protein lysates were analyzed using a nanoflow UPLC system (Easy nanoLC 1200, Thermo Scientific, Grand Island, NY, USA) interfaced with Orbitrap Fusion Lumos mass spectrometer (Thermo Scientific) equipped with an EasySpray ion source. In total, 39 fractions from different BioID interactome enrichment experiments, anti-pTyr immunoprecipitation and total protein experiments were analyzed by mass spectrometry. Three replicates of pTyr enrichment experiments, 12 basic reverse phase liquid chromatography total protein fractions, 3 replicate streptavidin captures (run twice per replicate) comprised the experiments carried out in Ba/F3 cells. Similarly, 3 replicates of pTyr enrichments, 12 basic reverse phase liquid chromatography total protein fractions, 6 replicates of label-free streptavidin captures comprised experiments in multipotent progenitor (MPP) cells. A detailed description of the mass spectrometry methods used is provided in Supplementary Methods.
Mass spectrometry data analysis
Proteome Discoverer software suite (v 2.1; Thermo Fisher Scientific, Grand Island, NY, USA) was used for quantitation and database searches. The mass spectrometry (MS/MS) data were searched using the SEQUEST search algorithm against a Mouse RefSeq database (v73 containing 58 485 entries) supplemented with frequently observed contaminants. In addition, the BirA-p190BCR-ABL sequence, which represents the common sequence of p190BCR-ABL and p210BCR-ABL, and the p210BCR-ABL-specific sequence (DH and PH domain) were added to the database, as separate entries. The search parameters used for SEQUEST are listed in Supplementary Methods. The peptide spectral matches, peptides and proteins were filtered at 1% false discovery rate cut-off calculated using decoy database searches. After database searching, SILAC ratios of peptides were quantified with PyQuant.18 For label-free analysis of the MPP BioID experiment, MaxQuant software package with default settings was used.19
Post processing and bioinformatics
In-house python scripts (https://github.com/pandeylab/pythomics) were used for post-processing annotation, protein inference and normalization of the SILAC data. To correct for loading biases in the SILAC mixes in the pTyr experiments, we normalized the data based on the total protein measurements from 12 basic reverse phase liquid chromatography fractions. Total protein was normalized to the median and that correction factor was applied to all values in pTyr data. After normalization, the total protein SILAC ratios for all peptides mapping to individual proteins were grouped and the median value was calculated for protein level measurements. For pTyr data, SILAC ratios were maintained at the peptide level and grouped by site, and median values were calculated, SILAC ratios for pTyr values were adjusted based on the corresponding abundance value from the total protein data that the phosphosite mapped. Additional median normalization was applied to the p190BCR-ABL/p210BCR-ABL ratio as p190BCR-ABL cells were observed to have a slight increase in global phosphorylation compared to p210BCR-ABL cells in the Ba/F3 experiment, the opposite was observed in MPP experiment and was normalization similarly. Finally, when a phosphorylation site was sampled in all three replicates, the median was calculated, in the case of phosphorylation sites sampled in only two replicates, the average was taken. The SILAC BioID data followed a similar scheme although based on the ratios of known endogenously biotinylated proteins being near equally represented in the p190BCR-ABL/p210BCR-ABL cells, no normalization was applied to the data. Median values of replicates were calculated when sampled in all three replicates and average was used for sampling in two replicates. No correction was applied for abundance values differences from the total protein. BioID data went through a series of filtering steps to eliminate potential false-positive interactors. Specifically, we first compared all putative interacting proteins (irrespective of BCR-ABL variant) to a negative control streptavidin capture consisting of non-BirA Ba/F3 lysate to screen for endogenously biotinylated proteins. Next, we referenced the CRAPome database20 to identify and remove proteins with a higher likelihood of being non-specific in pull-down assays, to further filter potential non-specific protein interactors. Then proteins were filtered if they were not sampled in at least two of the replicates. We followed these initial filtering steps with STRING analysis,21 a tool to search interaction networks between proteins based on previous studies, on the remaining protein identities to identify those proteins with known interactions by biochemical evidence. This analysis revealed a major node comprising of many known BCR-ABL interactors, indicating the robustness of our assay in detecting these interactions. Our final set of protein interactors after the filtering was comprised of 90 proteins.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository with the data set identifier PXS005209.
Results
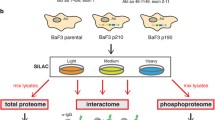
Both p190BCR-ABL and p210BCR-ABL are constitutively active kinases with similar molecular makeup that differ only by the presence (p210BCR-ABL) or absence (p190BCR-ABL) of a DH and PH domain (Figure 1a). Given the differences in clinical outcomes, we hypothesized that these two proteins display differential binding of partner proteins and phosphorylation signatures leading to distinct downstream signaling pathways. We therefore employed a two-pronged approach by expressing the variants in the same cellular background, the well-studied murine hematopoietic Ba/F3 cell line. The first part of our approach relied on detecting differential protein–protein interactions ('interactome') of these two proteins by using BioID coupled with mass spectrometry and SILAC-based quantitation (Figure 1b). The second part involved investigation of differential global phosphorylation signatures ('phosphoproteome') via pTyr peptide enrichment followed by mass spectrometry and SILAC-based quantitation22 (Figure 1c).
Strategy for investigating BCR-ABL interactome and phosphoproteome. (a) Domain structures of p190BCR-ABL and p210BCR-ABL are shown. The lack of DH and PH domain in the BCR part of the p190BCR-ABL variant is indicated by dashed lines. The biotin ligase, BirA, was cloned in-frame at the N-terminus of both variants as indicated. The domains shared by the two variants are coiled-coiled (CC), BCR serine/threonine kinase (S/T Kinase), Src homology 3 (SH3), Src homology 2 (SH2) Abl tyrosine Kinase (TyrK) and F-actin-binding domain (F-actin BD). (b) The experimental workflow for interactome analysis by BioID in Ba/F3 cells. Both BCR-ABL cell lines were grown in SILAC containing K4R6 (medium) or K8R10 (heavy) amino acids, exogenous biotin was added to both cultures as indicated and incubated for 24 h. The resulting lysates were subjected to streptavidin capture followed by trypsin digestion and liquid chromatography (LC)–MS/MS. (c) The experimental workflow for phosphoproteome analysis in Ba/F3 cells. Cells were grown in SILAC media containing K0R0 (light), K4R6 (medium) or K8R10 (heavy) amino acids. The parental line was cytokine starved and the cultures harvested, lysed, mixed and trypsin digested. A small fraction of the lysate was fractionated by basic reverse phase liquid chromatography (bRPLC) and analyzed by LC–MS/MS. The remaining peptides were immunoprecipitated with pY1000 antibody and subjected to LC–MS/MS analysis. (d) Western blot analysis with antibodies against Abl and β-actin showing the relative expression levels of the two variants in Ba/F3 cells. (e) Addition of BirA does not alter the ability of BCR-ABL to drive cytokine independence in Ba/F3 cells: flow cytometry plots monitoring GFP expression versus side scatter in Ba/F3 cells expressing BCR-ABL isoforms with or without BirA after retroviral transduction and post IL-3 withdrawal are shown.
