Abstract
Increased systemic vascular resistance and coronary microvascular dysfunction are well-documented in essential hypertension (EH). We investigated the effect of additional vasodilating treatment on coronary and peripheral resistance circulation in EH patients with high systemic vascular resistance index (SVRI) despite well-treated blood pressure (BP). We enroled patients on stable antihypertensive treatment that were given intensified vasodilating therapy (ACE inhibitor, angiotensin II receptor blocker or calcium channel blocker). Before and following 6 months of intensified therapy, coronary resting and maximal artery flow were measured by transthoracic Doppler echocardiography to calculate coronary flow reserve (CFR) and minimum vascular resistance (C-Rmin). Cardiac output was estimated by inert gas rebreathing to calculate SVRI. Maximal forearm blood flow was determined by venous occlusion plethysmography to calculate minimum vascular resistance (F-Rmin). Patients were assigned into two groups: high-SVRI and low-SVRI subgroups, based on a median split at baseline. Following additional treatment SVRI decreased more in the high-SVRI group than in the low-SVRI group (14.4 vs −2.2%: P=0.003), despite similar baseline ambulatory BP (132/81 mm Hg) and BP reduction (6.5 and 4.6%: P=0.19). F-Rmin remained unchanged (6.5 vs −2.0%: P=0.30), while C-Rmin decreased by 22 and 24% (P=0.80) and CFR increased by 23 and 17% (P=0.16). Thus, intensified vasodilating therapy improved SVRI more in patients with high SVRI than in those with low SVRI. Regardless of SVRI status, the treatment improved cardiac but not forearm dilatation capacity. The substantial improvement of the hypertensive cardiac microvascular dysfunction was not related to the reduction in SVRI.
Similar content being viewed by others
Introduction
Microvascular impairment is considered a pathophysiological hallmark in essential hypertension (EH). The lumen diameter of the small arteries and arterioles is reduced and the media-to-lumen ratio increased but with no change in the total amount of wall material, a process termed eutrophic inward remodelling.1 The structural alterations of the microvasculature lead to a systemic increase in minimum vascular resistance2 and a reduced vasodilator capacity3 that may compromise the ability of the local circulation to respond to an increase in tissue oxygen demand and thus to demand-related organ dysfunction.4 Remodelling of resistance arteries occurs early in the development of hypertension5 and has been demonstrated in numerous vascular beds including the forearm circulation6 and in the heart,7 where the coronary flow reserve (CFR) is reduced due to both remodelling and fibrosis.8 This may lead to reduced exercise capacity and microvascular angina and eventually to hypertensive heart failure. In addition, abnormal resistance artery structure has been found to predict cardiovascular events independently of blood pressure (BP) and of the degree of large artery disease progression9 in individuals with EH10, 11 even during ongoing therapy12 suggesting that correction of the vascular structure could serve as a supplemental treatment goal in addition to BP reduction.
A central issue is the effect of various antihypertensive drugs on the ability to correct vascular structure. Microvascular structure adapts to vasodilation rather than BP.13 In agreement, vasodilating therapy with ACE inhibitors (ACEI),14 angiotensin II receptor blockers (ARB)15 and calcium channel blockers (CCB)16 have been found superior to non-vasodilating β-blockers with regard to normalising media-to-lumen ratio of resistance arteries,14, 15, 16 despite similar reductions in BP. Comparative findings exist from the myocardial circulation, where ACEI increase CFR and cause regression of hypertensive resistance artery structure when compared with β-blockers.17
Many recent studies have focused on the effects of vasodilating treatment in previously untreated patients with EH; however, despite a sizeable residual cardiovascular risk in patients receiving antihypertensive treatment,18 little prospective information exists on correction of microvascular structure and systemic vascular resistance by vasodilating treatment in these patients.
The primary aim of the present study was to improve the understanding of how structural changes in the microvasculature are corrected in hypertensive patients during antihypertensive treatment. First, we aimed to investigate whether patients with high systemic vascular resistance index (SVRI), despite well-treated BP, would benefit from additional vasodilator therapy in terms of a reduction in systemic vascular resistance. Second, we investigated whether changes in SVRI could predict correction of vascular structure, assessed indirectly by CFR, minimum coronary resistance (C-Rmin) and minimum forearm vascular resistance (F-Rmin). The study was designed as a prospective cohort study as the purpose was not to investigate the effect of specific antihypertensive drugs, but rather the association between changes in the hemodynamic parameters. Patients were stratified into two subgroups according to baseline SVRI: those with SVRI above median (group 1), and those with SVRI below median (group 2).
