Abstract
Blood collection is a common experimental procedure for which there are many different methods, each with its own advantages and disadvantages. Researchers should use methods that minimize pain, suffering, distress and lasting harm to animals while meeting study requirements. The authors evaluated stress, activity and tissue damage in BALB/cO1aHsd mice after collecting blood using one of six methods: retrobulbar bleeding with thin or thick capillaries, tail vein bleeding, saphenous vein bleeding, facial vein bleeding or jugular vein bleeding. The authors compared in-cage activity, corticosterone concentration and performance in open-field tests between treatment groups and collected histologic samples at 1 h, 3 d and 14 d after bleeding. Mice that underwent retrobulbar bleeding with a thick capillary had a smaller change in corticosterone concentration and higher in-cage activity immediately after blood collection, whereas mice that underwent jugular vein bleeding had a greater change in corticosterone concentration and lower in-cage activity and open-field activity. Mice that underwent saphenous vein bleeding had a high incidence of histological change at 1 h, 3 d and 14 d after blood collection, but few indicators of histological change were present in other groups at 14 d after blood collection. These results suggest that, when collecting a small volume of blood, retrobulbar bleeding with a thick capillary and without anesthesia causes the least stress in mice, whereas jugular vein bleeding and facial vein bleeding cause the most stress and saphenous vein bleeding causes the most lasting damage in mice.
Similar content being viewed by others
Main
Various methods can be used to collect blood samples from rats and mice. When selecting which method to use, researchers must consider many factors, including the welfare of the subject animals. It is a researcher's responsibility to select an effective method that minimizes pain, suffering, distress and lasting harm1. Research and institutional guidelines recommend the use of several blood collection methods with rats and mice2,3,4.
In retrobulbar bleeding (RBB), also known as retro-orbital bleeding, a capillary tube is used to disrupt the retrobulbar venous sinus located behind the eye. This technique can be used to collect a large volume of blood with or without anesthesia, typically without detriment to the subject animal's general health. It is widely used for bleeding or exsanguinating rats and mice5,6,7 and meets animal welfare guidelines2, but its effects on stress and animal welfare depend on the speed and ability of the technician. An experienced technician can avoid severe tissue damage8, but RBB remains controversial owing to the risk of damaging the Harderian gland and other tissues surrounding the eyes9,10.
In tail vein bleeding (TVB), a needle is used to puncture one of the veins located in the tail. It is a simple technique for collecting small volumes of blood from unanesthetized mice or rats11,12, but this procedure requires that animals be warmed to dilate the vein before bleeding13. It can also take much more time to collect a given volume of blood by TVB than by RBB or SVB14,15 owing to the low blood pressure and flow rate of the tail. This prolongs handling of the rodent, which can cause stress; consequently, corticosterone concentrations can be higher in blood samples collected by TVB than in those collected by RBB16.
In saphenous vein bleeding (SVB), a needle is used to puncture the saphenous vein at a prepared site near the ankle. It is a fast technique for serial blood collections from unanesthetized mice or rats, but it requires that the puncture site be shaved first. The superficial location of the vein facilitates accurate venipuncture and observation of any post-collection hemorrhage17. Persistent (minor) bleeding is the only reported complication for this technique3. Anesthesia is not required but conscious animals experience some stress associated with restraint, whether by hand or by restraint tube. Multiple studies have examined how SVB affects behavior, body weight and plasma concentrations of corticosterone and glucose in mice13,15,18,19.
In facial vein bleeding (FVB), also known as submandibular bleeding, a lancet is used to puncture the facial vein located near the back of the jaw. This is a commonly used, fast technique for blood collection from unanesthetized mice or rats. If carried out improperly, this technique can penetrate and damage the inner ear or lacerate facial muscles and nerves20,21, although commercially developed lancets are designed to reduce these risks by limiting the depth of puncture below the skin22. A few studies have found this technique to be more stressful than RBB6,23, TVB13,21 and SVB19.
In jugular vein bleeding (JVB), a needle mounted on a syringe is used to puncture the jugular vein in the craniocervical region. This is an effective technique for repeated bleeding of small animals and may cause fewer negative effects than RBB or TVB in mice or rats5,16,24,25. When used for serial blood collections from unanesthetized mice, it causes stress responses similar to those caused by the tail incision technique, in which a razor blade is used to incise the lateral tail vein26. JVB is recommended by Gesellschaft für Versuchstierkunde/ Society of Laboratory Animal Science2 only when carried out by well-trained persons on anesthetized animals. There is little detailed information about the effects of this technique in mice and rats.
Some research has examined the effects of different blood collection techniques on physiology and behavior in rats5,7,8,9,12,27,28,29,30, but most evaluations with mice have focused on blood quality or refinement of a method, especially for repeated bleeding31,32,33. None has carried out a comprehensive evaluation of the effects of different blood collection techniques on the welfare of mice. This study compares the physiological, behavioral and histopathological effects of recommended bleeding techniques2 in mice.