Establishment of BioID system to examine BCR-ABL interactome in Ba/F3 cells
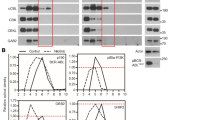
BioID can detect both transient interactions as well as more stable interactions. When the promiscuous biotin ligase (BirA) is cloned in-frame with a protein of interest, it biotinylates interacting proteins including those that are transiently bound, enabling detection of interacting proteins via enrichment by streptavidin.23 We cloned BirA onto the N-terminus of both p190BCR-ABL and p210BCR-ABL because we reasoned that this positions the BirA moiety most proximal to differing domains of p190BCR-ABL and p210BCR-ABL (Figure 1a). Relative expression levels of BirA-tagged p190BCR-ABL and p210BCR-ABL proteins were analyzed by immunoblot (Figure 1d). In agreement with previous studies in which expression of BCR-ABL in Ba/F3 cells induced IL-3 independence,24 the BirA-tagged versions of both p190BCR-ABL and p210BCR-ABL also induced cytokine independence (Figure 1e). In order to detect interacting proteins, these cells were cultured in the presence of exogenous biotin to induce in vivo labeling of BCR-ABL variant interactors. These cells were then lysed, mixed and three independent experiments were performed to enrich biotinylated proteins by streptavidin capture under denaturing conditions, followed by trypsin digestion. The resulting peptide mixture was then subjected to liquid chromatography–MS/MS analysis (Figure 1b). As expected, a substantial enrichment of biotinylated proteins as well as the capture of BCR-ABL variants was observed in the streptavidin isolates (Supplementary Figure 1a). A total of 814 proteins were identified by liquid chromatography–MS/MS analysis, of which 308 proteins were detected in all three replicates and their corresponding SILAC ratios are plotted in Figure 2a (Supplementary Table 1 for complete data). Additional streptavidin isolates were generated for immunoblot-based validation of targets identified in the mass spectrometry data (Figure 2b).
Interactome analysis of BCR-ABL variants using the BioID system. (a) A plot of SILAC ratios (log2 scale) for the detected proteins in Ba/F3 interactome analysis. Endogenously biotinylated proteins are shown. (b) Western blot validation of Ba/F3 mass spectrometry BioID data. Biotinylated proteins captured by streptavidin from the indicated cells were probed with antibodies against Cbl, Inpp5d/SHIP1, Stat1, Was/WASP and β-actin as shown in the right panel. The whole-cell lysate is also probed in parallel (left panel). (c) Heat map of select BCR-ABL-interacting proteins along with any observed enrichment in one or the other BCR-ABL isoform is shown. (d) Venn diagram of the BCR-ABL core interactome observed in our analysis of Ba/F3 and MPP cells. Within the green are overlapping interactors that were detected in both MPP and Ba/F3 cells. Interactors in white are either cell type-specific or not sampled in the cell type. Gene symbols for overlapping p190BCR-ABL-enriched and p210BCR-ABL-enriched interactions are underlined. (e) Western blot validation of MPP mass spectrometry BioID data. Biotinylated proteins captured by streptavidin from the indicated cells were probed with antibodies against Wipf1, Ubash3b, Stat1, Cbl and β-actin as shown in the right panel. The whole-cell lysate is also probed in parallel (left panel).
A common BCR-ABL interactome signature
First, we identified the overall BCR-ABL interactome within our BioID experiment by employing filtering steps to remove non-specific interactions and identify well-supported interactions using literature-based tools (see Materials and Methods section and Supplementary Figure 1b for depiction of STRING analysis). This analysis resulted in a subset of 90 proteins, whose SILAC ratios are represented in the heat map in Figure 2c. This subset includes many known BCR-ABL interactors including Nck adaptor protein 1 (Nck1), Nck adaptor protein 2 (Nck2), docking protein 1 (Dok1), tyrosine protein phosphatase non-receptor type 11 (Ptpn11), Son of sevenless homolog 1 and 2 (Sos1, Sos2), Abl interactor 1 (Abi1), Grb2-associated-binding protein 2 (Gab2) and pseudopodium-enriched atypical kinase (Peak1), among others. We also confirmed previously defined components of the BCR-ABL interactome15 in our analysis: growth factor receptor-bound protein 2 (Grb2), SHC-transforming protein 1 (Shc1), CRK proto-oncogene adaptor protein (Crk), Cbl proto-oncogene (Cbl), phosphoinositide 3-kinase regulatory subunit 2, Ubash3b and inositol polyphosphate phosphatase-like 1 (Inppl1 or SHIP2) (Figure 2d; Supplementary Table 1).
p190BCR-ABL and p210BCR-ABL display differential interactions with proteins in different cellular compartments
In order to characterize the differential interactome of p190BCR-ABL and p210BCR-ABL, we measured SILAC ratios of peptides from interacting biotinylated proteins captured from the two variants. We defined p190BCR-ABL-enriched interactions as proteins with SILAC ratios indicating >1.8-fold enrichment in p190BCR-ABL, whereas ratios indicating >1.8-fold enriched in p210BCR-ABL were defined as p210BCR-ABL-enriched interactors. We also defined those proteins displaying ratios that indicated >1.5-fold but <1.8-fold difference between the cell types as trending toward an enrichment of interaction. As a control we monitored the SILAC ratios of known endogenously biotinylated proteins commonly found in BioID experiments23 and those were not enriched in either cell type (Figure 2a). We then examined the differential interactors within the subset of 90 proteins identified after filtering steps. Many of the molecules identified to be enriched in their interactions with p190BCR-ABL are cytoskeletal proteins. Members of the Wiskott–Aldrich syndrome protein family, specifically, WASP, WAS/WASL-interacting protein family member 1 (Wipf1) and 2 (Wipf2), and WAS protein family member 2 (Wasf2) all displayed >1.8-fold increased association with p190BCR-ABL relative to p210BCR-ABL. Interestingly, BCR-ABL has been previously shown to interact with the cytoskeleton and has been implicated in inducing cytoskeletal-related phenotypes, although these alterations were not assigned to a specific variant.25, 26, 27, 28, 29 The differential interaction with Wipf1 identified through our quantitative mass spectrometry data were confirmed by immunoblot analysis of streptavidin-enriched isolates from p190BCR-ABL, p210BCR-ABL and parental (as a negative control) cell lysates (Figure 2b). Adaptor proteins, such as Nck1, Nck2 and intersectin 2, also known as SH3p18-like WASP-associated protein (Itsn2), are not explicitly cytoskeletal but have been shown to mediate cytoskeleton signaling pathways, also displayed an increased interaction with p190BCR-ABL in our analysis. Finally, signal-induced proliferation-associated protein 1 (Sipa1) and signal-induced proliferation-associated 1-like protein 1 (Sipa1l1), also modulators of the cytoskeleton, exhibited a modest preferential interaction with p190BCR-ABL. Sipa1 has been previously shown to interact with BCR-ABL and displays co-localization to uropod actin structures of migrating cells, suggesting that BCR-ABL is likely found at these structures.30 Here, we show that these important cytoskeletal re-modeling factors preferentially interact with the p190BCR-ABL variant.
In contrast to the findings for p190BCR-ABL, many of the proteins that displayed increased association with p210BCR-ABL (Figure 2c) are plasma membrane proximal proteins or display an increased activity at the plasma membrane. For example, Ubash3b, a membrane proximal adapter protein with phosphatase activity that targets Src and Syk family kinases and regulates plasma membrane-bound receptor tyrosine kinases,31, 32 was found to be 15-fold enriched in its interaction with p210BCR-ABL compared to p190BCR-ABL. Ubash3b is also a known interactor and negative regulator of Cbl31 and, intriguingly, Cbl and its homolog Casitas B-lineage lymphoma b (Cblb), were both enriched for p210BCR-ABL interaction in our analyses (seven-fold and four-fold, respectively). Cbl has previously been shown to form a complex with BCR-ABL and mediate the degradation of SHIP1 via polyubiquitination.33, 34 Our data confirm the downregulation of SHIP1 upon expression of BCR-ABL, (compare inputs of both variant expressing cells to the parental line in Figure 2b). Moreover, in addition to SHIP1, total protein levels of Cbl and Cblb are also downregulated compared to parental cells. Interestingly, the levels of SHIP1/Cbl/Cblb are downregulated in p190BCR-ABL to a greater extent than p210BCR-ABL suggesting that both forms are engaged in this complex but p190BCR-ABL might be mediating increased degradation of SHIP1 compared to p210BCR-ABL. The preferential interaction of Cbl and SHIP1 with p210BCR-ABL along with assessment of their protein abundance was confirmed by immunoblotting streptavidin isolates and whole-cell lysates (Figure 2b). A combination of higher total protein levels of SHIP1 in p210BCR-ABL cells and the presence of the p210BCR-ABL-enriched interaction of Ubash3b could also play an inhibitory role in the degradation SHIP1. Ubash3b is a ubiquitin binding protein and could be binding to ubiquitinated SHIP1, thus attenuating its degradation. Other membrane proximal proteins identified to be enriched in interaction with p210BCR-ABL include the phosphatase SHIP2 and adaptor proteins Crk and Shc1.