Materials and methods
The study was approved by the locally appointed Ethics Committee and the Danish Medicines Agency and conducted in accordance with the Helsinki II Declaration and Title 45, US Code of Federal Regulations, part 46, Protection of Human Subjects, revised 13 November 2001, effective 13 December 2001. Written informed consent was obtained from all participating subjects and the study was conducted according to good clinical practice guidelines (ICH-GCP Guideline (CPMP/ICH/135/95, Directive 2001/20/EC)), registered at www.clinicaltrials.gov (NCT01180413) and monitored by the Good Clinical Practice unit at Aarhus University Hospital, Denmark.
Essential hypertensive patients (age 25–80 years), on antihypertensive treatment, were recruited from the Hypertension Outpatient Clinic or by advertising in the local newspaper. Clinical characteristics are listed in Table 1. Subjects were eligible for inclusion if they had received unaltered antihypertensive medication for a minimum of 3 months and if daytime ambulatory BP (ABP) was above 120/75 mm Hg on current treatment (to prevent symptomatic hypotension). Patients underwent a clinical examination, electrocardiogram, blood and urine analyses. Twenty-four-hour ABP was then determined and baseline recordings were performed if patients met the inclusion criteria. Main exclusion criteria were secondary forms of hypertension, body mass index >35 kg m−2, signs or a history of ischaemic heart disease, valvular heart disease, atrial fibrillation/flutter, diabetes, renal disease, neurological disease, ejection fraction ⩽45% by echocardiography or ongoing antihypertensive treatment with a combination of either a CCB+ACEI or a CCB+ARB.
Experimental protocol
The investigation was designed as a single centre open-label prospective cohort study. Six months of intensive vasodilating therapy was given as add-on to the ongoing antihypertensive treatment. Patients who received neither CBB nor ACEI had 5 mg amlodipine and 5 mg ramipril added to their treatment. If the ongoing treatment included a CCB, only 5 mg ramipril was added, and if treatment included an ACEI/ARB and no CCB, 5 mg amlodipine was added. One month following inclusion, office BP was measured. Patients who had ramipril added to their ongoing treatment and who did not show signs or symptoms of hypotension had the dose of ramipril increased to 10 mg. In the case of dry cough, 5 or 10 mg ramipril was substituted with 50 or 100 mg losartan. Amlodipine was substituted with 10 mg lercanidipine in the case of ankle oedema.
BP measurements
24-h ABP monitoring was performed with validated and calibrated monitors (Spacelab 90217; Spacelabs Healthcare, Issaquah, Washington, USA). Daytime (0700–2300 hours) ABP measurements were performed automatically at 20 min intervals and every 30 min during the night (2300–0700 hours). Patients were asked to perform normal activities of daily living except physical training.
Echocardiography
Two-dimensional and Doppler echocardiograms were recorded with the Vivid 7 Dimension Ultrasound System (General Electric Healthcare, Buckinghamshire, UK) with a standard adult probe (4 S GE ultrasound probe 1.7/3 MHz) according to the recommendations of the American Society of Echocardiography guidelines.19
Coronary flow reserve
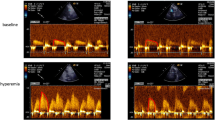
CFR is the ratio between blood flow velocity during rest and during maximal dilation. Doppler echocardiography was used to assess beat-to-beat coronary flow velocity in left anterior descending artery (LAD) under basal resting conditions and during pharmacologically induced coronary vasodilation with adenosine (140 μg min−1 kg−1, Adenosine Life Medical Sweden AB). The distal part of LAD was visualised from a modified apical long axis view and maximum resting flow velocity measured with a 7 MHz broadband probe (7 S GE Healthcare 3–8 MHz).20 A two-dimensional view of LAD during rest and hyperaemia was stored and used for measurement of LAD cross-sectional area (CSA). Two flow velocities were measured during rest and hyperaemia and the mean values were used for calculation of CFR. CFR was successfully determined in 45 patients, but could not be determined in three individuals because of suboptimal image quality.