Corticosterone is the principal glucocorticoid involved in regulation of fuel metabolism, immune reactions and stress responses13. It is therefore a useful parameter for measuring acute stress, and, in mice, corticosterone levels continue to increase for as long as 15 min after exposure to a stressor34,35. In-cage activity can be used as a parameter for assessing the influence of experimental treatment, genetic mutations or drug effects on rodent behavior36. The open-field test is also a common measure of exploratory behavior and general activity in mice and rats37. Stress can decrease locomotion and exploration activity and increase anxiety in mice38. Reserpine-induced pain also can decrease the total travel distance of mice during open-field tests39. We quantified changes in in-cage activity, corticosterone concentration and open-field test performance in mice to evaluate the stress response associated with each method of blood collection.
Methods
Mice and husbandry
We purchased 72 6-week-old, female, specific pathogen–free40 BALB/cO1aHsd mice from Harlan Laboratories (Borchen, Germany) for this study. The mice were marked by ear puncturing, randomly allotted to six experimental groups (n = 12) and housed in groups of six in Makrolon type III cages (Tecniplast, Buguggiate, Italy), such that each cage housed one mouse from each experimental group. Mice were given ad libitum access to tap water in drinking bottles and pelleted food containing 19.0% protein, 4.0% fat, 6% fiber and 7% ash (No. 1324, Altromin GmbH, Lage, Germany). Soft wood shavings were provided in each cage as bedding (Type 5, Altromin GmbH, Lage, Germany). Cages and bedding were changed every 7 d and nest material (Nestlets, EBECO, Castrop-Rauxel, Germany) was provided in each cage after changing as an environmental improvement. Cages were kept inside a Scantainer ventilated cabinet (Scanbur AS, Lellingegaard, Denmark) with the air exchanged 10–16 times per hour and a light intensity of 50 ± 10 lux on a 12-h:12-h light:dark cycle (with the lights coming on at 6:00 a.m.). Room temperature was 22 ± 2 °C with 55 ± 10% relative humidity. All husbandry and experimental procedures were carried out in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes and the Animal Welfare Act of Germany.
Mice were handled daily between 9:00 a.m. and 12:00 p.m. during a 14-d acclimatization period before treatment to reduce later handling stress during the experiment. During handling each animal was placed on a handler's arm for at least 1 min.
Pretreatment blood collection
Five days after the mice arrived at the facility, we began collecting blood samples to measure pretreatment corticosterone concentration in each mouse. We collected blood from a different mouse from each cage every day for 6 d, which minimized and standardized the stress associated with opening the cage. We carried out these blood collections between 9:00 a.m. and 12:00 p.m. each day and collected 150 μl blood from each mouse into a 0.5-ml centrifuge tube (Eppendorf, Hamburg, Germany). We collected blood by RBB from the left eye without anesthesia, using two thick capillaries, each with an outer diameter of 1.5–1.6 mm (volume of 75 μl, Hirschmann, Eberstadt, Germany). This type of capillary was used in all blood collections during this study, except RBBB and JVB treatments. We centrifuged the blood samples at 12,000 r.p.m. for 5 min and extracted the blood plasma. Plasma was stored in a freezer at − 20 °C until all blood samples were collected and was then thawed for corticosterone analysis.
Blood collection treatment
At the time of treatment, the mice were 8 weeks old and weighed 19.26 ± 1.21 g (mean ± s.d.). We collected blood from the mice in each of the six groups using a different bleeding method: one of two different RBB techniques, TVB, SVB, FVB or JVB. We collected blood from a different mouse from each cage every day for 6 d between 9:00 a.m. and 12:00 p.m. For each blood collection treatment, the subject mouse was removed from its housing cage and transported to the adjacent experimental room in a Makrolon type II cage (Tecniplast, Buguggiate, Italy) with bedding. A technician began the blood collection procedure immediately after the mouse arrived in the experimental room. We recorded the amount of time spent on the entire treatment procedure, beginning with the mouse's removal from its home cage and ending when the mouse was awake in the transportation cage after blood collection. We also recorded the amount of time spent on just the bleeding technique, beginning with the mouse's restraint and immobilization and ending when 75 μl blood was collected.
All blood collection techniques were carried out by experienced technicians or researchers, who routinely apply the technique in their respective institutions, to minimize possible tissue damage8 or stress in mice18. Five technicians or researchers traveled from different institutions in Germany to the Institute for Animal Welfare and Behavior of the University of Veterinary Medicine Hannover to participate in this study. Each bleeding technique was carried out by a single technician every time. We prepared and arranged blood collection equipment for all technicians such that working conditions were the same in this study as at their home institutions.