Finally, our data suggest that p190BCR-ABL and p210BCR-ABL engage with Stat proteins differently. For example, Stat1 showed a two-fold enrichment in p190BCR-ABL-specific interactions, which was confirmed by immunoblotting (Figure 2b). In addition, both Stat5 and Stat3 were found to be enriched (2.5-fold and 1.5-fold) in p210BCR-ABL-mediated interactions. Importantly, previous studies have indicated that Stat5 is a direct substrate of BCR-ABL.35
p190BCR-ABL and p210BCR-ABL interaction analysis in MPP cells
In an effort to confirm our findings, we interrogated p190BCR-ABL and p210BCR-ABL interaction differences in an alternate cell type. We chose an ex vivo expanded primary murine hematopoietic progenitor cell derived from early B-cell factor 1 (Ebf1) null fetal livers. These cells exhibit a 'multi-potent progenitor' phenotype and have been shown to be an excellent model for hematopoiesis prior to B-cell specification as these cells can be induced in culture to differentiate toward the B, T and myeloid lineage.36, 37, 38, 39 The p190BCR-ABL and p210BCR-ABL interaction analysis in MPP cells is diagramed in Supplementary Figure 2a. Interrogating the MPP cells expanded our experimentally derived BCR-ABL interactome and the overlap with the Ba/F3 experiment is shown in Figure 2d. Many of the same molecules were identified in the interactome analysis in both cell types and these analyses also uncovered some potentially cell type-specific interactions (Figure 2d). Importantly, many of the specific differential interactions observed in the Ba/F3 cells were also observed in these cells. For example, the preferential interaction of Dok1, WASP, Wipf1, Stat1 and Nck1 with p190BCR-ABL found in the MPP cells was in agreement with the Ba/F3 experiment, although the enrichment for these molecules was not as pronounced as was observed in Ba/F3 cells, perhaps due to cell type-specific differences or due to the sensitivity of the label-free quantitation used in MPP experiments. In order to confirm these interactions, we measured Wipf1, Stat1 and Ubash3b levels by subjecting the streptavidin isolates to immunoblotting and these data highlighted a clear p190BCR-ABL skewed interaction in MPP cells (Figure 2e). Cbl and Cblb, which had shown robust increased interactions with p210BCR-ABL relative to p190BCR-ABL in Ba/F3 cells, were found to also display specific differential interactions in the MPP experiment, although, again, with more modest enrichments (Figure 2e; Supplementary Table 2). As in the experiments in the Ba/F3 system, p210BCR-ABL expressing MPPs displayed increased interactions with SHIP1, Ubash3b, SHIP2, Stat5 and Shc1 relative to p190BCR-ABL.
Global phosphorylation signatures of p190BCR-ABL and p210BCR-ABL in Ba/F3 cells
It is possible that increased interaction of the p190BCR-ABL and p210BCR-ABL variants with a specific set of proteins leads to the increased phosphorylation of those targeted proteins to induce unique signaling through downstream pathways. To identify and quantify pTyr sites that are induced or altered in either p190BCR-ABL or p210BCR-ABL harboring cells, we employed a three-state SILAC strategy. Specifically, we isolated lysates from Ba/F3 cells harboring BirA-tagged versions of p190BCR-ABL and p210BCR-ABL (as used in the BioID experiments) and, as a control for basal phosphorylation levels, from Ba/F3 cells containing no exogenous protein. Not surprisingly, global phosphorylation was observed to be higher in cells ectopically expressing the constitutively active kinases, p190BCR-ABL and p210BCR-ABL relative to the parental cells. This was observed when lysates were enriched for pTyr-containing proteins by immunoprecipitation with a pTyr-specific antibody (Figure 3a). Three technical replicates of pTyr enrichments were subjected to liquid chromatography–MS/MS and subsequent analyses yielded quantitation of 1568 pTyr sites on 965 proteins, 746 sites of which were sampled in all three replicates. We measured phosphorylation ratios by calculating the median of replicate SILAC ratio p190BCR-ABL/p210BCR-ABL values of sites (all SILAC ratios are plotted in Figure 3b and all data is provided in Supplementary Table 3). Phosphosites displaying ratio values greater than two-fold enriched in p190BCR-ABL were defined as p190BCR-ABL hyperphosphorylated, whereas values with two-fold enriched in p210BCR-ABL were defined as p210BCR-ABL hyperphosphorylated. We also define those sites displaying ratios that indicate >1.5-fold but less than two-fold difference between the cell types as trending toward hyperphosphorylation. For a select number of phosphorylation sites, the mass spectrometry results were confirmed by immunoblotting with tyrosine phosphorylation-specific antibodies (Figure 3c).
Tyrosine phosphorylation analysis of BCR-ABL variants. (a) Tyrosine phosphorylated proteins were immunoprecipitated from whole-cell lysates (WCL) of parental, p190BCR-ABL and p210BCR-ABL Ba/F3 cells using anti-phosphotyrosine antibody and probed with the same antibody as indicated. (b) Relative abundance of normalized tyrosine phosphopeptides based on their SILAC ratios (log2 scale) in p190BCR-ABL and p210BCR-ABL expressing Ba/F3 cells. Selected proteins are labeled by name and corresponding phosphosite. (c) Validation of tyrosine phosphorylation by Western blot analysis in Ba/F3 cells. All antibodies used were phosphosite specific with the exception of Ubash3b (in which case anti-phosphotyrosine antibody was used for the IP). Antibodies against the proteins were used to detect the overall protein expression in each case. (d) A list of notable tyrosine containing peptides hyperphosphorylated in either p190BCR-ABL or p210BCR-ABL Ba/F3 cells. The sites are grouped into categories of protein function; GEFs and GAPs, cytoskeletal, membrane-associated proteins, adapters and kinases/phosphatases. (e) Plot of SILAC ratio values of MPP, the p190BCR-ABL/p210BCR-ABL versus Ba/F3 the p190BCR-ABL/p210BCR-ABL. Selected proteins are labeled by name and corresponding phosphosite.
Cytoskeleton proteins hyperphosphorylated in p190BCR-ABL expressing cells
Strikingly, in very strong agreement with our analysis of p190BCR-ABL-skewed protein–protein interactions, we identified many sites on cytoskeletal proteins and cytoskeletal signal transduction pathway members to be hyperphosphorylated in response to p190BCR-ABL expression (Figure 3d). The hyperphosphorylation of the Wiskott–Aldrich syndrome family member proteins WASP, its homolog Neural Wiskott–Aldrich syndrome protein (Wasl or N-WASP) and their interacting proteins Wipf1/2 are of particular interest as we also observed WASP and Wifp1 to be enriched in p190BCR-ABL interaction. This combination of interaction and hyperphosphorylation not only implicates Wiskott–Aldrich syndrome family members as direct substrates of p190BCR-ABL but also further suggests that cytoskeletal pathways are being activated differently in p190BCR-ABL-expressing cells compared to p210BCR-ABL, as these proteins are potent effectors of these pathways.40 The specific function of the sites on the Wiskott–Aldrich syndrome family members observed to be p190BCR-ABL hyperphosphorylated further support the activation of this pathway. For example, sites Tyr293 and Tyr253 on WASP and N-WASP, respectively, are essential for the activation of both molecules.40 WASP-Tyr293 hyperphosphorylation was confirmed by immunoblotting with a phospho-specific antibody and shown in Figure 3c. Activated N-WASP is essential for actin assembly and, via recruitment of Nck1 and Grb2, promotes actin-based movements.41 In agreement with the previous finding, we observed hyperphosphorylation of many sites on Nck1, Nck2 and Grb2 in p190BCR-ABL-expressing cells. Both Nck1 and Nck2 were also identified as preferentially interacting with p190BCR-ABL, suggesting that this BCR-ABL variant is co-located with all these proteins. The protein complex of Nck1/Grb2/Wiskott–Aldrich syndrome family members has been shown to be mediated by Abl-dependent phosphorylation of Tyr361 on Dok1. Phosphorylation of Dok1 recruits Nck1 to interact with Wiskott–Aldrich syndrome family members, which initiates actin polymerization and filopodia in mouse embryonic fibroblasts.42 Remarkably, we identified nine tyrosine sites, including Tyr361, on Dok1 that are hyperphosphorylated in p190BCR-ABL-expressing cells, further implicating enriched association of p190BCR-ABL with the cytoskeleton compared to p210BCR-ABL. Importantly, Dok1 trended toward an increase of p190BCR-ABL interaction in the interactome data. Taken together, the combination of preferential interaction and hyperphosphorylation of key residues of Wiskott–Aldrich syndrome family members, Nck1/2, Grb2 and Dok1 suggest that p190BCR-ABL is profoundly influencing the cytoskeleton.
