Abstract
Autonomic dysfunction is implicated in prehypertension, and previous studies have suggested that therapies that improve modulation of sympathovagal balance, such as biofeedback and slow abdominal breathing, are effective in patients with prehypertension at rest. However, considering that psychophysiological stressors may be associated with greater cardiovascular risk in prehypertensives, it is important to investigate whether heart rate variability biofeedback (HRV-BF) results in equivalent effects on autonomic cardiovascular responses control during stressful conditions in prehypertensives. A total of 32 college students with prehypertension were enrolled and randomly assigned to HRV-BF (n=12), slow abdominal breathing (SAB, n=10) or no treatment (control, n=10) groups. Then, a training experiment consisting of 15 sessions was employed to compare the effect of each intervention on the following cardiovascular response indicators before and after intervention: heart rate (HR); heart rate variability (HRV) components; blood volume pulse amplitude (BVPamp); galvanic skin response; respiration rate (RSP); and blood pressure. In addition, the cold pressor test and the mental arithmetic challenge test were also performed over two successive days before and after the invention as well as after 3 months of follow-up. A significant decrease in HR and RSP and a significant increase in BVPamp were observed after the HRV-BF intervention (P<0.001). For the HRV analysis, HRV-BF significantly reduced the ratio of low-frequency power to high-frequency power (the LF/HF ratio, P<0.001) and increased the normalized high-frequency power (HFnm) (P<0.001) during the stress tests, and an added benefit over SAB by improving HRV was also observed. In the 3-month follow-up study, similar effects on RSP, BVPamp, LF/HF and HFnm were observed in the HRV-BF group compared with the SAB group. HRV-BF training contributes to the beneficial effect of reducing the stress-related cardiovascular response in prehypertensives by improving autonomic sympathovagal modulation.
Similar content being viewed by others
Introduction
It is widely accepted that prehypertension (PHT) is associated with a high risk of progression to hypertension and cardiovascular disease later in life and leads to an increased risk of cardiac morbidity and mortality.1, 2, 3, 4 Many factors contribute to the pathology of PHT, such as genetics, hormones, age, lifestyle, psychophysiological factors and metabolic factors. However, psychophysiological stress is currently suggested to be one of the most important risk factors in the initiation and progression of high blood pressure (BP).5, 6, 7 Acute stress impairs endothelial function and produces short-lasting increases in arterial pressure and HR,8, 9 and individuals with hypertension or PHT10 display enhanced cardiovascular responses to stress.11
Mechanistic studies have shown that acute12 and chronic stress13, 14, 15 most likely affect the hypothalamic–pituitary–adrenal axis and the autonomic nervous system directly leading to enhanced sympathetic cardiovascular activity, which contributes to increased arterial pressure16, 17 or arrhythmias.18 Moreover, a growing body of evidence suggests the existence of autonomic dysregulation such as a decrease in heart rate variability (HRV) and baroreflex sensitivity as well as an increase in sympathetic activity in stressed individuals13, 14, 19 and prehypertensives exposed to psychophysiological or psychosocial stress.15, 20, 21 Although these studies strongly imply the effect of stress in the progression of hypertension, the lack of epidemiologic evidence and clinical guidelines for stress management has encouraged patients to seek alternative medicine.
Our previous studies have demonstrated the benefits of heart rate variability biofeedback (HRV-BF) and slow abdominal breathing (SAB) on BP control in subjects with hypertension or PHT at rest.22, 23, 24 Enhanced parasympathetic activity and subdued sympathetic activity are also observed after both interventions. However, cardiovascular responsiveness and recovery to acute stressors are considered to be more preferred prognostic indicators in hypertension or PHT,25, 26, 27 and studies have been designed to assess the effects of HRV-BF or SAB on autonomic function in the presence of stress. Thus, the present study was conducted to examine whether prehypertensives exhibit signs of autonomic dysregulation and overreactivity of cardiovascular responses during acute stress. We also aimed to test the additional hypothesis that alternative medicine approaches such as HRV-BF or SAB modulate stress-related autonomic nervous system dysregulation and reduce arterial pressure.