When anesthetizing a mouse, the technician placed the mouse in a box measuring 15 cm × 13 cm × 28 cm and induced narcosis by introducing 8 ml ether vaporized with 5 l/min oxygen41 inside a gas-washing bottle. This setup was specifically designed to introduce gaseous anesthetic agents and included an extra exhaust outlet for human safety. An anesthetist attended this procedure and noted when the mouse's respiration became visibly depressed, whereupon the technician removed the mouse from the box. For JVB, each mouse was kept under inhalation anesthesia for the entire bleeding procedure. To maintain inhalation anesthesia, the technician placed the mouse's nose in a 5-ml tube containing two cotton balls soaked in 0.5 ml ether (Fig. 1). Once removed from anesthetic agents, mice were determined to have emerged from anesthesia once they had righted themselves from a supine position.
RBB. We compared two different RBB techniques. In each, the technician manually restrained each mouse at the neck and inserted a hematocrit capillary into the medial canthus of the right eye (Fig. 2). The technician then rotated the capillary along the bulbus of the eye, rupturing the retrobulbar venous sinus (sinus orbitalis). For RBBA the technician used a thick capillary with an outer diameter of 1.5–1.6 mm (volume of 75 μl; Hirschmann, Eberstadt, Germany) to collect blood without anesthetizing the mouse. The thick capillary remained in the eye until it collected 75 μl blood. For RBBB the technician induced anesthesia in the mouse and used a thin capillary with an outer diameter of 1.2 mm (volume of 20 μl; intraEND, Blaubrand, Wertheim, Germany). Once inserted, the thin capillary remained in the eye until it collected 75 μl blood into a 0.5-ml centrifuge tube (Eppendorf, Hamburg, Germany). The technician then removed the capillary and stopped bleeding with a cotton pad.
The technician rotates the capillary (1) along the bulb (2), disrupts the retrobulbar venous sinus (3) and draws blood into the capillary. Image is drawn based on ref. 45.
TVB. For TVB the technician first warmed each mouse in its transportation cage with a red light (250 W, 230–250 V; Albert Kerbl, Buchbach, Germany). After 10 min, the technician restrained the mouse in a custom-made restraint and used a 23-gauge needle (B. Braun, Melsungen, Germany) to puncture the right lateral tail vein (v. caudalis lateralis). The technician then collected 75 μl blood using a thick capillary (Fig. 3) and stopped bleeding with a cotton pad.
SVB. For SVB the technician restrained each mouse in a 50-ml tube (BD Biosciences, Heidelberg, Germany). The technician isolated the leg above the knee and shaved away fur with a GT420 electric shaver (Braun, Suhl, Germany) until the saphenous vein was visible and then used a 27-gauge needle (B. Braun, Melsungen, Germany) to puncture the lateral saphenous vein (v. saphena lateralis). The technician then collected 75 μl blood using a thick capillary (Fig. 4) and stopped bleeding with a cotton pad.
FVB. For FVB the technician manually restrained each mouse and punctured the facial vein (v. facialis) using an Accu-Check lancet (Roche, Mannheim, Germany; Fig. 5). The technician then collected 75 μl blood using a thick capillary and stopped bleeding with a cotton pad.
JVB. For JVB the technician first induced anesthesia in each mouse, then maintained anesthesia and placed the mouse on its back with its legs stretched caudally. The technician mounted a 27-gauge heparinized needle on a 1-ml syringe (B. Braun, Melsungen, Germany) and inserted the needle into the craniocervical region under the clavicle towards the opposite knee to puncture the venous angle (angulus venosus), as previously described in literature16 (Fig. 1). The technician aspirated the syringe until it contained 75 μl blood, then withdrew the needle and stopped bleeding with a cotton pad.
In-cage activity
Immediately after the blood collection treatment, we placed each mouse in the transportation cage and recorded its in-cage activity for 10 min using a Canon G10Hi camera with Hi8 tapes (Canon, Tokyo, Japan). We began recording immediately upon placing a mouse in the cage, unless the mouse had undergone RBBB or JVB, in which case we began recording activity upon emergence from anesthesia. We used a different transportation cage for each mouse and cleaned these cages between days. After the experiment, we reviewed each recording of in-cage activity, rating the activity level of each mouse on a scale of 1–7 and recording the amount of time spent by each mouse at each activity level. We then calculated the average activity level for the mouse over the entire 10-min recording (Table 1).
Post-treatment blood collection
Fifteen minutes after the blood collection treatment was complete, we collected 150 μl blood from the left eye of each mouse into a 0.5-ml centrifuge tube (Eppendorf, Hamburg, Germany) by RBB without anesthesia using two 75-μl capillaries. We centrifuged the blood samples at 12,000 r.p.m. for 5 min and extracted the blood plasma. Plasma was stored in a freezer at − 20 °C until all blood samples were collected and was then thawed for corticosterone analysis.
Corticosterone measurement
We carried out a competitive corticosterone ELISA (IBL, Hamburg, Germany) and measured plasma corticosterone concentrations using a microtiter reader at 450 nm. For each mouse, we calculated the change in corticosterone concentration by subtracting the pre-treatment corticosterone concentration from the post-treatment corticosterone concentration.