Phosphorylation signatures of p210BCR-ABL-expressing cells
p210BCR-ABL displayed preferential protein–protein interactions with some important membrane proximal proteins including Ubash3b, SHIP2, Cbl, Cblb and SHIP1. In agreement with this p190BCR-ABL/p210BCR-ABL differential interaction, we also observed differential phosphorylation signatures indicating that the variants are exerting increased influence on these proteins and pathways. In general, increased p210BCR-ABL interaction was coupled with increased phosphorylation. For example, a novel pTyr site on Ubash3b (Tyr9) was observed to be hyperphosphorylated in p210BCR-ABL cells. To validate this novel site, we used an anti-pTyr antibody to isolate all pTyr proteins in the cells, and followed this immunoprecipitation with immunoblotting for Ubash3b proteins (Figure 3c). A direct comparison of total protein versus pTyr enriched, shows that Ubash3b is indeed more highly phosphorylated in p210BCR-ABL-expressing cells (compare total protein to anti-pTyr enriched) leading us to conclude that Ubash3b is more active in p210BCR-ABL-expressing cells. Other examples of concurrent p210BCR-ABL interaction and hyperphosphorylation were found in SHIP1 and SHIP2. As for SHIP1 phosphorylation we observed p210BCR-ABL hyperphosphorylation on the sites Tyr917 and Tyr1020. The SHIP1 sites Tyr917 and Tyr1020 are interesting because Dok1/Shc1 compete for binding at Tyr1020.34, 43, 44 This might be noteworthy because, in our BioID analysis, Shc1 is favored by p210BCR-ABL and Dok1 by p190BCR-ABL, implicating some interesting differential interplay between Shc1/Dok1/SHIP1 with respect to the two BCR-ABL variants. SHIP2 showed a robust increase in phosphorylation on Tyr1161 (2.4-fold enrichment over p190BCR-ABL-expressing cells) and with additional phosphosites Tyr986, Tyr835 and Tyr662 all trending toward enrichment (>1.5-fold) in p210BCR-ABL-expressing cells. The site Tyr1136 (Tyr1135 in human) on SHIP2 showed a reciprocal relationship between interaction and phosphorylation as it was observed to be hyperphosphorylated in p190BCR-ABL-expressing cells. Phosphorylation of this site has previously been shown to translocate SHIP2 to focal adhesions,45 which further implicates a connection of p190BCR-ABL to the cytoskeleton and suggests that the two variants use SHIP2 in different ways to promote their unique signaling programs. Cbl and Cblb represent another example against the general trend of increased interaction leading to increased phosphorylation. Specifically, Cbl and Cblb display a shared phosphopeptide that is hyperphosphorylated in p190BCR-ABL cells (where the interaction is less). These sites, Tyr369/363 (Tyr371/363 in human) increase the ubiquitin ligase activity of Cbl when phosphorylated.46 This observation has particularly intriguing implications for polyubiquitination/degradation of SHIP1 by Cbl/BCR-ABL and could explain in part why SHIP1 is less abundant in p190BCR-ABL- expressing cells due to increased degradation.
The rhoGEF domain in p210BCR-ABL has been shown to be constitutively activated and mutations that disrupt the activity of this domain decrease the transforming potential of the oncogene.47 We wanted to investigate if any G-protein signaling pathway-related proteins were differentially regulated by phosphorylation, which could identify those potentially working in concert with p210BCR-ABL to produce specific rhoGEF pathway signaling programs. We did observe many tyrosine sites on GEFs and GTPase-activating proteins (GAPs) that were hyperphosphorylated in p210BCR-ABL cells (Figure 3d). For example, Arf-GAP with a rho-GAP domain, ANK repeat and PH domain-containing protein 1 and 3 (Arap1/CENTD2 and Arap3/CENTD3), Arf-GAP with coiled coil, ANK repeat and Acap1, rho GEF6 (Arhgef6), rho GDP-dissociation inhibitor 1 (Arhgdia) and proto-oncogene Vav1 (Vav1) are all hyperphosphorylated.
Differential regulation of Stat proteins
Previous work using immunoprecipitation and immunoblotting in the Ba/F3 system has revealed that Stat family members are differentially regulated by p190BCR-ABL and p210BCR-ABL.2, 11 In one study, Stat1 and Stat6 were found to show higher phosphorylation in p190BCR-ABL expressing Ba/F3 cells, whereas Stat5 phosphorylation was higher in p210BCR-ABL expressing Ba/F3 cells and Stat3 exhibited no change in phosphorylation levels. To confirm and extend this observation, which was limited by available antibodies and only detected overall levels of phosphorylation, we interrogated our differential pTyr phosphorylation data to determine differences at specific sites of phosphorylation induced by the BCR-ABL variants (Table 1). We identified a total of nine sites on Stat family proteins (Stat1, Stat2, Stat3, Stat5 and Stat6), six of which displayed hyperphosphorylation in either p190BCR-ABL or p210BCR-ABL-expressing cells. We confirmed several of these sites, identified in our mass spectrometry data, by immunoblotting extracts from p190BCR-ABL or p210BCR-ABL-expressing cells using commercially available phosphorylation site-specific antibodies (Figure 3c). These data confirm that sites Tyr701 in Stat1 and Tyr641 in Stat6 are hyperphosphorylated preferentially in p190BCR-ABL cells. Phosphorylation of Tyr701 and Tyr641, located just outside the SH2 domain, is required for dimerization, nuclear translocation and DNA-binding activity of both Stat1 and Stat6.48, 49 A similar site Tyr694, as well as neighboring sites Tyr668 and Tyr682/3 in the SH2 domain, in Stat5a/b showed no change by mass spectrometry or immunoblotting. However, in p210BCR-ABL-expressing cells, we identified an increase of phosphorylation at Tyr90 and Tyr114, which is located near the DNA-binding domain of Stat5a/b. Although the exact role of phosphorylation at Tyr90 and Tyr114 is not well understood, these tyrosine residues do reside within the tetramerization domain, which is important for protein–protein interactions and is involved in altering local chromatin structures upon DNA binding.50 Stat3 also had an N-terminal (Tyr45) phosphosite hyperphosphorylated in p210BCR-ABL cells similar to the pattern observed in Stat5. These data support that the Stat proteins are differentially engaged by the BCR-ABL variants, and we identify the differential and specific residues through which these proteins are inducing this pathway.
Correlation of global phosphorylation signatures between p190BCR-ABL and p210BCR-ABL in the MPP and Ba/F3 cells
Next, we chose to interrogate phosphorylation signatures in another cellular background as a means of biological validation. Just as we did with our interactome analysis, we leveraged BCR-ABL variant expressing MPP cells. Experimental workflow for phosphorylation analysis in MPP cells is shown in Supplementary Figure 2b. The correlation of p190BCR-ABL/p210BCR-ABL phosphorylation changes in Ba/F3 and MPP cells is plotted in Figure 3e and all MPP phosphorylation data are listed in Supplementary Table 4. Molecules with sites that showed the same trend of phosphorylation in both cell types, and were also enriched among p210BCR-ABL interactors were Ubash3b (Tyr8), SHIP1 (Tyr918/Y886), Crk (Tyr108) and Shc1 (Tyr423). We also observed the hyperphosphorylation of the ubiquitin ligase-activating site Tyr369/363 (discussed above) on Cbl/Cblb in MPP p190BCR-ABL cells. As for phosphorylation signatures of proteins enriched for p190BCR-ABL interaction, we observed similar hyperphosphorylation of WASP, N-WASP, Wipf2, Itsn2, Dok1 and Nck1 in MPP as Ba/F3 cells further implicating p190BCR-ABL specificity for these molecules.