Forearm plethysmography
With the patient in supine position, forearm blood flow was measured by strain gauge venous occlusion plethysmography (Hokanson EC6; Bellevue, WA, USA) on the non-dominant arm.21 The brachial cuff was inflated to 50 mm Hg above systolic BP or at least 200 mm Hg for 10 min to induce ischaemia. Hand circulation was simultaneously interrupted by inflating a wrist cuff to 220 mm Hg. After 5 min of ischaemia, 10 handgrips were performed to maximise the oxygen consumption in the forearm.22 Five venous occlusion measurements were performed in a rapid sequence with the brachial cuff deflated to 40 mm Hg. Concurrently, BP was measured on the contralateral arm. The percentage rise in forearm volume at each occlusive sequence was measured by a circumference adapted mercury-in-silastic strain gauge positioned at the widest part of the forearm.
Cardiac output
Noninvasive measurements of cardiac output (CO) were performed with a validated inert gas rebreathing method (Innocor; Innovision, Odense, Denmark).23 Patients were asked to avoid physical activity and food consumption for 1 h. Before starting, patients were instructed on correct breathing technique and three test rounds with atmospheric air were performed to accustom patients to the system.5 Patients then rebreathed an oxygen-enriched gas mixture containing an inert soluble gas (0.5% N2O) and an inert insoluble gas (0.1% SF6) at a respiration rate of 15–20 l min−1. N2O concentration decreases during rebreathing with a rate proportional to pulmonary blood flow, which in the absence of pulmonary shunts (defined as arterial O2 saturation >98%) equals CO.24 Respiration of insoluble SF6 allowed for determination of lung volume from which the soluble gas disappeared. Brachial cuff BP measurements were performed simultaneously and SVR calculated from (mean arterial pressure (MAP)−4.6 mm Hg)/CO, where 4.6 mm Hg is the estimated venous pressure.
Calculations and statistical analysis
The study population was stratified into two groups according to baseline SVRI (median SVRI 37.5±1.4 mm Hg min l−1 m−2 at baseline). Baseline SVRI above median (group 1) and baseline SVRI below median (group 2; Figure 1). Quantile–quantile plots were used to test for normal distribution. In the absence of Gaussian distribution, the variable was log-transformed. Between-group differences were analysed with unpaired two-tailed student’s t-test, while within-group differences were analysed with paired t-test. Student’s t-test was performed with GraphPad Prism 5.0 (GraphPad Software Inc., CA, USA). The relationship between changes in the hemodynamic indices was analysed with a simple linear regression analysis. SPSS 19.0 (SPSS, IL, USA) was used for Multivariate analysis to adjust for change in BP. All data are presented as means±s.e.m. Statistical significance was defined as P<0.05.
Left ventricular mass (LVM) and LVM index (LVMI) were calculated according to international guidelines.19 CSA of LAD was calculated from the diameter (d) assuming a circular model: CSA=πd2/4.25 Maximal coronary volume flow was calculated as maximal coronary flow velocity × CSA. C-Rmin pr. 100 g LVM was calculated as: MAP during adenosine infusion/(maximal coronary volume flow) × (LVM/100 g). Glomerular filtration rate (GFR) was estimated with the Cockcroft-Gault formula:26 GFR=((140–age) × body mass, kg × constant)/serum creatinine, μmol l−1, where the constant is 1.23 for men and 1.04 for women.
Results
Fifty-one patients were enroled and 48 patients completed the study (44% male and 56% female). One patient was excluded because of newly diagnosed diabetes, one because of moderate aortic regurgitation and one withdrew his informed consent. No severe adverse events occurred during the study.
Clinical characteristics and BP reduction
At inclusion, 28 patients received monotherapy and 20 patients received dual antihypertensive therapy. Taken together, 12 patients received treatment with CCB, 19 received thiazides, 5 received β-blockers, 18 ACEIs and 14 received ARBs as monotherapy or in combination. The majority of patients were non-smokers (96%). Biochemical parameters, body mass index and GFR were unaltered following treatment (Table 1).
Patients were on average well regulated with respect to baseline 24-h ABP: 132±1/81±1 mm Hg systolic and diastolic, respectively, with no significant difference between groups 1 and 2 (P=0.71). Following additional vasodilating treatment, 24-h MAP decreased significantly (P<0.001, Table 1) by 6.5±1.1% (group 1) and 4.6±0.9% (group 2; group 1 vs 2: P=0.19). Ambulatory systolic BP and diastolic BP decreased by the same proportion, with no significant change in HR (P=0.93).
