Abstract
Objective:
The objective of this study was to evaluate the association of amniotic fluid lactate dehydrogenase and glucose concentrations with microbial invasion of amniotic cavity and histologic chorioamnionitis before 37 pregnancy weeks in women with or without preterm premature rupture of membranes.
Study Design:
Amniocentesis was performed on 70 women with suspected intra-amniotic infection. Standard biochemical methods, molecular microbiology and culture techniques were used. Histopathological examination of the placenta was performed.
Results:
Thirty (48%) women had microbial invasion of the amniotic cavity (MIAC), 53 (76%) women had histological chorioamnionitis and 28 women had both. The cutoff values for MIAC and histological chorioamnionitis were 429 IU l−1 for lactate dehydrogenase and 0.7 mmol l−1 for glucose. Both end points occurred equally often regardless of the membrane status.
Conclusion:
Increased amniotic fluid lactate dehydrogenase and decreased glucose were associated with MIAC and histological chorioamnionitis. However, test performance was of limited value.
Similar content being viewed by others
Introduction
Chorioamnionitis is closely associated with preterm delivery and neonatal morbidity and mortality, especially at an early gestational age.1, 2, 3, 4 Early diagnosis of subclinical chorioamnionitis, that is, intra-amniotic infection (IAI), and optimal timing of delivery is a major obstetrical challenge. One manifestation of IAI is microbial invasion of the amniotic cavity (MIAC). Clinical chorioamnionitis occurs in only 5 to 10% of preterm deliveries,5 whereas silent MIAC and histologic chorioamnionitis (HCA) are much more common.6, 7, 8 HCA and MIAC do not necessarily coexist.9 Thus, rapid biomarkers to detect IAI would be useful in clinical practice.
The usefulness of a common, non-invasive biomarker to detect chorioamnionitis—maternal blood C-reactive protein—is limited in IAI.10 Thus, more accurate biomarkers are needed.
Previous studies have linked high concentration of amniotic fluid lactate dehydrogenase (am-LD) and low concentration of glucose (am-Gluc) with IAI or HCA.11, 12, 13 However, studies linking these biomarkers with MIAC detected by molecular microbiology techniques (am-PCR) are lacking.
We wanted to evaluate the association of am-LD and am-Gluc with both MIAC and HCA in singleton pregnancies with suspected chorioamnionitis with subsequent preterm birth regardless of membrane status at presentation.
Materials and Methods
This prospective study was performed at the Department of Obstetrics and Gynecology, University Hospital, Helsinki, Finland between March 2010 and March 2015. One hundred and four singleton pregnancies between 22+0 and 36+6 weeks of gestation with amniocentesis performed by our study group members for suspected chorioamnionitis were recruited, including women with or without preterm prelabor rupture of the membranes (PPROM). IAI was suspected in the presence of contractions and at least one of the following criteria: uterine tenderness, maternal fever, fetal tachycardia, malodorous discharge from cervix, increased C-reactive protein>10 mg l−1 and total white blood cell count >20 × 109 l−1, or sludge at ultrasound examination in those with intact membranes (Table 1). We excluded women with amniocentesis-to-delivery interval >7 days (n=34). PPROM was diagnosed by clinical speculum examination or a positive bedside dipstick test (ActimProm®, Medix Biochemica, Espoo, Finland). Gestational age was determined at the first trimester (12+0 to 13+6 weeks of gestation) ultrasound screening. After exclusions, 70 women were eligible for the final analysis.