In addition to directly phosphorylating interacting proteins, BCR-ABL induces downstream signaling pathways as well. Many downstream tyrosine kinases were observed in both Ba/F3 and MPP cells to be differentially phosphorylated between p190BCR-ABL and p210BCR-ABL, which suggests that the two variants could be collaborating with other kinases to induce unique signaling programs. Sites on kinases such as tyrosine protein kinase Lyn (Lyn), tyrosine protein kinase Csk (Csk), tyrosine protein kinase Tec (Tec), tyrosine protein kinase Syk (Syk) and tyrosine kinase non-receptor 2 (Tnk2) were observed to be differentially phosphorylated in p190BCR-ABL and p210BCR-ABL cells (Figure 3e). Tyr265, located near the N-terminal side of the kinase domain in Lyn, was hyperphosphorylated in p210BCR-ABL cells in both cellular backgrounds and Tyr193/194 located within in the SH2 domain was conversely hyperphosphorylated in p190BCR-ABL cells. The p190BCR-ABL hyperphosphorylation at residues Tyr64 and Tyr18 of Csk, a regulator of Src family kinases, could indicate increased activation.
The integration of interactome and phosphorylation data highlights differently regulated molecules by p190BCR-ABL and p210BCR-ABL
To better characterize the findings from both analyses, we integrated the differential interactome and differential phosphoproteome data (Figure 4). Common protein interactors represent molecules that display similar association with p190BCR-ABLand p210BCR-ABL, as determined by BioID and SILAC-based mass spectrometry analysis. These interactions likely represent general BCR-ABL interactions, as many of these same proteins are hyperphosphorylated in both BCR-ABL variant-expressing cells, as compared to cells not expressing any BCR-ABL protein (Supplementary Table 2). Strikingly, overlaying the differential interactome and phosphoproteome data reveals a general agreement between increased interaction and increased phosphorylation demonstrating the power of this approach (Figure 4). For instance, in p210BCR-ABL cells, SHIP2, SHIP1, Ubash3b, Pik3ap1 and Crk have concurrent enrichment for interaction and phosphorylation. Similarly, in p190BCR-ABL cells, WASP, Wipf1, Nck1, Dok1, Itsn2 and DAB2-interacting protein (Dab2ip) have concurrent enrichment for interaction and phosphorylation. The large majority of the interactors that are not enriched for either variant have near equal phosphorylation signatures. An interesting group of molecules are those that appear to be equal interactors yet have differential p190BCR-ABL/p210BCR-ABL phosphorylation signatures, such as the kinase leucine-rich repeat kinase 1 (Lrrk1) and the focal adhesion localized protein Zyxin (Zyx). Another interesting group of molecules are those that show modest interaction preference to one variant and have increased phosphorylation in the same direction. Examples include the Arap1 (a GEF protein discussed above), the non-receptor tyrosine kinase Peak1 and cytokine-dependent hematopoietic cell linker (Clnk), which is involved in the regulation of the B-cell antigen receptor (Figure 4). Interestingly, in the MPP BioID experiment Clnk was found to be two-fold enriched in p190BCR-ABL interaction which highlights the utility of combining both interaction and phosphorylation data to identify differentially regulated proteins.
Integrative analysis of p190BCR-ABL and p210BCR-ABL phosphorylation, and interactome signatures. Interacting proteins of p190BCR-ABL and p210BCR-ABL are represented by circles and are placed from left to right based on their SILAC ratios in the interactome analysis. A three-color gradient represents the degree of enrichment, with blue representing an enrichment with p210BCR-ABL interactions, yellow representing an enrichment with p190BCR-ABL and green representing equal interactions between the two variants. Circles are scaled based on the number of peptide spectral matches in the interactome experiment, which is suggestive of the relative strength of the interactions. Arrows point toward boxes containing tyrosine sites corresponding to proteins found in interaction analysis and the phosphorylation signature observed, with respect to p190BCR-ABL /p210BCR-ABL. Blue dots are used to denote at least greater than two-fold increase in phosphorylation enrichment in p210BCR-ABL cells, whereas yellow dots represents at least greater than two-fold increase in phosphorylation in p190BCR-ABL cells. Lighter shades of blue and yellow dots indicate >1.5-fold enriched in each cell type. Green dots signify no phosphorylation change between p190BCR-ABL or p210BCR-ABL cells.
In a multipotent background p190BCR-ABL and p210BCR-ABL regulates total protein levels of important hematopoietic drivers differently
Differential signaling programs employed by either p190BCR-ABL or p210BCR-ABL likely lead to differences in the regulation of the total proteome. In an effort to identify proteins whose abundance was differentially regulated as a result of p190BCR-ABL or p210BCR-ABL expression, we examined the total proteome data from Ba/F3 and MPP experiments (data is listed in Supplementary Tables 5 and 6, respectively). Notably, in the MPP experiment, we observed a number of important hematopoietic development/leukemia-related proteins that were differentially expressed between p190BCR-ABL and p210BCR-ABL. In p190BCR-ABL MPP cells, many hematopoietic developmentally regulated cell surface markers were upregulated in comparison to p210BCR-ABL cells. These included CD14, CD81, CD9, CD166 and CD44. An important driver of B-cell lymphopoiesis, transcription factor E2-alpha (E2A/Tcf3), was upregulated in p190BCR-ABL MPP cells. E2A is essential for initiating differentiation of uncommitted hematopoietic progenitors to pro-B cells and when translocated with the PBX1 gene is a potent driver of a subset of acute lymphoblastic leukemia.51 Notable proteins upregulated in p210BCR-ABL MPP cells are listed in Table 2, which includes the transcription factors AT-rich interactive domain-containing protein 3A (Arid3a) and runt-related transcription factors 1 and 3 (Runx1 and Runx3, respectively). Aberrant Arid3a expression levels have been shown to alter normal hematopoiesis, as overexpression of Arid3a results in inhibition of maturation of myeloid lineages, whereas downregulation of Arid3a results in decreased B-cell production.52 Both Runx1 and Runx3 have important roles in hematopoiesis and myeloid leukemia.53, 54 Myeloperoxidase, a common diagnostic marker differentiating myeloid from lymphoid leukemia was enriched in p210BCR-ABL MPP cells. Cell surface markers CD48, CD82 and the hematopoietic stem cell marker CD34 were found to be enriched in p210BCR-ABL MPP cells.
Discussion
Although BCR-ABL signaling has been intensively investigated for many years, a direct and systematic comparison of p190BCR-ABL and p210BCR-ABL signaling differences has never been undertaken. In this study, we directly compared p190BCR-ABL and p210BCR-ABL protein–protein interactions and global protein phosphorylation signatures in homogenous cell culture systems that model early hematopoietic differentiation. Our observations indicate many differences between p190BCR-ABL and p210BCR-ABL in signaling pathway regulation.
