Abstract
Objective:
In infants <35 weeks’ gestation, we sought to define the transcutaneous bilirubin (TcB) levels at which a total serum bilirubin (TSB) level suggesting the need for phototherapy is unlikely to occur and a TSB measurement can, therefore, be avoided.
Study Design:
Nursing staff performed 896 TcB measurements within 1 h of a TSB on 225 neonates 26 0/7–34 6/7 weeks’ postmenstrual age (PMA). Generalized linear models were fit with generalized estimating equations (GEEs) to model the probability of having a TSB level at or above the phototherapy initiation cutpoint as a function of the TcB; these methods allow for multiple tests per infant.
Results:
The mean difference between TcB and TSB measurements was <1 mg dl−1 for each PMA category. When the TcB was at least 3 mg dl−1 below the TSB cutpoint for phototherapy, there was a ⩾98% probability that the TSB was not at, or above, the recommended phototherapy level. The single exception to this was a phototherapy level of 6 mg dl−1 for infants of 28 0/7–29 6/7 weeks’ PMA, where a TcB of 4 mg dl−1 below the phototherapy level (ie a TcB ⩽2 mg dl−1) was necessary to achieve ⩾98% probability.
Conclusion:
Our data support the use of routine TcB screening for infants 28–34 6/7 weeks’ gestation. TcB screening in the neonatal intensive care unit can identify infants who require a TSB to confirm or exclude the need for phototherapy.
Similar content being viewed by others
Introduction
Multiple studies have documented the relationship between transcutaneous bilirubin (TcB) and total serum bilirubin (TSB) levels and the utility of TcB measurements in the care of term and late preterm infants is now well established.1 Several studies have also evaluated the relationship between TSB and TcB in preterm infants.1, 2 In a systematic review of 22 studies, Nagar et al.2 concluded that ‘….TcB devices reliably estimated bilirubin levels in preterm infants and could be used in clinical practice to reduce blood sampling’ but these authors do not provide specific guidelines for the use of TcB measurements in this population. The primary purpose of screening newborns with TcB measurements is to help the clinician to decide when to obtain a TSB measurement that, in turn, might suggest the need for phototherapy. Using recently recommended levels for initiating phototherapy at different postmenstrual ages, (PMA)3 we sought to define the TcB levels at which a TSB level suggesting the need for phototherapy3 is unlikely to occur and a TSB measurement (and heel stick) can, therefore, be avoided.
Methods
Between 1 October 2011 and 13 October 2012 the nursing staff in our neonatal intensive care unit (NICU) performed 896 TcB measurements on 225 neonates at 26 0/7–34 6/7 weeks PMA. TcB measurements were obtained from the lower sternum within 1 h of a TSB measurement that had been obtained in the course of routine clinical care. TSB measurements were performed in the hospital clinical laboratory with the Synchron Diazo method (Beckman Coulter, Fullerton, CA, USA). All TcB measurements were obtained using the Konica Minolta Dräger Air-Shields transcutaneous jaundice meter, model JM-103 (Draeger Medical,Telford, PA, USA). Three independent measurements were obtained, a maximum of the three values was recorded and used as the TcB measurement in this study. TcB measurements were discontinued if phototherapy was instituted. The study was approved by the hospital’s Human Investigation Committee and, because of the noninvasive nature of this measurement, the committee approved a waiver of consent.
Statistical methods
Because many infants had multiple bilirubin measurements, we estimated means and s.d. of the differences between TcB and TSB using a random effects model with infants as a random effect. We grouped infants by PMA and performed separate analyses for different recommended TSB cutpoint levels for initiation of phototherapy for each PMA group. To include a specific TSB cutpoint, we required at least five TSB readings at or above that cutpoint. Generalized linear models were fit with generalized estimating equations to model the probability of having a TSB level at or above the phototherapy initiation cutpoint as a function of the TcB; these methods allow for multiple tests per infant. Model fit was assessed through plots of cumulative residuals. If the model fit was satisfactory, probabilities and confidence intervals for the probability of TSB levels at or above the cutpoint for a given TcB level were obtained. The SAS System (SAS Institute, Cary, NC, USA) for Windows version 9.3 and StatXact release 10 (Cytel, Cambridge, MA, USA) were used for statistical analysis.