The laboratory tests performed included: am-Gluc, am-LD, am-16S rRNA sequencing (am-PCR) and amniotic fluid culture. Am-LD and am-Gluc were analyzed by using Modular P System (Roche Diagnostics, Penzberg, Germany) and according to IFCC (International Federation of Clinical Chemistry) recommendations. Am-LD was analyzed with a quantitative assay for the total enzymatic activity of lactate dehydrogenase (including the activity of all LD-isoenzymes, LD1-LD5) according to IFCC recommendations. The assay was developed for Roche automated clinical chemistry analyzers. The intra-assay coefficient of variation for am-LD was less than 2.3% and the detection limit of the assay was 5 IU l−1. The detection limit of am-Gluc was 0.50 mmol l−1. In the low concentration range (less than 4 mmol l−1), the intra-assay coefficient of variation was 4.7%, and at concentration levels higher than 4 mmol l−1 (linear range to 42 mmol l−1), the intra-assay coefficient of variation was higher than 2.0%. Values of am-Gluc below 0.5 mmol l−1 were recorded as 0 mmol l−1 for statistical analysis. MIAC was defined as positive am-PCR or culture. Amniotic fluid bacterial culture result was available in 46 cases and am-PCR result in 59 cases. Both were available in 43 cases. An amniotic fluid sample was cultured for aerobic and anaerobic bacteria on chocolate blood agar in 5% CO2 and on Fastidious Anaerobe Agar in anaerobic conditions at 35±1 ºC. A thioglycolate broth enrichment was used. The samples were in culture for 7 days and were inspected after 1, 2 and 7 days. The culture methods used enabled also the detection of common Candida species and Mycoplasma hominis, but not Ureaplasma species. A minimum of 500 μl of amniotic fluid was subjected to ceramic bead-beating cell lysis (Precellys24 tissue homogenizer, Bertin Technologies, Montigny le Bretonneux, France) followed by magnetic-bead-based DNA extraction method (NucliSENS kit with easyMAG automatic nucleic acid purification platform, bioMérieux, Marcy l'Etoile, France) as described by the manufacturer. The extracted DNA was amplified in duplicate by PCR using the following primers: 5′-TTG GAG AGT TTG ATC MTG GCT C-3′ (forward) and 5′-GTA TTA CCG CGG CTG CTG-3′ (reverse). DNA of λ-phage served as an inhibition control in the PCR reaction. Positive PCR product was verified by gel electrophoresis; 5 μl of the PCR product was sequenced in a core facility, and the obtained sequence was compared with NCBI BLAST sequence database (www.ncbi.nlm.nih.gov/blast). Mixed sequences were analyzed by Ripseq mixed analysis tool (https://www.ripseq.com/) when appropriate.
Histopathological examination of the placenta (n=70) was performed by a placental pathologist. Placental sampling sites were the chorionic plate, umbilical cord and extraplacental membranes. Sections of tissue blocks were stained with hematoxylin and eosin. HCA was defined as a diffuse infiltration of inflammatory cells (mainly neutrophils) in any part of the tissue. Inflammatory changes in the umbilical cord (funisitis) was recorded separately. The outcome data were analyzed in relation to comparing the presence or absence of histological chorioamnionitis.
The results of am-LD, am-Gluc, am-PCR and microbial culture were available to the clinicians. The cutoff values of am-Gluc <0.8 mmol l−1 and am-LD >419 were considered consistent with IAI during the study period.11
Women with PPROM were managed according to the local clinical guidelines. All patients with PPROM received intravenous antibiotics (Azithromycin 500 mg × 1 and cefuroxime 1.5 g × 3 for three consecutive days or until delivery in cases with PPROM before 33+0 gestational weeks. Cases with PPROM after 33+0 gestational weeks received cefuroxime 1.5 g × 3 for three consecutive days or until delivery). Women with PPROM had expectant management until 34 gestational weeks, after which the labor was induced, and earlier if symptoms and signs of clinical chorioamnionitis were present.
All calculations were carried out using Microsoft Statistical Package for Social Sciences (SPSS, Chicago, IL, USA) for Windows v22.0. Comparisons between proportions were performed with the Chi-square test or Fisher’s exact test when the number of cases was under five. Because the data did not follow a normal distribution, data with continuous variables were analyzed with Mann–Whitney U test. The receiver operating characteristics curves were derived and area under the curve was determined. The sensitivity, specificity, positive predictive value and negative predictive value were derived. All statistical tests were two-sided. A P-value <0.05 was considered statistically significant.
All patients provided a written informed consent for the study. The local Ethics Committee and the hospital district of Helsinki and Uusimaa approved the study protocol (75/03/03/2013).