In 1996, Ilaria and Etten,11 later confirmed in part by Druker and colleagues in 2009,2 revealed one of the first pathways to be differentially regulated by p190BCR-ABL and p210BCR-ABL. Both groups use immunoprecipitation and anti-pTyr immunoblotting to measure the total phosphorylation status of Stat proteins induced by both variants. However, these analyses lacked tyrosine site-specific resolution, which we now provide. Our site-specific analyses provide a deeper understanding of the nature of the differential regulation of Stat proteins. Specifically, our analysis identified p190BCR-ABL/p210BCR-ABL differential regulation of sites within the trans-activating domains of Stat proteins, that when phosphorylated, lead to dimerization and subsequent DNA binding. Our interaction analysis leads us to conclude that these differential phosphorylation signatures are likely mediated by differential interactions between Stat proteins and the two variants. Stat proteins have a major role in normal hematopoiesis—for example, when Stat1 is deleted in mice, B lymphoid development is significantly reduced.55 This is potentially relevant to the p190BCR-ABL interaction and phosphorylation signatures observed in our study as p190BCR-ABL could be engaging Stat1 differently than p210BCR-ABL to drive the associated B-cell phenotype. Furthermore, Stat proteins have been revealed to be incredibly important for BCR-ABL-mediated leukemogenesis. Specifically, both Stat3 and Stat5, found to exhibit preference for p210BCR-ABL interaction, are indispensable for the initiation of p210BCR-ABL leukemia and Stat5 is necessary for maintaining chronic myelogenous leukemia.56, 57 In addition, Janus kinase 2 (Jak2), the upstream kinase targeting Stat5, has also been shown to be required for p190BCR-ABL to initiate leukemic transformation, whereas Jak2 is dispensable for p210BCR-ABL transformation35 suggesting that p190BCR-ABL/p210BCR-ABL differential activation of Stat proteins is necessary for different phenotypes. This is particularly relevant to our findings as the preferential direct interaction of Stat5 with p210BCR-ABL could circumvent the need of Jak2, where p190BCR-ABL requires an intermediary, Jak2, for leukemia initiation because it lacks this robust Stat5 interaction. We conclude Stat protein differential interaction, and potentially activation, associated with p190BCR-ABL and p210BCR-ABL appears to play a role in how the two variants drive leukemia, in particular the apparent lineage preference (Acute myeloid leukemia versus chronic myelogenous leukemia), and certainly warrants further investigation.
Importantly, our analyses uncovered a potential differential subcellular localization as a feature of p190BCR-ABL and p210BCR-ABL unique signaling programs (Figure 5). Because p190BCR-ABL lacks the PH domain, which mediates interactions with the plasma membrane, it may be free to associate more with the cytoskeleton. In support of this, we observed increased interaction of p190BCR-ABL with cytoskeletal modifiers such as Wiskott–Aldrich syndrome family members, hyperphosphorylation of many cytoskeletal proteins as well as others such as Dok1, Nck1, Nck2, Grb2 and SHIP2 that are involved in cytoskeletal reorganization.41, 42 Differential interaction with cytoskeleton has previously been implicated in studies showing that p190BCR-ABL and p210BCR-ABL induced distinctly different cellular motilities.58, 59 Strikingly, in support of our findings, previous data provided evidence that deletion of the C-terminal F-actin-binding domain in p190BCR-ABL reduced its oncogenicity, whereas the same deletion in p210BCR-ABL appeared dispensable for establishing leukemia.26, 27
Model of differential p190BCR-ABL and p210BCR-ABL signaling. Interaction, determined by BioID interactome analysis, is by close proximity of ellipses. Yellow coloring indicates proteins that are enriched in p190BCR-ABL interaction and blue coloring denotes p210BCR-ABL-enriched interaction. Green/brown coloring indicates no detected interaction in BioID but are placed near p190BCR-ABL based on their p190BCR-ABL hyperphosphorylation signature. p210BCR-ABL is shown in dark blue to be anchored to the plasma membrane by the PH domain. p190BCR-ABL is shown in orange to be anchored to the cytoskeleton by the F-acting-binding domain interacting with molecules therein. Dotted lines with arrowheads refer to molecular functions observed in previous studies. Line with semicircle arrowhead represents the ubiquitin-binding function of Ubash3b. Ubiquitin modifications (UB) are represented by green circles.
In contrast to an apparent preference of p190BCR-ABL with cytoskeletal components and regulators, p210BCR-ABL, which contains the PH domain, tends to interact with molecules that suggest its localization to be more proximal to the plasma membrane (Figure 5). We identified p210BCR-ABL-enriched interaction with Cbl, Cblb, SHIP1, SHIP2, Shc1 and Ubash3b, which are all involved in the regulation of plasma membrane-bound receptors.31, 60, 61 Ubash3b is a particularly interesting molecule that could be playing some role in attenuating certain functions of p210BCR-ABL, including SHIP1 degradation by inhibiting Cbl and binding to ubiquitinated proteins. Moreover, Ubash3b has been shown to have phosphatase activity toward some Src and Syk family tyrosine kinases32, 62 and we found evidence of dysregulation of both Lyn and Syk in our analysis. Src kinases have been shown to be required for the establishment of BCR-ABL-positive B-acute lymphoblastic leukemia, whereas being dispensable for chronic myelogenous leukemia establishment in mouse models, stressing the importance of our data describing the p210BCR-ABL-specific preference for interactions with and regulation of Ubash3b and its downstream regulation of Src kinases.63
Another interesting level of differential p190BCR-ABL/p210BCR-ABL regulation was found at the level of total proteome control. In the MPP experiment we found a surprising amount of developmental- and leukemia-related proteins differentially regulated in p190BCR-ABL and p210BCR-ABL-expressing cells. Particularly, the presence of the myeloid/myeloid neoplasm centric proteins such as Runx1, Runx3 and myeloperoxidase associated with p210BCR-ABL cells and the presence of B-cell centric proteins such as Tcf3 and Pbxip1 associated with p190BCR-ABL cells is interesting. The presence of CD34 on p210BCR-ABL and not p190BCR-ABL MPP cells is perhaps consistent with the observation that p190BCR-ABL requires a B-cell differentiation event from the stem cell compartment to establish leukemia, whereas p210BCR-ABL leukemogenesis continually originates from stem cell compartment, in mouse models.64
Reckel et al.65 have reported a similar study in this issue where they also examine differential signaling by p190BCR-ABL and p210BCR-ABL. Both studies have identified many of the same molecules to be differentially regulated by p190BCR-ABL and p210BCR-ABL, underscoring the reproducibility of global proteomic approaches to study signaling pathways in two different laboratories. However, there are also some minor differences that we believe are attributable to two broad areas—technical and biological. The methods employed by each group had some differences; for example, different phosphopeptide enrichment strategies, mass spectrometers, search algorithms, quantification methods and methods for interactome analysis (BioID versus affinity purification) were used. Of the two approaches used for interactome analysis, the BioID system is likely to detect direct interactions and might allow for detection of more transient interactions, whereas affinity purification methods might favor stronger biochemical interactions. Thus, taken together, both approaches to detect protein-protein interactions are somewhat complementary, as each method has its own biases for particular subsets of interactions, as has been previously shown.17 An important contributing factor to biological variation observed between the two studies is likely the relative expression level of the two variants in Ba/F3 cells.
Regardless of the differences, the two studies were both able to highlight many of the same molecules and pathways, which have major relevance to understanding BCR-ABL biology. Most prominent of which are the identification of p210BCR-ABL-specific interaction of Ubash3b/Sts-1 by both affinity purification and BioID, differential regulation of members of the STAT and Src families, and the association of p190BCR-ABL with cytoskeletal elements, leading to a general conclusion that the variants may exhibit different subcellular localization or enrichment. The differential phosphorylation signatures of Stat and Src family members are particularly attractive, as inhibition of these molecules could be further evaluated in the context of current tyrosine kinase inhibitor-based therapies targeting BCR-ABL, a strategy that would be especially important in the treatment of BCR-ABL-positive ALL.
In summary, we have hypothesized that the disparate clinical and experimental phenotypes associated with the BCR-ABL variants likely originate from inherent differences in signaling. Our findings in Ba/F3 and MPP cell lines represent a first glimpse into the complexity of how BCR-ABL variants differentially interface with hematopoiesis and drive leukemogenesis via their unique signaling programs. We hypothesize that understanding how p190BCR-ABL and p210BCR-ABL differently regulate key signaling molecules will help explain the lineage preferences and lead to new therapeutic inroads. Our data provide a clear set of differently regulated molecules to test in specific hematopoietic stages and in more clinically directed assays.
References
Score J, Calasanz MJ, Ottman O, Pane F, Yeh RF, Sobrinho-Simões MA et al. Analysis of genomic breakpoints in p190 and p210 BCR-ABL indicate distinct mechanisms of formation. Leukemia 2010; 24: 1742–1750.