Materials and Methods
Participants
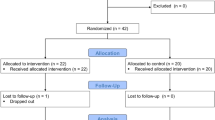
A total of 32 volunteers (8 females; aged 19–23) with a confirmed diagnosis of PHT according to the current criteria,10 which include systolic BP between 120 and 139 mm Hg and/or diastolic pressures between 80 and 89 mm Hg, were considered for participation in this study. Subjects with symptoms or clinical criteria suggestive of cardiopulmonary, metabolic or major psychiatric diseases were excluded. None of the participants were receiving medical treatment before enrollment, and they were required to abstain from food or drink for 2 h before the training procedure and from caffeine on the day of training to control for the impact of these variables on autonomic cardiovascular responses. A BioTrace Advanced Physiological Monitoring and Feedback System (Mind Media B.V., Herten, Netherlands) was used to process the data acquired from the Spirit Nexus-16B Biofeedback System (Mind Media B.V.), and the R–R-interval data from the electrocardiogram (ECG) as well as other physiological data such as galvanic skin response (GSR), respiratory rate (RSP), BP and BVPamp were simultaneously collected both at rest and during the training procedures and stress tests.
First, the baseline characteristics of the subjects with PHT were compared with 26 (6 females; aged 19–23) normal blood pressure healthy volunteers (NBP). The PHT subjects were then randomized to a HRV-BF group (n=12), a SAB group (n=10) or a control group (n=10). Ethics approval was granted by Sun Yat-Sen University Institutional Ethics Committee and all participants provided informed consent in accordance with the National Institutes of Health on medical research and ethical guidelines.
Materials and procedure
The experiment was conducted in a quiet, dimly lit room with a constant temperature of 26±2 °C and a relative humidity of 60–70% and included 15 training sessions performed twice a week over 2 months. Each session lasted 40 min with the initial 5 min for baseline recording, 30 min for training and 5 min for rest. The participants were also required to rest for ~15 min to get adapted and were examined at the same time of day to eliminate the impact of the fluctuation in physiological parameters. Details of the HRV-BF procedure have been described elsewhere.24 Briefly, subjects were instructed to breathe abdominally following the respiratory pacer at the particular frequency with the highest amplitude of low-frequency HR oscillations, which is a resonance frequency in the individual’s cardiovascular system. It is determined by characteristics of the baroreflex system, and is the respiratory frequency at which the amplitude of HRV is maximized.28 Spectral analysis was performed on cardiac interbeat interval data within the 0.005–0.4 Hz band. Spectral data were updated approximately every second.
Subjects were instructed to increase their respiratory sinus arrhythmia by breathing with the pacer to increase the power spectrum peak that occurred at approximately resonance frequency with the goal of maximally increasing the respiratory sinus arrhythmia amplitude. Throughout the sessions, subjects in the HRV-BF group were required to practice breathing at his or her own resonance frequency at home for a 30-min period twice a day using a respiratory audio guide. Subjects in the SAB group breathed at 6 cycles min−1 and were required to practice breathing at this respiratory rhythm at home for a 30-min period twice a day using a respiratory audio guide. Subjects in the control group were instructed to breathe spontaneously and sit in front of the computer screen with no intervention.
A stress reactivity–recovery test was performed preintervention and on the following 2 days after the interventions, as well as at the 3-month follow-up including the cold pressor test (CPT) and the mental arithmetic challenge test (MAT). Subjects were invited to sit in a comfortable armchair for a 10-min adaptation after placement of the sensors. ECG, BVP and RSP data were recorded during the resting phase (baseline, duration=10 min), CPT phase (CPT, duration=2 min), MAT phase (MAT, duration=2 min) and recovery phase after either the CPT or MAT (recovery, duration=10 min). The CPT and MAT were presented in a counterbalanced order over two successive days to prevent order effects. For the CPT, which served as a physical and passive stressor, participants were asked to immerse his/her left hand in icy cold water (5 °C) up to the wrist for 2 min. For the MAT, which served as a psychological and active stressor, participants were required to perform digit subtraction with audio-taped instructions.
During the stress tasks, subjects were instructed to breathe spontaneously and not to move, talk, laugh or mentally pace their breathing rate to exclude the confounding influence of slow-paced respiration.
HRV analysis
The electrocardiograph was recorded simultaneously with a sampling rate of 512 samples s−1 during each session to evaluate the R–R intervals. The electrocardiograph (lead II) signal was amplified and processed using a 24-bit converter (Mind Media B.V.) and was transformed as the R–R intervals. All the R–R-interval data were carefully checked through visual screening to exclude all the undesirable beats (that is, to ensure that the analysis of each segment was free of movement artifacts and/or sharp transients in the signal due to premature beats), which accounted for <1% in each subject. Extra or missing beats were replaced with R–R intervals calculated by linear interpolation from adjacent cycles. R–R intervals for each studied segment were assessed with fast Fourier transform to calculate the spectral components of HRV, from which high-frequency power was used as an indicator of parasympathetic activity. Normalized high-frequency power (HFnm) and the ratio of low-frequency power to high-frequency power (the LF/HF ratio) were employed as an indicator of sympathovagal balance,29 despite the presence of controversial results.30 In the present study, HFnm is calculated as HF/HF+LF+VLF.