Results
Of the 70 women, 32 (45.7%) were primiparous. The mean (s.d.) age was 30.9 (±5.9) years. The median (range) gestational age was 27.5 (range 22+5 to 36+5) weeks at the time of amniocentesis, and 27.5 (range 23+2 to 36+6) weeks at the time of delivery. Forty (57%) women had PPROM.
Microbiologic results were available in 62 (87%) women. Of those, 30 (48%) had MIAC. Table 2 shows the specific amniotic fluid microbiologic findings. Both am-PCR and culture were positive in eight women. Six women had only culture positive, and 16 women had only am-PCR result positive. Of all women, 53 had HCA (76%). A total of 28 women (45%) had both MIAC and HCA. Funisitis occurred in 19 (36%) cases, and all had HCA.
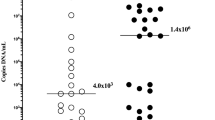
Am-LD and am-Gluc concentrations in relation to MIAC are shown in Figures 1 and 2. The median concentrations of am-LD were higher in those with MIAC than in those without MIAC (1514.5 IU l−1 (range from 91 to 9698 IU l−1) vs 694 IU l−1 (range from 62 to 3882 IU l−1), P= 0.012). The median concentrations of am-Gluc were lower among those with MIAC than among those without MIAC (0.05 mmol l−1 (range from 0 to 3.4 mmol l−1) vs 1.1 mmol l−1 (range from 0 to 6 mmol l−1), P= 0.002). In the prediction of MIAC and HCA, the cutoff value of am-LD based on receiver operating characteristic curve was 429 IU l−1 for both. The corresponding value for am-Gluc was 0.7 mmol l−1 (Figures 3 and 4). The overall performance of these cutoff values for MIAC and HCA is shown in Table 3. The concomitant use of both thresholds for am-LD (>429 IU l−1) and am-Gluc (<0.7 mmol l−1) were associated with MIAC (odds ratio 3.8, 95% confidence interval 1.3 to 10.9) and HCA (odds ratio 5.9, 95% confidence interval 1.5 to 23.0). If both biomarkers were normal (am-LD <429 IU l−1 and am-Gluc ⩾0.7 mmol l−1), MIAC occurred in 6/19 (32%) cases and HCA in 13/23 (56%) cases.
(a and b) Receiver operating characteristic (ROC) curves of amniotic fluid lactate dehydrogenase (am-LD) (a) and amniotic fluid glucose (am-Gluc) (b) for predicting microbial invasion of the amniotic cavity (MIAC). The most optimal cutoff value for am-LD was 429 IU l−1 (AUC 0.69 (95% confidence interval (CI) 0.55 to 0.82)) and for am-Gluc 0.7 mmol l−1 (AUC 0.72 (95% CI 0.59 to 0.85)). The sensitivity and 100–specificity points for am-LD values of 429, 637 and 1550 IU l−1 and for am-Gluc values of 0.7, 1.0 and 1.6 mmol l−1 are marked.
(a and b) Receiver operating characteristic (ROC) curves of amniotic fluid lactate dehydrogenase (am-LD) (a) and amniotic fluid glucose (am-Gluc) (b) for predicting histologic chorioamnionitis (HCA). The most optimal cutoff value for am-LD was 429 IU l−1 (AUC 0.76 (95% confidence interval (CI) 0.62 to 0.9)) and for am-Gluc 0.7 mmol l−1 (AUC 0.70 (95% CI 0.58 to 0.83)). The sensitivity and 100–specificity points for am-LD values of 236, 429 and 919 IU l−1 and for am-Gluc values of 0.7, 1.1 and 3.5 mmol l−1 are marked.
The overall rates of MIAC or HCA did not differ between those with or without PPROM (for MIAC 43 vs 56%, P=0.32; for HCA 78 vs 73%, P=0.69). The median concentrations of am-LD (1092 IU l−1 vs 1297 IU l−1, P=0.64) and am-Gluc (0.7 mmol l−1 vs 0.6 mmol l−1, P=0.5) did not differ either. Both in cases with HCA and in cases without HCA, the membrane status did not have any effects on the rate of MIAC or the am-LD and am-Gluc concentrations (Table 4).