Results
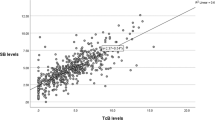
The population consisted of 62% white, 18% African–American, 6% Asian, 4% Hispanic, 10% unknown and 54% male. The infant’s age at testing ranged from day 1 to day 29 of the hospital stay. Table 1 shows the data for measurements according to the infants’ PMA. Other than in infants <28 weeks, where we obtained only 15 samples, the correlation between TcB and TSB was good. The Pearson correlation coefficient (r) was 0.85 for the total population and ranged from 0.77–0.83 for each postmenstrual category (Figure 1). The mean difference between TcB and TSB measurements was small, 0.47 ±1.55 mg dl−1 (s.d.), and was <1 mg dl−1 for each PMA (Table 1). The day-to-day variability in the differences between repeat TcB and TSB measurements in individual infants (using the square root of the one-way ANOVA estimate of variance) was slightly smaller than the s.d. of these measurements between infants in the corresponding PMA groups, ranging from 0.59–1.44 mg dl−1. The s.d. shown in Table 1 incorporate both within infant and between infant variability.
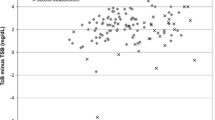
Figure 1 provides linear regression analysis for the total population and for each PMA category. Figure 2 illustrates the effect of increasing TSB on the difference between TcB and TSB. Contrary to our findings in infants of ⩾35 weeks,4 there is a weak and clinically reassuring positive relationship between increasing TSB levels and the difference between TcB and TSB for the total population and most PMA categories. There was no greater tendency for the TcB to underestimate the TSB as TSB levels increase, although, because of the preterm population studied, very few TSB levels were >13 mg dl−1 and none were >15 mg dl−1 (Tables 1 amd 2). Table 2 shows the number of recorded observations (not infants) above each phototherapy level for each PMA group. Figure 3 illustrates that the age of the infant at the time of measurement also had a mild positive but clinically unimportant effect on the difference between TcB and TSB (r=0.266). Table 3 shows the probability of having a TSB level at or above the phototherapy initiation cutpoint as a function of the TcB level and Table 4 provides the data derived from Table 3 but presented in a more user friendly format for the clinician.
Discussion
The primary purpose of TcB measurements is to identify when a TSB should be measured, and when used to screen both term and preterm infants in this manner TcB has been shown to be a reliable and effective tool.1, 2
In addition to reducing the need for heel sticks in tiny infants, it is important that screening with TcB should avoid missing a TSB that might indicate the need for phototherapy. To minimize this possibility, we have always used the highest of three independent TcB measurements as the level used for screening purposes and have shown that, compared with the average of three measurements, using the highest value will reduce the number of false negative TcB’s by about 50% but increase false positives by a similar amount.4 Using the TSB phototherapy levels that have recently been recommended for infants of <35 weeks of gestation,3 we provide TcB levels that permit screening of infants in the NICU with some degree of confidence while saving the need for a heel stick TSB measurement in many infants. Among all 896 TcB measurements on 225 infants, the TcB was ⩾3 mg dl−1 below the TSB in only 13 measurements from 11 infants. In only 1 infant could this difference possibly have misled the clinician with regard to the need for phototherapy (a 33 1/7wk PMA infant whose TcB was 7.2 mg dl−1 and TSB 10.7 mg dl−1). If we include this single example among the total population of 226 infants, we can calculate a 95% confidence interval of 0.0002–0.023 for the probability of being ‘led astray’ in an infant by at least one TcB measurement. But if we measure TcB levels daily, both the estimate of the probability and the upper bound of the confidence interval of 2.3/100 are likely an over estimate of the risk of not measuring the TSB in a particular infant who has an elevated TSB. With daily measurements, there is a high probability that if the TSB remains at a level suggesting the need for phototherapy a subsequent TcB measurement within a day or two will identify the need for a TSB measurement and, perhaps, phototherapy. Our data do suggest a useful ‘rule of thumb:’ when the TcB is at least 3 U below the TSB cutpoint for phototherapy, there is a high probability that the TSB is not at or above the recommended phototherapy level and a heel stick can be avoided. The single exception to this is the phototherapy level of 6 mg dl−1 in the 28 0/7–29 6/7 week PMA category (Table 3) where a TcB of 4 mg dl−1 below the phototherapy level (that is, a TcB⩽2 mg dl−1) was necessary to achieve >98% probability. As only one infant had a TSB that exceeded the upper recommended limits for phototherapy for the PMA group (Table 2), we do not know if this rule of thumb applies to those elevated levels.
Other investigators have studied similar populations in the US and elsewhere and provided TcB values that predicted different TSB levels 2, 5, 6 or used a TcB level of 70% of the recommended phototherapy level in that country as the criterion for measuring the TSB.7, 8 Using these and other cutoffs investigators have, without exception, documented the clinical utility and acceptable accuracy of TcB measurements and shown that they can reduce blood sampling and spare the need for multiple heel sticks in this fragile population.2 TcB measurements in these studies have been obtained from the forehead and sternum. In our first study of infants ⩾35 weeks with the JM-103 device,9 we found a better correlation between TcB and TSB when TcB was measured at the sternum, a finding that has been subsequently confirmed by others.2 In a recent study of 24–34 week neonates, however, Yaser et al.10 found that measurements of TcB in the interscapular area provided fewer false negatives than measurements from the forehead or sternum for the prediction of TSB levels that suggested the need for phototherapy.