Open-field test
Immediately after collecting blood for corticosterone analysis, we placed the subject mouse into the center of an open-field apparatus similar to one previously described42, measuring 60 cm × 60 cm square with a wall height of 50 cm. We recorded the behavior of the mouse for 5 min using a Model ICD-47E camera (Ikegami, Tokyo, Japan) with a TL 550 video recorder (Panasonic, Osaka, Japan). We analyzed each recording after the experiment using EthoVision 3.1 software (Noldus, Wageningen, the Netherlands) and calculated the latency of the mouse to move toward the edge of the apparatus, the total distance traveled by the mouse and the average speed of the mouse. After open-field testing, we returned the subject mouse to its home cage and cleaned the open-field apparatus.
Histological examination
We collected histopathological samples of puncture sites 1 h, 3 d and 14 d after the blood collection treatment. We euthanized four mice from each experimental group at each time point (n = 4) by carbon dioxide inhalation, according to a previously described method43. Carbon dioxide was introduced into home cages through a customized lid to produce a homogenous distribution and at a rate of 13 l/min until breathing ceased. After euthanasia, we isolated the puncture site for each mouse, fixed tissues with 10% formaldehyde solution and prepared histologic slides. We embedded tissues in paraffin, cut three sections of 3–4 μm at 100-μm intervals using a rotary microtome (Mikrotom HM 360, Microm, Walldorf, Germany) and stained these sections with hematoxylin and eosin.
We examined these sections under a microscope and noted the presence of eight indicators of histological change (hemorrhage, blood clots, polymorphonuclear inflammatory cells, mononuclear inflammatory cells, degenerative or necrotic changes, fibrosis, edema and thrombus) in four types of tissue (skin tissue, blood vessels, muscle tissue and connective and adipose tissue). For mice that underwent RBB, we evaluated two additional tissue types: Harderian gland and optic nerve. We assigned one point for the presence of each indicator in each tissue type in each mouse and calculated the incidence of histological change in each group as a percentage of the total possible score for that group, which was 128 points for groups that underwent TVB, SVB, FVB and JVB and 192 points for groups that underwent RBB.
Data analysis
We analyzed all data using StatView software version 5.0 (SAS Institute Inc., Cary, NC). We used the Lilliefors test to check all data for normality and confirmed that all data were normally distributed, including average in-cage activity scores. We carried out one-way analysis of variance tests (ANOVAs) to compare bleeding methods with respect to the following variables: time spent on the entire treatment procedure, time spent on just the bleeding technique, in-cage activity level of mice, change in corticosterone concentrations in mice, latency of mice to move to the edge of the field in the open-field test, total distance traveled by mice in the open-field test and average speed of mice in the open-field test. For these analyses we carried out post-hoc pairwise comparisons using Scheffé tests. We carried out paired t-tests to compare plasma corticosterone concentrations in pre-treatment samples and post-treatment samples for each treatment group. For all analyses P < 0.05 was considered statistically significant and data are expressed as mean ± s.d.
Results
Blood collection
The length of time spent on the entire treatment procedure differed significantly between treatments (F5,66 = 99.326, P < 0.0001). This length of time was shortest for RBBA (9.42 ± 1.24 s) followed by FVB (20.50 ± 11.80 s), SVB (63.17 ± 16.12 s), RBBB (118.50 ± 24.33 s), JVB (136.75 ± 21.64 s) and finally TVB (716.91 ± 25.38 s). The length of time spent on just the bleeding technique also differed significantly between treatments (F5,66 = 29.597, P < 0.0001). This length of time was significantly shorter for RBBA (6.43 ± 1.24 s) and FVB (13.33 ± 11.33 s) compared with SVB (40.67 ± 13.82 s) and JVB (40.58 ± 16.58 s; P < 0.05 for these comparisons). The length of time spent on just the bleeding technique of RBBB (18.67 ± 9.89 s) was also generally shorter than SVB (P = 0.0727) and JVB (P = 0.0747). TVB took significantly longer (79.09 ± 31.52 s) than all other techniques (P < 0.001 for these comparisons; Fig. 6).
In-cage activity
Average in-cage activity level differed significantly between treatment groups (F5,66 = 65.752, P < 0.0001). Activity level was significantly lower in mice that underwent FVB and JVB than in mice that underwent RBBA, SVB or TVB (P < 0.0001 for these comparisons). Activity level in mice that underwent RBBB was also significantly lower than that in mice that underwent RBBA or TVB (P < 0.05 for both comparisons) but significantly higher than that in mice that underwent FVB or JVB (P < 0.0001 for both comparisons; Fig. 7).