Discussion
Our principal finding was that increased am-LD and decreased am-Gluc concentrations were associated with MIAC and HCA regardless of the membrane status. The use of both am-PCR and bacterial culture almost doubled the number of MIAC cases we found. The rate of MIAC was high regardless of the membrane status, higher than in one previous study,14 although in the present study, most women were not asymptomatic. Therefore, amniocentesis is indicated also in women with intact membranes with nonspecific symptoms and signs. Despite the fact that am-LD levels were significantly higher and am-Gluc levels lower in cases with MIAC and HCA, these markers were not accurate enough to augment clinical decision-making. Nevertheless, the high positive predictive value of the am-LD and am-Gluc combination for HCA might justify the use of the combination as a provisional diagnostic tool for IAI.
We acknowledge the relatively small sample size. However, the clinical setting strengthens the data and the results. Antibiotics, tocolysis and glucocorticoids were universally used according to the uniform clinical guidelines. Therefore, we did not specifically analyze the role of these potential confounding factors on the biomarker levels.
The strength of the study was the use of both am-PCR and bacterial culture instead of culture only. Most earlier studies have used only bacterial culture or Gram stain to detect MIAC.15 By am-PCR, we were able to detect cases not detected by culture.16 The overall number of MIAC cases found nearly doubled. In the future, larger studies with am-PCR, culture and placental histopathological examination are certainly needed.
Some women had HCA in the absence of MIAC. However, we did not study the role of viral infections. This or other inflammatory conditions, such as so called sterile inflammation, could explain some of the cases.17, 18, 19
Clinical chorioamnionitis is only the tip of the iceberg of all IAI cases. Most cases are subclinical.1 Amniocentesis remains the most accurate test to detect such cases. In our study, three quarters of clinically suspected IAI patients had HCA and almost half had intact membranes. On the other hand, 1 in 10 symptomatic patients had neither HCA nor PPROM. In such situations, the result of amniocentesis provides additional information and augments clinical decision-making. Previous observational studies have already suggested that implementation of amniocentesis in the management of the preterm labor and PPROM is important.20 The biomarker results may improve neonatal outcome, 21, 22, 23, 24 which is the ultimate goal of our approach. Amniocentesis is an invasive but safe procedure.25, 26 If PPROM occurs following amniocentesis, it may in fact reflect infection or inflammation in the amniotic membranes at the time of amniocentesis and not necessarily be a complication of the procedure.27
One major problem in current clinical practice is the lack of highly sensitive and specific biomarkers for MIAC and HCA. Am-LD reflects the inflammation and neutrophil activation in the amniotic cavity. Garry et al.28 were the first to describe that am-LD exceeding 419 IU l−1 predicts MIAC. This cutoff value has also been used in other studies.13, 29, 30 Our cutoff was almost similar. In a previous study by Kidokoro et al.,31 the optimal cutoff value for HCA was 250 IU l−1. They performed amniocentesis between 16 and 35 gestational weeks which may have affected the results.31 In our study, the sensitivity and positive predictive value of am-LD for HCA were relatively high, but the negative predictive value was still low which is an obvious limitation.
Am-Gluc has been previously linked to MIAC with values less than 0.8 mmol l−1 (refs. 11, 32) and to HCA with values less than 1.1 to 1.6 mmol l−1.33 In our study, low am-Gluc concentration predicted HCA with lower cutoff value than previously used. We found that am-Gluc was not a sensitive marker for HCA. This is in line with one previous study.34
HCA can occur with MIAC (infectious) or without MIAC (sterile).19, 35 In sterile inflammation, am-Gluc concentration may stay within the normal range.34 We found that am-Gluc was associated with MIAC only in the presence of HCA.
Over half of those with normal am-LD and normal am-Gluc had HCA and almost one-third had MIAC. This is the subgroup for which more accurate biomarkers of inflammation are desperately needed.36
Conclusion
MIAC and HCA occurred equally often regardless of the membrane status. Increased am-LD and decreased am-Gluc levels were associated with MIAC and HCA, but the diagnostic accuracy was limited. Better biomarkers for identifying inflammatory changes potentially harmful to the fetus are needed.