Demehri S, O’Hare T, Eide CA, Smith CA, Tyner JW, Druker BJ et al. The function of the pleckstrin homology domain in BCR-ABL-mediated leukemogenesis. Leukemia 2009; 24: 226–229.
Foà R, Vitale A, Vignetti M, Meloni G, Guarini A, Propris MSD et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia. Blood 2011; 118: 6521–6528.
Gurion R, Raanani P, Vidal L, Leader A, Gafter-Gvili A . First line treatment with newer tyrosine kinase inhibitors in chronic myeloid leukemia associated with deep and durable molecular response – systematic review and meta-analysis. Acta Oncol 2016; 55: 1077–1083.
Tala I, Chen R, Hu T, Fitzpatrick ER, Williams DA, Whitehead IP . Contributions of the RhoGEF activity of p210 BCR/ABL to disease progression. Leukemia 2013; 27: 1080–1089.
Li S, Ilaria RL, Million RP, Daley GQ, Etten RAV . The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia–like syndrome in mice but have different lymphoid leukemogenic activity. J Exp Med 1999; 189: 1399–1412.
Lugo TG, Pendergast AM, Muller AJ, Witte ON . Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science 1990; 247: 1079–1082.
Hantschel O . Structure, regulation, signaling, and targeting of Abl kinases in cancer. Genes Cancer 2012; 3: 436–446.
Cilloni D, Saglio G . Molecular pathways: BCR-ABL. Clin Cancer Res 2012; 18: 930–937.
Harnois T, Constantin B, Rioux A, Grenioux E, Kitzis A, Bourmeyster N . Differential interaction and activation of Rho family GTPases by p210bcr-abl and p190bcr-abl. Oncogene 2003; 22: 6445–6454.
Ilaria RL, Etten RAV . P210 and P190BCR/ABL induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J Biol Chem 1996; 271: 31704–31710.
Goss VL, Lee KA, Moritz A, Nardone J, Spek EJ, MacNeill J et al. A common phosphotyrosine signature for the Bcr-Abl kinase. Blood 2006; 107: 4888–4897.
Rubbi L, Titz B, Brown L, Galvan E, Komisopoulou E, Chen SS et al. Global phosphoproteomics reveals crosstalk between Bcr-Abl and negative feedback mechanisms controlling Src signaling. Sci Signal 2011; 4: ra18.
Titz B, Low T, Komisopoulou E, Chen SS, Rubbi L, Graeber TG . The proximal signaling network of the BCR-ABL1 oncogene shows a modular organization. Oncogene 2010; 29: 5895–5910.
Brehme M, Hantschel O, Colinge J, Kaupe I, Planyavsky M, Köcher T et al. Charting the molecular network of the drug target Bcr-Abl. Proc Natl Acad Sci USA 2009; 106: 7414–7419.
Roux KJ, Kim DI, Burke B . BioID: a screen for protein-protein interactions. Curr Protoc Protein Sci 2013; 74, 19.23.1–19.23.14.
Lambert J-P, Tucholska M, Go C, Knight JDR, Gingras A-C . Proximity biotinylation and affinity purification are complementary approaches for the interactome mapping of chromatin-associated protein complexes. J Proteomics 2015; 118: 81–94.
Mitchell CJ, Kim M-S, Na CH, Pandey A . PyQuant: a versatile framework for analysis of quantitative mass spectrometry data. Mol Cell Proteomics 2016; 15: 2829–2838.
Cox J, Mann M . MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 2008; 26: 1367–1372.
Mellacheruvu D, Wright Z, Couzens AL, Lambert J-P, St-Denis NA, Li T et al. The CRAPome: a contaminant repository for affinity purification-mass spectrometry data. Nat Methods 2013; 10: 730–736.
Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res 2015; 43: D447–D452.
Kim M-S, Zhong Y, Yachida S, Rajeshkumar NV, Abel ML, Marimuthu A et al. Heterogeneity of pancreatic cancer metastases in a single patient revealed by quantitative proteomics. Mol Cell Proteomics 2014; 13: 2803–2811.
Roux KJ, Kim DI, Raida M, Burke B . A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol 2012; 196: 801–810.
Daley GQ, Baltimore D . Transformation of an interleukin 3-dependent hematopoietic cell line by the chronic myelogenous leukemia-specific P210bcr/abl protein. Proc Natl Acad Sci USA 1988; 85: 9312–9316.
Bhatia R, Munthe HA, Verfaillie CM . Role of abnormal integrin-cytoskeletal interactions in impaired β1 integrin function in chronic myelogenous leukemia hematopoietic progenitors. Exp Hematol 1999; 27: 1384–1396.
Wertheim JA, Perera SA, Hammer DA, Ren R, Boettiger D, Pear WS . Localization of BCR-ABL to F-actin regulates cell adhesion but does not attenuate CML development. Blood 2003; 102: 2220–2228.
Heisterkamp N, Voncken JW, Senadheera D, Gonzalez-Gomez I, Reichert A, Haataja L et al. Reduced oncogenicity of p190 Bcr/Abl F-actin-binding domain mutants. Blood 2000; 96: 2226–2232.
Salgia R, Li JL, Ewaniuk DS, Pear W, Pisick E, Burky SA et al. BCR/ABL induces multiple abnormalities of cytoskeletal function. J Clin Invest 1997; 100: 46–57.
Hantschel O, Wiesner S, Güttler T, Mackereth CD, Rix LLR, Mikes Z et al. Structural basis for the cytoskeletal association of Bcr-Abl/c-Abl. Mol Cell 2005; 19: 461–473.
Yi S-J, Lee H-T, Groffen J, Heisterkamp N . Bcr/Abl P190 interaction with Spa-1, a GTPase activating protein for the small GTPase Rap1. Int J Mol Med 2008; 22: 453–458.
Kowanetz K, Crosetto N, Haglund K, Schmidt MHH, Heldin C-H, Dikic I . Suppressors of T-cell receptor signaling Sts-1 and Sts-2 bind to Cbl and inhibit endocytosis of receptor tyrosine kinases. J Biol Chem 2004; 279: 32786–32795.
Mikhailik A, Ford B, Keller J, Chen Y, Nassar N, Carpino N . A phosphatase activity of Sts-1 contributes to the suppression of TCR signaling. Mol Cell 2007; 27: 486–497.
Ruschmann J, Ho V, Antignano F, Kuroda E, Lam V, Ibaraki M et al. Tyrosine phosphorylation of SHIP promotes its proteasomal degradation. Exp Hematol 2010; 38: 392–402.
Sattler M, Verma S, Byrne CH, Shrikhande G, Winkler T, Algate PA et al. BCR/ABL directly inhibits expression of SHIP, an SH2-containing polyinositol-5-phosphatase involved in the regulation of hematopoiesis. Mol Cell Biol 1999; 19: 7473–7480.
Hantschel O, Warsch W, Eckelhart E, Kaupe I, Grebien F, Wagner K-U et al. BCR-ABL uncouples canonical JAK2-STAT5 signaling in chronic myeloid leukemia. Nat Chem Biol 2012; 8: 285–293.
Medina KL, Pongubala JMR, Reddy KL, Lancki DW, DeKoter R, Kieslinger M et al. Assembling a gene regulatory network for specification of the B cell fate. Dev Cell 2004; 7: 607–617.
Gwin K, Frank E, Bossou A, Medina KL . Hoxa9 regulates Flt3 in lymphohematopoietic progenitors. J Immunol 2010; 185: 6572–6583.
Pongubala JMR, Northrup DL, Lancki DW, Medina KL, Treiber T, Bertolino E et al. Transcription factor EBF restricts alternative lineage options and promotes B cell fate commitment independently of Pax5. Nat Immunol 2008; 9: 203–215.
Heydarian M . Prediction of gene activity in early B cell development based on an integrative multi-omics analysis. J Proteomics Bioinform 2014; 7: 50–63.
Massaad MJ, Ramesh N, Geha RS . Wiskott–Aldrich syndrome: a comprehensive review. Ann NY Acad Sci 2013; 1285: 26–43.