Statistical analysis
Data were analyzed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). The results are reported as the mean±s.e.m. unless otherwise indicated. Log10 transformation was applied for variables derived from the spectral HRV analysis (HF, HFnm) to control for skewness.
A one-way analysis of variance was applied to compare the baseline characteristics between NBP and PHT; a mixed-effects model analysis of variance was applied to compare the cardiovascular responses and autonomic changes during the stress reactivity–recovery tests between NBP and PHT, and statistical significance was defined by two-tailed tests at P<0.05.
Repeated measures linear mixed models (LMM) analyses were applied to examine the effects of interventions on the autonomic changes across each 2-min segment of stress–reactivity–recovery, with random intercepts and adjustments for age, sex, body mass index and stress tasks. LMM analysis was also adjusted for the serious correlation between repeated measurement across the testing protocol. Each LMM tested the main effect for interventions, stages, the stressor-by-stage interaction and the time-by-treatment interaction. Only those effects with P<0.1 were included in the final model. Random intercepts were used to control for variability between individuals.
Results
Baseline characteristics of NBP and PHT
As shown in Table 1, no significant differences were observed in age, gender and height between the NBP and PHT subjects. However, weight, body mass index, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were significantly higher in the PHT subjects (P<0.05). HR and GSR were higher in the PHT group, whereas HF and HFnm were lower compared with subjects in the NBP group (P<0.05) at rest.
During the stress reactivity–recovery tests, subjects in the PHT group exhibited intense HR, RSP and GSR responses (P<0.01) during MAT and slower post-MAT recovery was observed in HR, GSR and BVPamp (P<0.01). Slower post-CPT recovery was observed in BVPamp (P<0.01) in the PHT subjects compared with the NBP subjects as shown in Figure 1. Furthermore, prehypertensives also displayed a marked increase in the LF/HF ratio during MAT (P<0.01) and post-CPT recovery (P<0.01) as well as a significant reduction in HF during post-CPT recovery (P<0.01) compared with the NBP subjects (Figure 2). These findings suggested that prehypertensives exhibit sympathovagal balance dysfunction with decreased vagal activity and increased sympathetic arousal.
Effects of HRV-BF and SAB on BP
As shown in Figure 3, HRV-BF produced a marked decrease in both systolic blood pressure (from 131.58±8.41 mm Hg to 116.17±9.25 mm Hg, P<0.01) and DBP (from 81.33±3.06 mm Hg to 71.17±7.12 mm Hg) compared with the control group. Reductions in SBP (from 126.70±5.56 mm Hg to 115.10±4.18 mm Hg, P<0.01) and DBP (from 80.30±6.27 mm Hg to 72.90±3.96 mm Hg, P<0.01) were also observed post-SAB intervention compared with the controls. However, no significant difference between the HRV-BF and SAB interventions was found in the reduction of BP, whereas based on the 3-month follow-up study, HRV-BF exhibited a tendency toward added benefits over SAB in SBP (P<0.05) and DBP (P<0.05) reduction.
Effects of HRV-BF and SAB on HR, BVPamp, GSR and RSP during the stress reactivity–recovery test
We applied repeated measures LMM to access the main effect of HRV-BF and SAB on autonomic cardiovascular responses in prehypertensives in stress tests. The results are shown in Table 2 and indicated that HR and RSP were significantly decreased and BVPamp was increased following 15 sessions of HRV-BF intervention (P<0.01) and compared with the control group (P<0.01). Identical effects of reduction in HR and RSP and an increase in BVPamp were also observed after SAB intervention (P<0.01) and compared with the control group (P<0.01). However, no superiority was shown for HRV-BF compared with SAB in the post-intervention study. More detailed information could be found in Supplementary Figure 1.
The 3-month follow-up study also showed a significant reduction in RSP (P<0.01) and an increase in BVPamp (P<0.01) after HRV-BF intervention, whereas a similar effect in HR was not observed. A significant decrease in RSP (P<0.05) and a significant increase in BVPamp (P<0.05) in the SAB group were observed in the follow-up study. No significant change was observed in GSR in either the post-intervention or follow-up study. However, HRV-BF exhibited a significant decrease in RSP (P<0.05) and a significant increase in BVPamp (P<0.05) compared with SAB based on the 3-month follow-up study. More detailed information could be found in Supplementary Figure 2.