Systemic hemodynamic responses to intensified vasodilation
Table 2a summarizes systemic cardiovascular parameters at baseline and following intensified treatment. Baseline SVRI was significantly higher in group 1 (P<0.001) and decreased by 14.4±3.1% (P<0.001) compared with −2.2±4.1% (P=0.76) in group 2 (group 1 vs group 2: P=0.003, Figure 1). Heart rate did not change significantly during cardiac output measurement.
Coronary and forearm microcirculation
Coronary minimum vascular resistance (C-Rmin) decreased by 22±8% (group 1, P=0.02) and 24±4% (group 2, P<0.001) during the 6 months of additional treatment (group 1 vs 2: P=0.80, Figure 2). CFR increased by 23±3% (group 1, P<0.001) and 17±3% (group 2, P<0.001; group 1 vs 2: P=0.16). This was caused by a combination of decreased resting coronary flow velocity and increased flow velocity during adenosine-induced hyperaemia. No significant change was observed in forearm minimum vascular resistance; 6.5±6.3% (group 1, P=0.79) vs −2.0±4.8% (group 2, P=0.38; group 1 vs 2: P=0.30), see Figure 2, Table 2b.
Echocardiography
LVMI decreased significantly (P<0.001) by 9.9±2.0% (group 1) and 8.4±1.9% (group 2) during treatment (group 1 vs group 2: P=0.60, Table 3). Indices of left ventricular systolic and diastolic function were unchanged (EF, TEI-index, E/A-ratio and E/E’; Supplementary Echocardiographic Data). Likewise, atrial size was unchanged.
Correlations
There was a significant but weak correlation between changes in CFR and SVRI (r2=0.12, P=0.02; data not shown). No significant correlation was found between CFR and MAP (r2=0.03, P=0.24). Changes in SVRI did not correlate to changes in either C-Rmin (r2=0.02, P=0.41) or F-Rmin (r2=0.002, P=0.83).
Discussion
The present study shows that for patients already well treated for EH but with high SVRI, aggressive vasodilator treatment causes reduction in SVRI that was greater than that seen in patients with lower SVRI. Regardless of SVRI status, treatment caused lower coronary minimum vascular resistance and greater maximum vasodilator capacity in the heart. Unexpectedly, this occurred independently of SVRI and of BP. No changes were seen in the forearm circulation. Although several previous studies have demonstrated that antihypertensive treatment improves coronary microvascular dysfunction in EH, to our knowledge there are no previous data on the impact of additional vasodilating treatment in patients with mild hypertension and well-regulated BP. Thus, the present study is the first to demonstrate that coronary, but not forearm, microvascular dysfunction can be further improved despite BP control and that this may not be predicted from changes in SVRI.
Systemic response to intensified vasodilator treatment
The main aim of the current study was to evaluate whether mildly hypertensive patients with high SVRI despite well-regulated BP, would have a more pronounced improvement in coronary and peripheral microcirculation as a response to intensified vasodilator therapy compared with patients with low SVRI. This was based on the fact that systemic vascular resistance is increased in EH and previous observations that antihypertensive therapy did not reduce peripheral vascular resistance (F-Rmin) in all EH patients despite reduced BP.13 Hence, determination of SVRI and secondary identification of high-SVRI individuals could be of potential clinical interest, in order to target treatment specifically to reduce SVRI using additional vasodilating therapy. However, despite our current finding that SVRI is significantly more reduced in the high-SVRI group than in the low SVRI group, we observed comparable improvements in the coronary and peripheral microcirculation and no clinical relevant correlations between SVRI and microvascular structure. Only a weak linear correlation between changes in SVRI and CFR was found, that was non-significant following subdivision per SVRI group. We have previously reported similar findings in untreated patients with mild hypertension following initiation of antihypertensive treatment (r=−0.30, P=0.04 vs r=−0.35, P=0.02).22 Thus, current results are in agreement with previous findings and supports a lack of clinical important association between SVRI and non-invasive indices of vascular structure. Although a large-scale prospective clinical study is needed for final conclusions regarding the correlation between changes in SVRI and CFR, current and previous findings, does not support assessment of SVRI to be helpful in determining cardiac microvascular dysfunction in EH.