Corticosterone concentration
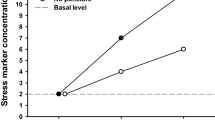
Plasma corticosterone concentration was significantly higher after treatment than before treatment in each treatment group (P < 0.05 for all comparisons; Fig. 8). The increase in corticosterone concentration after treatment differed significantly between groups (F5,66 = 2.523, P = 0.0382). This increase was greatest in mice that underwent JVB (3,006.86 ± 2,101.61 ng/l), followed by mice that underwent FVB (2,386.50 ± 2,260.98 ng/l) or TVB (2,208.87 ± 1,853.51 ng/l), mice that underwent SVB (1,688.41 ± 811.86 ng/l) or RBBB (1,643.53 ± 675.49 ng/l) and finally mice that underwent RBBA (887.62 ± 481.07 ng/l). The increase in corticosterone concentration was significantly smaller in mice that underwent RBBA than in mice that underwent RBBB, TVB, SVB, FVB or JVB (P < 0.05 for these comparisons).
Open-field test
Latency in the open-field test differed significantly between treatment groups (F5,66 = 4.700, P = 0.0010). Latency was significantly longer in mice that underwent JVB (20.13 ± 24.36 s) than in any other treatment group (P < 0.05 for these comparisons). By contrast, latency in mice that underwent RBBB was the shortest (2.05 ± 2.06 s). Latency did not differ significantly between any other treatment groups (P > 0.05 for these comparisons; Fig. 9).
Total distance traveled in the open-field test also differed significantly between treatment groups (F5,66 = 8.336, P < 0.0001). Total distance traveled was longest in mice that underwent TVB (2,183.27 ± 348.65 cm) or SVB (2,110.21 ± 443.21 cm) followed by mice that underwent RBBA (1,807.78 ± 861.96 cm), RBBB (1,699.41 ± 498.42 cm), FVB (1,225.54 ± 697.78 cm) and finally JVB (839.54 ± 371.71 cm). Mice that underwent JVB or FVB traveled significantly less than mice that underwent TVB or SVB (P < 0.05 for these comparisons), and mice that underwent JVB traveled significantly less than mice that underwent RBBA (P < 0.05). Total distance traveled did not differ significantly between mice that underwent TVB, SVB, RBBB or RBBA (P > 0.05 for these comparisons; Fig. 10).
Average speed in the open field test differed significantly between treatment groups (F5,66 = 8.334, P < 0.0001). Average speed of mice that underwent JVB (2.80 ± 1.24 cm/s) was generally lower than that of any other group and was significantly lower than that of mice that underwent RBBA, TVB or SVB (P < 0.05 for these comparisons). Average speed of mice that underwent TVB (7.28 ± 2.16 cm/s) or SVB (7.04 ± 1.48 cm/s) was generally higher than that of any other group. Mice that underwent SVB were significantly faster than mice that underwent FVB or JVB (P < 0.05 for both comparisons), and mice that underwent TVB were significantly faster than mice that underwent SVB or JVB (P < 0.05 for both comparisons).
Histological examination
At 1 h after RBBA most indicators of histological change were observed in the retrobulbar connective tissue and Harderian gland. Comparatively fewer indicators of histological change were observed at 1 h after RBBB. At 1 h after TVB most indicators of histological change were observed in subcutaneous connective and adipose tissue. At 1 h after SVB lesions were observed primarily in subcutaneous connective tissue and muscles. At 1 h after FVB indicators of histological change were observed in all examined tissues including the eye. At 1 h after JVB indicators of histological change were observed in muscles and in subcutaneous connective and adipose tissue. At 3 d after RBBA, RBBB, FVB and JVB the incidence of histological change was similar to that observed at 1 h after blood collection. At 3 d after TVB and SVB the incidence of histological change (5.47% and 19.53%, respectively) had decreased relative to the incidence observed at 1 h after treatment (13.97% and 29.41%, respectively; Table 2). At 14 d after blood collection no indicator of histological change was observed in most of the samples. In a few samples, indicators of histological change with low qualitative scores were observed (median score = 0 for almost all parameters). The incidence of histological change at this time was very low (<6%) for each treatment group except that which underwent SVB (10.94%).
Discussion
We assessed changes in corticosterone concentration, behavior and histology of mice following six different blood collection methods. Many factors can produce acute stress during blood collection, and we noted potential stressors that differed between methods such as anesthesia (RBBB and JVB), heating (TVB) and shaving (SVB). These stressors likely interacted with the actual bleeding technique to produce the different physiological, behavioral and histological changes we observed in each group.
The change in corticosterone concentration after blood collection was greatest in mice that underwent JVB, indicating that this group experienced the most physiological stress throughout blood collection. Mice that underwent JVB also had low in-cage activity and long latency, low speed and short total distance traveled in the open-field test. All of these results indicate that JVB was the most stressful method of blood collection with the greatest effect on physiology and behavior in mice.
The change in corticosterone concentration after blood collection was lowest in mice that underwent RBBA, indicating that this group experienced the least physiological stress throughout blood collection. Mice that underwent RBBA also showed high in-cage activity and short latency, moderate speed and moderate distance traveled in the open-field test relative to other groups. These results indicate that RBBA was a comparatively less stressful method of blood collection with a small effect on physiology and behavior in mice. This was likely because mice that underwent RBBA were restrained for the shortest period of time and without anesthesia.