References
Wu HC, Shen CM, Wu YY, Yuh YS, Kua KE . Subclinical histologic chorioamnionitis and related clinical and laboratory parameters in preterm deliveries. Pediatr Neonatol 2009; 50: 217–221.
Goldenberg RL, Culhane JF, Iams JD, Romero R . Epidemiology and causes of preterm birth. Lancet 2008; 371: 75–84.
Roescher AM, Timmer A, Erwich JJ, Bos AF . Placental pathology, perinatal death, neonatal outcome, and neurological development: a systematic review. PLoS One 2014; 9: e89419.
Garcia-Munoz Rodrigo F, Galan Henriquez GM, Ospina CG . Morbidity and mortality among very-low-birth-weight infants born to mothers with clinical chorioamnionitis. Pediatr Neonatol 2014; 55: 381–386.
Edwards RK . Chorioamnionitis and Labor. Obstet Gynecol Clin North Am 2005; 32: 287–296.
Xie A, Zhang W, Chen M, Wang Y, Wang Y, Zhou Q et al. Related factors and adverse neonatal outcomes in women with preterm premature rupture of membranes complicated by histologic chorioamnionitis. Med Sci Monit 2015; 21: 390–395.
Menon R, Taylor RN, Fortunato SJ . Chorioamnionitis – A complex pathophysiologic syndrome. Placenta 2010; 31: 113–120.
Lahra MM, Jeffery HE . A fetal response to chorioamnionitis is associated with early survival after preterm birth. Am J Obstet Gynecol 2004; 190: 147–151.
Vajrychova M, Kacerovsky M, Tambor V, Hornychova H, Lenco J . Microbial invasion and histological chorioamnionitis upregulate neutrophil-gelatinase associated lipocalin in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2016; 29: 12–21.
Dulay AT, Buhimschi IA, Zhao G, Bahtiyar MO, Thung SF, Cackovic M et al. Compartmentalization of acute phase reactants interleukin-6, C-reactive protein and procalcitonin as biomarkers of intra-amniotic infection and chorioamnionitis. Cytokine 2015; 76: 236–243.
Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol 1990; 163: 968–974.
Ford C, Genc MR . Optimized amniotic fluid analysis in patients suspected of intrauterine infection/inflammation. J Perinat Med 2011; 40: 33–37.
Buhimschi CS, Bhandari V, Hamar BD, Bahtiyar MO, Zhao G, Sfakianaki AK et al. Proteomic profiling of the amniotic fluid to detect inflammation, infection, and neonatal sepsis. PLoS Med 2007; 4: e18.
Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014; 71: 330–358.
Pettker CM, Buhimschi IA, Magloire LK, Sfakianaki AK, Hamar BD, Buhimschi CS . Value of placental microbial evaluation in diagnosing intra-amniotic infection. Obstet Gynecol 2007; 109: 739–749.
DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol 2010; 64: 38–57.
Payne MS, Bayatibojakhi S . Exploring preterm birth as a polymicrobial disease: an overview of the uterine microbiome. Front Immunol 2014; 5: 595.
Gervasi MT, Romero R, Bracalente G, Chaiworapongsa T, Erez O, Dong Z et al. Viral invasion of the amniotic cavity (VIAC) in the midtrimester of pregnancy. J Matern Fetal Neonatal Med 2012; 25: 2002–2013.
Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014; 72: 458–474.
Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM . Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol 2015; 213 (4 Suppl): S29–S52.
Hitti J, Tarczy-Hornoch P, Murphy J, Hillier SL, Aura J, Eschenbach DA . Amniotic fluid infection, cytokines, and adverse outcome among infants at 34 weeks' gestation or less. Obstet Gynecol 2001; 98: 1080–1088.
Porreco RP, Heyborne KD, Shapiro H . Amniocentesis in the management of preterm premature rupture of the membranes: a retrospective cohort analysis. J Matern Fetal Neonatal Med 2008; 21: 573–579.