Mechanisms for the improvement in CFR and decrease in minimal coronary resistance
Coronary microvascular dysfunction is documented in EH and even in patients with prehypertension. In the present study, coronary microvascular function was improved following intensified long-term vasodilator therapy in patients with both high and lower SVRI. The reduction in C-Rmin and increase in maximal coronary flow velocity combined with decreased left ventricular mass index indicate this is caused by a combination of functional and structural improvements of the coronary microcirculation. First, the lowering of resting coronary flow velocity contributed significantly to the substantial improvement in CFR following vasodilator therapy. As resting coronary flow velocity primarily relates to the myocardial oxygen consumption,27 the reduction in resting flow velocity observed in this study may be associated with the lowering of mean arterial pressure and hence afterload. This is consistent with previous findings by Rossen et al.,28 who showed that changes in heart rate or BP are accompanied by changes in resting coronary perfusion. Second, intensified vasodilator therapy has been shown to improve coronary perfusion by correction of structural alterations of intramyocardial arterioles in hypertensive rodents,29 which is consistent with the increase in maximal coronary flow velocity in the current study. In addition, improvements in diastolic function may have contributed since myocardial perfusion manly occurs in diastole (Supplementary Echocardiographic Data). Although, no linear correlation was found between changes in MAP and CFR, the multifactorial mechanisms of impaired CFR in EH (increased resting flow due to LV hypertrophy and impaired hyperaemic response due to remodelling and diastolic dysfunction) suggests some degree of relation. However, the improvement of coronary reserve could not be predicted from changes in SVRI and no significant correlation between MAP and coronary reserve was found in the current study, supporting a dissociation between microvascular structure and arterial pressure.4
Forearm and coronary vascular structure
Previous cross-sectional studies have indicated that the structure of the peripheral vasculature is correlated with the vascular resistance in the coronary microcirculation.30 This is further supported by a recent prospective study, which demonstrated that structural changes in the forearm and coronary microcirculation occur in parallel during initiation of antihypertensive treatment.22 Surprisingly our data showed no change in F-Rmin despite marked improvements in the coronary perfusion, indicating that changes in F-Rmin do not predict changes in CFR in well-treated patients with hypertension during intensive vasodilating treatment. This suggests that there may be a limit to the reduction in F-Rmin that can be obtained by vasodilator treatment.
Clinical implications of improved coronary microcirculation
Although the additional treatment reduced MAP and improved CFR, these two parameters were not correlated. Thus, reduction in MAP could not be taken to imply an improvement in coronary reserve, and vice versa. Moreover, despite the gain in coronary perfusion with today’s first-choice antihypertensive drug classes,17 the data indicate that even though the recommended treatment goals for BP have already been attained, further improvements in the coronary perfusion may still be achieved with intensified vasodilator treatment. This was found in spite of the fact that the majority (92%) of the included patients already received at least one vasodilator drug at baseline. The current study indicates that improved coronary perfusion can be obtained with intensified vasodilator therapy, but prognostic data are needed to establish whether this improves exercise capacity and cardiovascular outcome. Currently such data are lacking in relation to CFR in uncomplicated EH, but reduced CFR is a sensitive predictor of target-organ damage31 and has been associated with adverse clinical outcome in patients with chest pain,9 hypertrophic cardiomyopathy32 and LAD stenosis33 suggesting a prognostic role of CFR. In addition, CFR closely correlates with maximal exercise capacity,34 and exercise capacity is a strong predictor of subsequent cardiac events.35 So to the extent that an improvement in CFR is advantageous, there is a potential to improve outcome with intensified vasodilating treatment, but certainly also to improve angina in symptomatic microvascular disease.
Limitations
Although the study has provided a clear result, there are limitations. First, CFR and C-Rmin were determined non-invasively and no direct measurements of the coronary microvascular structure were made. The improved coronary vasodilator reserve could thus be related to mechanisms other than correction of vascular structure. In addition, CFR is a surrogate marker of global coronary perfusion and cannot fully reveal the distribution of blood flow in the entire myocardium, although there were no clinical indications of coronary artery disease in these mildly hypertensive patients. The investigated population was exclusively Caucasian and limitations exist when extrapolating the results to humans of different ethnic-origin.36 Arterial stiffness was not determined. Although, arterial stiffness is predominantly related to ageing of conduit arteries some influence may exist on the microvasculature that may affect the current results. BP was measured in the brachial artery, not centrally. Antihypertensive agents have been reported to have varying effects on peripheral and central arteries.37
In conclusion, the current study demonstrates that for patients with high SVRI, additional vasodilating treatment can cause a decrease in SVRI. Furthermore, regardless of SVRI status, hypertensive coronary microvascular dysfunction can be improved by additional vasodilating treatment, even in mildly hypertensive patients with well-regulated BP. Moreover, coronary microvascular changes occurred independently of the change in BP and SVRI. In contrast, no changes in forearm microvascular dysfunction were seen. We conclude that, the status of the coronary microvasculature should be assessed directly rather than using information on BP and SVRI to assess the need for further treatment.