Anesthesia probably caused a large amount of stress in mice that underwent JVB or RBBB. By design, our study allows us to compare two very similar techniques, RBBA and RBBB, which differ primarily in their use of anesthesia. Although RBBB used a thinner capillary and caused a lower incidence of histological change compared with RBBA, RBBB involved anesthesia and, correspondingly, mice that underwent RBBB showed a greater change in corticosterone concentration and lower in-cage activity after blood collection than mice that underwent RBBA. Mice that underwent RBBB seemed to recover quickly from short anesthesia and performed similarly to mice that underwent RBBA in open-field tests, but mice that underwent JVB received anesthesia for a longer period of time, which might have contributed to their stronger and longer stress response.
The change in corticosterone concentration was also large and in-cage activity and performance in open-field tests were generally low in mice that underwent FVB compared with all other groups except for mice that underwent JVB. These results indicate that FVB is also a very stressful method of blood collection. FVB took much less time than other techniques, except for RBBA, and did not involve anesthesia, but in-cage activity after blood collection was significantly lower in mice that underwent FVB than in any other group except mice that underwent JVB. This agrees with previous findings that FVB can cause more stress than TVB, SVB or RBB13,19,44. Whereas restraint and anesthesia cause stress in other methods, FVB uses no anesthesia and only brief restraint. For this reason, we believe that mice that underwent FVB experienced more stress due to laceration of facial muscles and nerves, even though we used a short lancet.
No previous study has compared all six of the techniques that we used in this study, but previous studies have compared TVB, SVB and FVB, with mixed conclusions. Aasland et al.15 found higher stress in mice after TVB than after SVB, in agreement with our corticosterone measurements after blood collection. By contrast, Madetoja et al.13 found higher stress in mice after SVB than after FVB or TVB, whereas our findings showed lower stress in mice after SVB than after FVB or TVB. This might be explained by the fact that Madetoja et al. included a warming period before SVB and a shorter warming period before TVB, whereas we included no warming period before SVB and a longer warming period before TVB. If warming mice before treatment elevates stress levels during blood collection, then the differences between our protocols and those of Madetoja et al. could account for the differences in our results.
The change in corticosterone concentration in mice that underwent TVB was similar to that in mice that underwent FVB, even though the time spent on TVB was significantly longer than the time spent on FVB. Mice that underwent TVB also showed significantly higher in-cage activity and faster speed and longer total distance traveled in the open-field test compared with mice that underwent FVB. In our study, TVB and FVB both caused histological changes in subcutaneous connective and adipose tissue, and FVB also caused changes in muscle tissue. A previous comparison found that mice that underwent FVB lost significantly more body weight and had elevated levels of plasma corticosterone after 1 d, compared to mice that underwent TVB21. Together with these findings, our results suggest that, despite inducing comparable changes in corticosterone concentrations, FVB causes greater acute stress than TVB and leads to a slower recovery and more extensive histological damage because it punctures a sensitive site.
The incidence of histological change at 14 d after blood collection was very low in all treatment groups except in mice that underwent SVB. This agrees with previous research that compared RBBA and TVB to JVB16,25 and compared JVB to TVB26. The incidence of histological change at 14 d after blood collection was unexpectedly high for mice that underwent SVB for reasons that are not known. We generally found few changes during histological examination, and we consider these associated effects of blood collection to be of less concern and importance than our measured indicators of stress.
During procedures that involve blood collection, researchers should try to minimize restraint time and routine handling to avoid unnecessary stress15. They should also minimize acute stress from pain and possible tissue damage. Depending on the focus and requirements of an experiment, researchers must carefully select a blood sampling method from among the established options, giving consideration to the different effects that we observed in this study.
Our results suggest that FVB and JVB led to higher acute stress and that RBBA and RBBB caused less stress than other techniques, based on corticosterone concentrations, behavioral indicators of stress and incidences of histological change after blood collection. Although mice that underwent TVB and SVB had higher incidences of histological change at 14 d, they also had relatively lower corticosterone concentrations and low behavioral indicators of stress, indicating that these methods are also acceptable. We note that only small volumes of blood were collected in this study. These findings might not apply to procedures for collecting greater volumes of blood, as these could require longer restraint and cause higher stress and incidence of histological changes in mice.
References
European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union L276, 33–79 (2010).
Gesellschaft für Versuchstierkunde/Society of Laboratory Animal Science. Empfehlung zur Blutentnahme bei Versuchstieren, insbesondere kleinen Versuchstieren (Gesellschaft für Versuchstierkunde/Society of Laboratory Animal Science, 2009). http://www.gv-solas.de/fileadmin/user_upload/pdf_publikation/tie_blutentnahme09.pdf.
Diehl, K.H. et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 21, 15–23 (2001).
US National Institutes of Health. Guidelines for Survival Bleeding of Mice and Rats (US National Institutes of Health, Bethesda, MD, 2012).
Fitzner Toft, M., Petersen, M.H., Dragsted, N. & Hansen, A.K. The impact of different blood sampling methods on laboratory rats under different types of anaesthesia. Lab. Anim. 40, 261–274 (2006).