Benesch S, Lommel S, Steffen A, Stradal TEB, Scaplehorn N, Way M et al. Phosphatidylinositol 4,5-biphosphate (PIP2)-induced vesicle movement depends on N-WASP and involves Nck, WIP, and Grb2. J Biol Chem 2002; 277: 37771–37776.
Woodring PJ, Meisenhelder J, Johnson SA, Zhou G-L, Field J, Shah K et al. c-Abl phosphorylates Dok1 to promote filopodia during cell spreading. J Cell Biol 2004; 165: 493–503.
Sattler M, Verma S, Pride YB, Salgia R, Rohrschneider LR, Griffin JD . SHIP1, an SH2 domain containing polyinositol-5-phosphatase, regulates migration through two critical tyrosine residues and forms a novel signaling complex with DOK1 and CRKL. J Biol Chem 2001; 276: 2451–2458.
Lamkin TD, Walk SF, Liu L, Damen JE, Krystal G, Ravichandran KS . Shc interaction with Src homology 2 domain containing inositol phosphatase (SHIP) in vivo requires the Shc-phosphotyrosine binding domain and two specific phosphotyrosines on SHIP. J Biol Chem 1997; 272: 10396–10401.
Deneubourg L, Elong Edimo W, Moreau C, Vanderwinden J-M, Erneux C . Phosphorylated SHIP2 on Y1135 localizes at focal adhesions and at the mitotic spindle in cancer cell lines. Cell Signal 2014; 26: 1193–1203.
Kassenbrock CK, Anderson SM . Regulation of ubiquitin protein ligase activity in c-Cbl by phosphorylation-induced conformational change and constitutive activation by tyrosine to glutamate point mutations. J Biol Chem 2004; 279: 28017–28027.
Sahay S, Pannucci NL, Mahon GM, Rodriguez PL, Megjugorac NJ, Kostenko EV et al. The RhoGEF domain of p210 Bcr-Abl activates RhoA and is required for transformation. Oncogene 2007; 27: 2064–2071.
Frank DA . STAT signaling in the pathogenesis and treatment of cancer. Mol Med 1999; 5: 432–456.
Mikita T, Campbell D, Wu P, Williamson K, Schindler U . Requirements for interleukin-4-induced gene expression and functional characterization of Stat6. Mol Cell Biol 1996; 16: 5811–5820.
Kornfeld J-W, Grebien F, Kerenyi MA, Friedbichler K, Kovacic B, Zankl B et al. The different functions of Stat5 and chromatin alteration through Stat5 proteins. Front Biosci 2008; 13: 6237–6254.
Kee BL, Quong MW, Murre C . E2A proteins: essential regulators at multiple stages of B-cell development. Immunol Rev 2000; 175: 138–149.
Ratliff ML, Mishra M, Frank MB, Guthridge JM, Webb CF . The transcription factor ARID3a is important for in vitro differentiation of human hematopoietic progenitors. J Immunol 2016; 196: 614–623.
Ichikawa M, Yoshimi A, Nakagawa M, Nishimoto N, Watanabe-okochi N, Kurokawa M . A role for RUNX1 in hematopoiesis and myeloid leukemia. Int J Hematol 2013; 97: 726–734.
Lam K, Zhang D-E . RUNX1 and RUNX1-ETO: roles in hematopoiesis and leukemogenesis. Front Biosci J Virtual Libr 2012; 17: 1120–1139.
Strobl B, Moriggl R . Editorial: recovery from chemotherapy depends on STAT1 for replenishment of B lymphopoiesis. J Leukoc Biol 2014; 95: 849–851.
Hoelbl A, Schuster C, Kovacic B, Zhu B, Wickre M, Hoelzl MA et al. Stat5 is indispensable for the maintenance of bcr/abl‐positive leukaemia. EMBO Mol Med 2010; 2: 98–110.
Warsch W, Kollmann K, Eckelhart E, Fajmann S, Cerny-Reiterer S, Hölbl A et al. High STAT5 levels mediate imatinib resistance and indicate disease progression in chronic myeloid leukemia. Blood 2011; 117: 3409–3420.
Daubon T, Chasseriau J, El Ali A, Rivet J, Kitzis A, Constantin B et al. Differential motility of p190bcr-abl- and p210bcr-abl-expressing cells: respective roles of Vav and Bcr-Abl GEFs. Oncogene 2008; 27: 2673–2685.
Rochelle T, Daubon T, Troys MV, Harnois T, Waterschoot D, Ampe C et al. p210bcr-abl induces amoeboid motility by recruiting ADF/destrin through RhoA/ROCK1. FASEB J 2013; 27: 123–134.
Thien CBF, Langdon WY . Cbl: many adaptations to regulate protein tyrosine kinases. Nat Rev Mol Cell Biol 2001; 2: 294–307.
Rohrschneider LR, Fuller JF, Wolf I, Liu Y, Lucas DM . Structure, function, and biology of SHIP proteins. Genes Dev 2000; 14: 505–520.
Chen X, Ren L, Kim S, Carpino N, Daniel JL, Kunapuli SP et al. Determination of the substrate specificity of protein-tyrosine phosphatase TULA-2 and identification of Syk as a TULA-2 substrate. J Biol Chem 2010; 285: 31268–31276.
Hu Y, Liu Y, Pelletier S, Buchdunger E, Warmuth M, Fabbro D et al. Requirement of Src kinases Lyn, Hck and Fgr for BCR-ABL1-induced B-lymphoblastic leukemia but not chronic myeloid leukemia. Nat Genet 2004; 36: 453–461.
Kovacic B, Hoelbl A, Litos G, Alacakaptan M, Schuster C, Fischhuber KM et al. Diverging fates of cells of origin in acute and chronic leukaemia: cells of origin of BCR/ABL+CML and B-ALL. EMBO Mol Med 2012; 4: 283–297.
Reckel S, Hamelin R, Georgeon S, Armand F, Jolliet Q, Chiappe D et al. Differential signaling networks of Bcr-Abl p210 and p190 kinases in leukemia cells defined by functional proteomics. Leukemia 2017; e-pub ahead of print 24 February 2017.
Acknowledgements
This study was supported by NCI’s Clinical Proteomic Tumor Analysis Consortium Initiative (U24CA160036) and a shared instrumentation grant (S10OD021844). JC was supported by NIGMS Training Grant 5T32GM07814. We thank Saradhi Mallampati for his support, the Center for Proteomics Discovery at Johns Hopkins, Rieke Jenson for graphic design consultation and all the members of the Reddy and Pandey laboratories.
Author contributions
JAC completed all experiments and carried out data analysis. JAC, KR and AP wrote the manuscript. JAC, T-CH, KR and AP conceived the experimental idea. JAC, KR and AP designed and planned all experiments. RT and SS helped optimize experimental conditions. RT, SKS, XWu, AHP and CM assisted with data analysis. RT and XWu helped optimize validation effort. MH helped establish and optimize the BCR-ABL MPP system. XWo, KR, M-SK and RSN helped optimize BioID protocols. SR carried out mass spectrometry analysis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Cutler, J., Tahir, R., Sreenivasamurthy, S. et al. Differential signaling through p190 and p210 BCR-ABL fusion proteins revealed by interactome and phosphoproteome analysis. Leukemia 31, 1513–1524 (2017). https://doi.org/10.1038/leu.2017.61
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.61
- Springer Nature Limited
This article is cited by
-
Jak2/STAT6/c-Myc pathway is vital to the pathogenicity of Philadelphia-positive acute lymphoblastic leukemia caused by P190BCR-ABL
Cell Communication and Signaling (2023)
-
Distinct outcomes, ABL1 mutation profile, and transcriptome features between p190 and p210 transcripts in adult Philadelphia-positive acute lymphoblastic leukemia in the TKI era
Experimental Hematology & Oncology (2022)
-
Deciphering molecular interactions by proximity labeling
Nature Methods (2021)
-
Characterization of p190-Bcr-Abl chronic myeloid leukemia reveals specific signaling pathways and therapeutic targets
Leukemia (2021)
-
Integrative phosphoproteome and interactome analysis of the role of Ubash3b in BCR-ABL signaling
Leukemia (2020)