Maki Y, Furukawa S, Kodama Y, Sameshima H, Ikenoue T . Amniocentesis for threatened preterm labor with intact membranes and the impact on adverse outcome in infants born at 22 to 28 weeks of gestation. Early Hum Dev 2015; 91: 333–337.
Shinjo A, Otsuki K, Sawada M, Ota H, Tokunaka M, Oba T et al. Retrospective cohort study: a comparison of two different management strategies in patients with preterm premature rupture of membranes. Arch Gynecol Obstet 2012; 286: 337–345.
Akolekar R, Beta J, Picciarelli G, Ogilvie C, D'Antonio F . Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review and meta-analysis. Ultrasound Obstet Gynecol 2015; 45: 16–26.
Yeast JD, Garite TJ, Dorchester W . The risks of amniocentesis in the management of premature rupture of the membranes. Am J Obstet Gynecol 1984; 149: 505–508.
Lee SY, Park KH, Jeong EH, Oh KJ, Ryu A, Kim A . Intra-amniotic infection/inflammation as a risk factor for subsequent ruptured membranes after clinically indicated amniocentesis in preterm labor. J Korean Med Sci 2013; 28: 1226–1232.
Garry D, Figueroa R, Aguero-Rosenfeld M, Martinez E, Visintainer P, Tejani N . A comparison of rapid amniotic fluid markers in the prediction of microbial invasion of the uterine cavity and preterm delivery. Obstet Gynecol 1996; 175: 1336–1341.
Buhimschi CS, Buhimschi IA, Abdel-Razeq S, Rosenberg VA, Thung SF, Zhao G et al. Proteomic biomarkers of intra-amniotic inflammation: relationship with funisitis and early-onset sepsis in the premature neonate. Pediatr Res 2007; 61: 318–324.
Dulay AT, Buhimschi CS, Zhao G, Oliver EA, Mbele A, Jing S et al. Soluble TLR2 is present in human amniotic fluid and modulates the intraamniotic inflammatory response to infection. J Immunol 2009; 182: 7244–7253.
Kidokoro K, Furuhashi M, Kuno N, Ishikawa K . Amniotic fluid neutrophil elastase and lactate dehydrogenase: association with histologic chorioamnionitis. Acta Obstet Gynecol Scand 2006; 85: 669–674.
Gauthier DW, Meyer WJ, Bieniarz A . Correlation of amniotic fluid glucose concentration and intraamniotic infection in patients with preterm labor or premature rupture of membranes. Am J Obstet Gynecol 1991; 165: 1105–1110.
Odibo AO, Rodis JF, Sanders MM, Borgida AF, Wilson M, Egan JF et al. Relationship of amniotic fluid markers of intra-amniotic infection with histopathology in cases of preterm labor with intact membranes. J Perinatol 1999; 19: 407–412.
Greig PC, Ernest JM, Teot L . Low amniotic fluid glucose levels are a specific but not a sensitive marker for subclinical intrauterine infections in patients in preterm labor with intact membranes. Am J Obstet Gynecol 1994; 171: 365–370 discussion 370-1.
Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2015; 28: 1394–1409.
Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 2014; 210: 125.e1–125.e15.
Acknowledgements
The study was funded by Helsinki University Hospital Research grant (TYH2013340), by The Finnish Medical Foundation, and by the SalWe Research Program ‘Get it Done’ (Tekes- The Finnish Funding Agency for Technology and Innovation grant 3986/31/2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Myntti, T., Rahkonen, L., Tikkanen, M. et al. Amniotic fluid rapid biomarkers are associated with intra-amniotic infection in preterm pregnancies regardless of the membrane status. J Perinatol 36, 606–611 (2016). https://doi.org/10.1038/jp.2016.59
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.59
- Springer Nature America, Inc.
This article is cited by
-
Glucocorticoids, sodium transport mediators, and respiratory distress syndrome in preterm infants
Pediatric Research (2021)
-
Comparison of amniotic fluid matrix metalloproteinase-8 and cathelicidin in the diagnosis of intra-amniotic infection
Journal of Perinatology (2016)