Holmberg, H., Kiersgaard, M.K., Mikkelsen, L.F. & Tranholm, M. Impact of blood sampling technique on blood quality and animal welfare in haemophilic mice. Lab. Anim. 45, 114–120 (2011).
Sharma, A. et al. Safety and blood sample volume and quality of a refined retro-orbital bleeding technique in rats using a lateral approach. Lab Anim. (NY) 43, 63–66 (2014).
Van Herck, H. et al. Orbital sinus blood sampling in rats as performed by different animal technicians: the influence of technique and expertise. Lab. Anim. 32, 377–386 (1998).
Van Herck, H. et al. Histological changes in the orbital region of rats after orbital puncture. Lab. Anim. 26, 53–58 (1992).
Heimann, M., Kasermann, H.P., Pfister, R., Roth, D.R. & Burki, K. Blood collection from the sublingual vein in mice and hamsters: a suitable alternative to retrobulbar technique that provides large volumes and minimizes tissue damage. Lab. Anim. 43, 255–260 (2009).
Bazare Jr., J., Leamons, M.L. & Young, J.F. Sampling methods for pharmacokinetic studies in the mouse. J. Pharmacol. Methods 5, 99–120 (1981).
Omaye, S.T., Skala, J.H., Gretz, M.D., Schaus, E.E. & Wade, C.E. Simple method for bleeding the unanaesthetized rat by tail venipuncture. Lab. Anim. 21, 261–264 (1987).
Madetoja, J., Madetoja, M., Mäkinen, J., Riuttala, E. & Jokinen, J. Blood Sampling from the tail vein, in comparison with two other techniques, causes less stress to mice. Scand. J. Lab. Anim. Sci. 36, 215–221 (2009).
Hui, Y.H. et al. Pharmacokinetic comparisons of tail-bleeding with cannula- or retro-orbital bleeding techniques in rats using six marketed drugs. J. Pharmacol. Toxicol. Methods 56, 256–264 (2007).
Aasland, K.E., Skjerve, E. & Smith, A.J. Quality of blood samples from the saphenous vein compared with the tail vein during multiple blood sampling of mice. Lab. Anim. 44, 25–29 (2010).
Müller, B. Blutentnahme aus dem retroorbitalen Venenplexus, den Schwanzvenen und dem jugularen Venenwinkel bei Ratte und Maus: Vergleichende Hämatologisch/Biochemische und Klinische Untersuchungen (Justus-Liebig-Universität, Giessen, Germany, 1999).
Hem, A., Smith, A.J. & Solberg, P. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guinea pig, ferret and mink. Lab. Anim. 32, 364–368 (1998).
Abatan, O.I., Welch, K.B. & Nemzek, J.A. Evaluation of saphenous venipuncture and modified tail-clip blood collection in mice. J. Am. Assoc. Lab. Anim. Sci. 47, 8–15 (2008).
Mazlan, N.H. et al. Comparison of two blood sampling methods in mice in order to increase animal welfare and the reliability of experimental results. Exp. Anim. 60, 226 (2011).
Mitchell, S. Venipuncture techniques in pet rodent species. J. Exotic Pet Med. 20, 284–293 (2011).
Teilmann, A.C., Kalliokoski, O., Sorensen, D.B., Hau, J. & Abelson, K.S. Manual versus automated blood sampling: impact of repeated blood sampling on stress parameters and behavior in male NMRI mice. Lab. Anim. 48, 278–291 (2014).
Golde, W.T., Gollobin, P. & Rodriguez, L.L. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab. Anim. (NY) 34, 39–43 (2005).
Schlichting, A., Tsai, P.-P., Stelzer, H.D. & Hackbarth, H. Vergleich der retrobulbären Blutentnahme mit der Punktion der V. facialis im Hinblick auf Tierschutzgerechtigkeit (45th Scientific meeting of GV-SOLAS, Dresden, Germany, 2008).
Kassel, R. & Levitan, S. A jugular technique for the repeated bleeding of small animals. Science 118, 563–564 (1953).
Meyer-Eilers, S. Vergleichende Histologische Untersuchung nach Wiederholter Blutentnahme aus dem Jugularen Venenwinkel bei Mäusen und Ratten (Luwig-Maximilians-Universität, Munich, Germany, 2000).
Shirasaki, Y., Ito, Y., Kikuchi, M., Imamura, Y. & Hayashi, T. Validation studies on blood collection from the jugular vein of conscious mice. J. Am. Assoc. Lab. Anim. Sci. 51, 345–351 (2012).
Van Herck, H. et al. Endocrine stress response in rats subjected to singular orbital puncture while under diethyl-ether anaesthesia. Lab. Anim. 25, 325–329 (1991).
Van Herck, H. et al. Orbital bleeding in rats while under diethylether anaesthesia does not influence telemetrically determined heart rate, body temperature, locomotor and eating activity when compared with anaesthesia alone. Lab. Anim. 31, 271–278 (1997).