References
Mulvany MJ . Small artery remodeling in hypertension. Curr Hypertens Rep 2002; 4: 49–55.
Conway J . Hemodynamic aspects of essential hypertension in humans. Physiol Rev 1984; 64: 617–660.
Jacobsen JC, Hornbech MS, Holstein-Rathlou NH . Significance of microvascular remodelling for the vascular flow reserve in hypertension. Interface Focus 2011; 1: 117–131.
Christensen KL, Buus NH . Dissociation of blood pressure and resistance artery structure: potential clinical implications. Basic Clin Pharmacol Toxicol 2012; 110: 73–79.
Eftekhari A, Mathiassen ON, Buus NH, Gotzsche O, Mulvany MJ, Christensen KL . Disproportionally impaired microvascular structure in essential hypertension. J Hypertens 2011; 29: 896–905.
Folkow B, Grimby G, Thulesius O . Adaptive structural changes of the vascular walls in hypertension and their relation to the control of the peripheral resistance. Acta Physiol Scand 1958; 44: 255–272.
Strauer BE . Ventricular function and coronary hemodynamics in hypertensive heart disease. Am J Cardiol 1979; 44: 999–1006.
Vogt M, Strauer BE . Systolic ventricular dysfunction and heart failure due to coronary microangiopathy in hypertensive heart disease. Am J Cardiol 1995; 76: 48D–53D.
Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M, Picano E . Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near-normal coronary arteries. Am J Cardiol 2009; 103: 626–631.
Mathiassen ON, Buus NH, Sihm I, Thybo NK, Morn B, Schroeder AP et al. Small artery structure is an independent predictor of cardiovascular events in essential hypertension. J Hypertens 2007; 25: 1021–1026.
Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML et al. Prognostic significance of small-artery structure in hypertension. Circulation 2003; 108: 2230–2235.
Buus NH, Mathiassen ON, Fenger-Gron M, Praestholm MN, Sihm I, Thybo NK et al. Small artery structure during antihypertensive therapy is an independent predictor of cardiovascular events in essential hypertension. J Hypertens 2013; 31: 791–797.
Mathiassen ON, Buus NH, Larsen ML, Mulvany MJ, Christensen KL . Small artery structure adapts to vasodilatation rather than to blood pressure during antihypertensive treatment. J Hypertens 2007; 25: 1027–1034.
Thybo NK, Stephens N, Cooper A, Aalkjaer C, Heagerty AM, Mulvany MJ . Effect of antihypertensive treatment on small arteries of patients with previously untreated essential hypertension. Hypertension 1995; 25: 474–481.
Schiffrin EL, Deng LY, Larochelle P . Progressive improvement in the structure of resistance arteries of hypertensive patients after 2 years of treatment with an angiotensin I-converting enzyme inhibitor. Comparison with effects of a beta-blocker. Am J Hypertens 1995; 8: 229–236.
Schiffrin EL, Pu Q, Park JB . Effect of amlodipine compared to atenolol on small arteries of previously untreated essential hypertensive patients. Am J Hypertens 2002; 15: 105–110.
Buus NH, Bottcher M, Jorgensen CG, Christensen KL, Thygesen K, Nielsen TT et al. Myocardial perfusion during long-term angiotensin-converting enzyme inhibition or beta-blockade in patients with essential hypertension. Hypertension 2004; 44: 465–470.
Blacher J, Evans A, Arveiler D, Amouyel P, Ferrieres J, Bingham A et al. Residual cardiovascular risk in treated hypertension and hyperlipidaemia: the PRIME Study. J Hum Hypertens 2010; 24: 19–26.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18: 1440–1463.