A strength of our study is the relatively large number of infants studied and the number of TcB levels obtained. In addition, all measurements were made by our regular nursing staff during the course of routine NICU care, rather than by research staff, thus providing data that should have application to other similar units. This is also the first study, to our knowledge, that has examined the effect of chronological age on TcB measurements in a preterm population. Tables 3 and 4 provide, for the first time, confidence intervals for the probability of a TcB predicting a TSB that meets criteria for initiating phototherapy according to recently published guidelines,3 and Table 4 shows the probability of avoiding a false negative TcB. A weakness is the potential bias that resulted from the initiation of phototherapy at levels similar to those recommended3 by the neonatologists caring for these infants. As a result, infants with higher TSB levels relative to PMA were already receiving phototherapy and could not be included in the study and this prevented us from calculating the predictive confidence of TcB measurements for the higher range of phototherapy TSB levels.3
Conclusion
Routine screening with TcB measurements in a level III NICU can identify infants who do or do not require a TSB to rule in or out the need for phototherapy and can eliminate the need for many heel stick blood samples. With the single exception of infants with a PMA of 28 0/7–29 6/7 weeks and a phototherapy level of 6 mg dl−1, if the TcB is at least 3 mg dl−1 below the TSB cutpoint for phototherapy, there is a high probability that the TSB is not at or above the recommended phototherapy level. Together with the data already published, our data now provide sufficient information to support the use of routine TcB screening for infants of <35 weeks but at least of 28 weeks’ gestation.
References
De Luca D, Engle W, Jackson G . Transcutaneous Bilirubinometry: hepatology Research and Clinical Developments. Nova Biomedical: New York, NY, USA, 2013.
Nagar G, Vandermeer B, Campbell S, Kumar M . Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics 2013; 132: 871–881.
Maisels MJ, Watchko JF, Bhutani VK, Stevenson DK . An approach to the management of hyperbilirubinemia in the preterm infant less than 35 weeks of gestation. J Perinatol 2012; 32 (9): 660–664.
Maisels MJ, Engle W, Wainer S, Jackson GL, McManus S, Artinian F . Transcutaneous bilirubin levels in an outpatient and office population. J Perinatol 2011; 31: 621–624.
Schmidt ET, Wheeler CA, Jackson GL, Engle WD . Evaluation of transcutaneous bilirubinometry in preterm neonates. J Perinatol 2009; 29: 564–569.
De Luca D, Zecca E, De Turrist P, Barbato G, Marras M, Romagnoli C . Using bilicheck for preterm neonates in a sub-intensive unit: diagnostic usefulness and suitability. Early Hum Dev 2007; 83: 313–317.
Willems WA, van den Berg LM, de Wit H, Molendijk A . Transcutaneous bilirubinometry with the bilicheck in very premature newborns. J Matern Fetal Neonatal Med 2004; 16: 209–214.
Ebbesen F, Rasmussen LM, Wimberley PD . A new transcutaneous bilirubinometer, BiliCheck, used in the neonatal intensive care unit and the maternity ward. Acta Paediatr 2002; 91: 203–211.
Maisels MJ, Ostrea E Jr, Touch S et al. Evaluation of a new transcutaneous bilirubinometer. Pediatrics 2004; 113: 1628–1635.
Yaser A, Tooke L, Rhoda N . Interscapular site for transcutaneous bilirubin measurement in preterm infants: a better and safer screening site. J Perinatol 2014; 34: 209–212.
Acknowledgements
We are profoundly grateful to the nursing staff of our NICU for obtaining and documenting the TcB measurements on these infants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr. Maisels is an unpaid consultant to Draeger Medical Inc., the US supplier of the JM-103. The other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Maisels, M., Coffey, M. & Kring, E. Transcutaneous bilirubin levels in newborns <35 weeks’ gestation. J Perinatol 35, 739–744 (2015). https://doi.org/10.1038/jp.2015.34
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2015.34
- Springer Nature America, Inc.
This article is cited by
-
Transcutaneous bilirubin levels in extremely preterm infants less than 30 weeks gestation
Journal of Perinatology (2023)
-
Transcutaneous bilirubin measurements: useful, but also reproducible?
Pediatric Research (2021)
-
Screening methods for neonatal hyperbilirubinemia: benefits, limitations, requirements, and novel developments
Pediatric Research (2021)
-
Reliability of transcutaneous bilirubin determination based on skin color determined by a neonatal skin color scale of our own
European Journal of Pediatrics (2021)
-
Repetitive bilirubin measurements in preterm infants prior to phototherapy: is it wise to use the rate of rise?
Pediatric Research (2020)