Van Herck, H. et al. Blood sampling from the retro-orbital plexus, the saphenous vein and the tail vein in rats: comparative effects on selected behavioural and blood variables. Lab. Anim. 35, 131–139 (2001).
Vachon, P. & Moreau, J.P. Serum corticosterone and blood glucose in rats after two jugular vein blood sampling methods: comparison of the stress response. Contemp. Top. Lab. Anim. Sci. 40, 22–24 (2001).
Christensen, S.D., Mikkelsen, L.F., Fels, J.J., Bodvarsdottir, T.B. & Hansen, A.K. Quality of plasma sampled by different methods for multiple blood sampling in mice. Lab. Anim. 43, 65–71 (2009).
Fernández, I., Pena, A., Del Teso, N., Perez, V. & Rodriguez-Cuesta, J. Clinical biochemistry parameters in C57BL/6J mice after blood collection from the submandibular vein and retroorbital plexus. J. Am. Assoc. Lab. Anim. Sci. 49, 202–206 (2010).
Chan, Y.K. et al. Influence of tail versus cardiac sampling on blood glucose and lipid profiles in mice. Lab. Anim. 46, 142–147 (2012).
Flint, M.S. & Tinkle, S.S. C57BL/6 mice are resistant to acute restraint modulation of cutaneous hypersensitivity. Toxicol. Sci. 62, 250–256 (2001).
Nyuyki, K.D., Maloumby, R., Reber, S.O. & Neumann, I.D. Comparison of corticosterone responses to acute stressors: chronic jugular vein versus trunk blood samples in mice. Stress 15, 618–626 (2012).
Ganea, K., Liebl, C., Sterlemann, V., Muller, M.B. & Schmidt, M.V. Pharmacological validation of a novel home cage activity counter in mice. J. Neurosci. Methods 162, 180–186 (2007).
Gould, T.D., Dao, D.T. & Kovacsics, C.E. The open field test. in Mood and Anxiety Related Phenotypes in Mice (ed. Gould, T.D.) 1–20 (Humana, New York, 2009).
Kale, P.P., Addepalli, V. & Ghadawale, S.R. Impact of pre-exposure of tail suspension on behavioural parameters like locomotion, exploration, and anxiety in mice. Indian J. Exp. Biol. 51, 732–738 (2013).
Liu, S.B. et al. Attenuation of reserpine-induced pain/depression dyad by gentiopicroside through downregulation of GluN2B receptors in the amygdala of mice. Neuromolecular Med. 16, 350–359 (2014).
Nicklas, W. et al. Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab. Anim. 36, 20–42 (2002).
Schlichting, A., Haberstroh, H., Tsai, P.-P., Stelzer, H.D. & Hackbarth, H. Stress Response of Mice under Different Volatile Anaesthesia (11th FELASA Symposium, Helsinki, Finland, 2010).
Tsai, P.P., Stelzer, H.D., Hedrich, H.J. & Hackbarth, H. Are the effects of different enrichment designs on the physiology and behaviour of DBA/2 mice consistent? Lab. Anim. 37, 314–327 (2003).
Corbach, S. Investigation of Carbon Dioxide–Euthanasia of Laboratory Mice Regarding Animal Welfare Aspects (School of Veterinary Medicine Hannover, Hannover, Germany, 2006).
Teilmann, A.C. et al. Physiological and pathological impact of blood sampling by retro-bulbar sinus puncture and facial vein phlebotomy in laboratory mice. PLoS One. Published online 26 November 2014 (10.1371/journal.pone.0113225).
Weiss, J., Maeß, J. & Nebendahl, K. in Haus- und Versuchstierpflege 2nd edn. (Enke, Stuttgart, Germany, 2003).
Acknowledgements
We thank the institutes that sent their well-trained technicians or researchers to Hannover to participate in this study for their support. Parts of these results were presented as a poster at the 12th FELASA SECAL congress held 10–13 June 2013 in Barcelona, Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tsai, PP., Schlichtig, A., Ziegler, E. et al. Effects of different blood collection methods on indicators of welfare in mice. Lab Anim 44, 301–310 (2015). https://doi.org/10.1038/laban.738
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/laban.738
- Springer Nature America, Inc.
This article is cited by
-
Protective effects of Bifidobacterium bifidum FL-228.1 on dextran sulfate sodium-induced intestinal damage in mice
European Journal of Nutrition (2023)
-
Comparison of murine retroorbital plexus and facial vein blood collection to mitigate animal ethics issues
Laboratory Animal Research (2021)
-
Stress and behavioral correlates in the head-fixed method: stress measurements, habituation dynamics, locomotion, and motor-skill learning in mice
Scientific Reports (2020)
-
Recent Advances in Experimental Models of Breast Cancer Exosome Secretion, Characterization and Function
Journal of Mammary Gland Biology and Neoplasia (2020)
-
Sex differences in variability across timescales in BALB/c mice
Biology of Sex Differences (2017)