Dimitrow PP, Galderisi M, Rigo F . The non-invasive documentation of coronary microcirculation impairment: role of transthoracic echocardiography. Cardiovasc Ultrasound 2005; 3: 18.
Mathiassen ON, Buus NH, Olsen HW, Larsen ML, Mulvany MJ, Christensen KL . Forearm plethysmography in the assessment of vascular tone and resistance vasculature design: new methodological insights. Acta Physiol (Oxf) 2006; 188: 91–101.
Eftekhari A, Mathiassen ON, Buus NH, Gotzsche O, Mulvany MJ, Christensen KL . Changes in blood pressure and systemic vascular resistance do not predict microvascular structure during treatment of mild essential hypertension. J Hypertens 2012; 30: 794–801.
Damgaard M, Norsk P . Effects of ventilation on cardiac output determined by inert gas rebreathing. Clin Physiol Funct Imaging 2005; 25: 142–147.
Agostoni P, Cattadori G, Apostolo A, Contini M, Palermo P, Marenzi G et al. Noninvasive measurement of cardiac output during exercise by inert gas rebreathing technique: a new tool for heart failure evaluation. J Am Coll Cardiol 2005; 46: 1779–1781.
Nitenberg A, Ledoux S, Valensi P, Sachs R, Attali JR, Antony I . Impairment of coronary microvascular dilation in response to cold pressor—induced sympathetic stimulation in type 2 diabetic patients with abnormal stress thallium imaging. Diabetes 2001; 50: 1180–1185.
Cockcroft DW, Gault MH . Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41.
Wieneke H, von Birgelen C, Haude M, Eggebrecht H, Mohlenkamp S, Schmermund A et al. Determinants of coronary blood flow in humans: quantification by intracoronary Doppler and ultrasound. J Appl Physiol 2005; 98: 1076–1082.
Rossen JD, Winniford MD . Effect of increases in heart rate and arterial pressure on coronary flow reserve in humans. J Am Coll Cardiol 1993; 21: 343–348.
Amenta F, Peleg E, Tomassoni D, Sabbatini M, Rosenthal T . Effect of treatment with lercanidipine on heart of Cohen-Rosenthal diabetic hypertensive rats. Hypertension 2003; 41: 1330–1335.
Rizzoni D, Palombo C, Porteri E, Muiesan ML, Kozakova M, La Canna G et al. Relationships between coronary flow vasodilator capacity and small artery remodelling in hypertensive patients. J Hypertens 2003; 21: 625–631.
Schwartzkopff B, Motz W, Frenzel H, Vogt M, Knauer S, Strauer BE . Structural and functional alterations of the intramyocardial coronary arterioles in patients with arterial hypertension. Circulation 1993; 88: 993–1003.
Cortigiani L, Rigo F, Gherardi S, Galderisi M, Sicari R, Picano E . Prognostic implications of coronary flow reserve on left anterior descending coronary artery in hypertrophic cardiomyopathy. Am J Cardiol 2008; 102: 1718–1723.
Meimoun P, Benali T, Elmkies F, Sayah S, Luycx-Bore A, Doutrelan L et al. Prognostic value of transthoracic coronary flow reserve in medically treated patients with proximal left anterior descending artery stenosis of intermediate severity. Eur J Echocardiogr 2009; 10: 127–132.
Schachinger V, Britten MB, Elsner M, Walter DH, Scharrer I, Zeiher AM . A positive family history of premature coronary artery disease is associated with impaired endothelium-dependent coronary blood flow regulation. Circulation 1999; 100: 1502–1508.
Roger VL, Jacobsen SJ, Pellikka PA, Miller TD, Bailey KR, Gersh BJ . Prognostic value of treadmill exercise testing: a population-based study in Olmsted County, Minnesota. Circulation 1998; 98: 2836–2841.
Gupta AK . Racial differences in response to antihypertensive therapy: does one size fits all? Int J Prev Med 2010; 1: 217–219.
Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006; 113: 1213–1225.
Acknowledgements
The project was funded by a grant from the Danish Council for Independent Research
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Journal of Human Hypertension website
Supplementary information
Rights and permissions
About this article
Cite this article
Engholm, M., Mulvany, M., Eftekhari, A. et al. Positive effects of aggressive vasodilator treatment of well-treated essential hypertensive patients. J Hum Hypertens 30, 690–696 (2016). https://doi.org/10.1038/jhh.2016.13
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2016.13
- Springer Nature Limited