Effects of HRV-BF and SAB on HRV components during the stress reactivity–recovery test
Table 3 shows the HRV parameters for the pre-treatment, post-HRV-BF, post-SAB and post-control groups. Significant increases in HF and HFnm and decreases in LF/HF were observed following 15 sessions of HRV-BF intervention (P<0.01) and compared with the control group (P<0.01). Similar effects were observed after SAB intervention (P<0.01). HRV-BF also revealed the superiority over SAB in modulating cardiac autonomic activity by increasing HF or HFnm and decreasing LF/HF (P<0.01). More detailed information could be found in Supplementary Figure 3.
The 3-month follow-up study on HRV components suggested that the significant effect on parameters that is reflective of sympathovagal balance such as LF/HF, HF or HFnm was sustainable for at least 3 months after both HRV-BF and SAB interventions (P<0.05). Meanwhile, HRV-BF also exhibited added benefits over SAB in modulating LF/HF, HF or HFnm at the 3-month follow-up (P<0.01). More detailed information could be found in Supplementary Figure 4.
Discussion
Sympathetic overreactivity, which is described as an excessive hypothalamic drive due to excessive environmental stimuli,31 has been persistently discussed in essential-hypertensive subjects or even in subjects with early-stage hypertension. Conflicting findings on cardiovascular reactivity to laboratory stressors in hypertensive and prehypertensive subjects have been reported on autonomic cardiovascular regulation in hypertension, possibly because differing methods of autonomic assessment were used, including muscle sympathetic nerve activity, variability of R–R intervals and baroreflex sensitivity.32, 33 Multiple indices reflecting cardiovascular reactivity were investigated in the present study, which showed a delayed BVPamp recovery in both CPT and MAT in prehypertensives and indicated relatively more lasting sympathetic reactivity to laboratory stressors.34 The results of enhanced HR, RSP and GSR in MAT rather than CPT were also consistent with the work of Eliasson et al.,35 which demonstrated that mental stressors may lead to a stronger cardiovascular and adrenosympathetic reaction than physical stressors, and this enhanced autonomic reaction in the presence of mental stressors might further extend to everyday emotional occurrences, which could be one of the most common causes in subjects with high BP.36, 37, 38, 39 These findings also indicated that sympathetic overreactivity might occur in the prehypertensive stage.
Moreover, analysis of HRV offered a further indication of sympathetic–vagal imbalance in prehypertensives. There are two peaks involved in the frequency domain of HRV analysis called the low-frequency peak and the high frequency peakHF, which are consistently assumed to be mediated, respectively, by cardiac sympathetic and parasympathetic activity predominantly; therefore, despite the controversy,40 the LF/HF ratio as well as HFnm is logically applied to evaluate the balance between sympathetic and vagal modulation.41 Moreover, it is believed that normalized units or the LF/HF ratio of R–R-interval variability provides the strongest correlations with attendant changes in muscle sympathetic nerve activity.42 Our results revealed an increased LF/HF ratio during both CPT and MAT as well as decreased HF or HFnm during recovery in CPT, which illustrates the combination of increased sympathetic and reduced vagal drive.
The effectiveness of stress reduction and relaxation, including HRV-BF and SAB, in lowering BP has been investigated for over 40 years. However, the mechanisms responsible for the BP-lowering effect induced by biofeedback or SAB remains incompletely understood. Our results suggested that both HRV-BF and SAB regulate and rebalance the sympathetic–vagal balance, resulting in significantly decreased HR and RSP and increased BVPamp during stress tests, which are largely maintained during the 3-month follow-up, suggested that favorable alterations in autonomic nervous system balance may be due to breathing at higher cardiorespiratory fitness, which is also correlated with lower resting BP.43, 44 No significant changes in GSR during stress after either HRV-BF or SAB might be due to the higher sensitivity of GSR to external stimuli and psychological activity compared with electrocardiogram and electroencephalogram. Moreover, individual differences in baseline might also affect the analysis of the present results that major differences in GRS were observed among individuals in the same environment as well as on different experimental days.
Consistent with the clinical findings in hypertension or coronary heart diseases,45, 46 we found that the HF or HFnm was increased, whereas the LF/HF ratio was decreased under stressful conditions after both HRV-BF and SAB; a similar effect was observed in the follow-up study, which indicated that HRV-BF enhances the capacity for tolerance and recovery from stressful conditions.24, 47, 48 Furthermore, HRV-BF also offered advantages over SAB in increasing vagal activity, which was particularly significant in the 3-month follow-up. The benefits of biofeedback over SAB regarding automatic rebalancing indicated that the participation of direct or indirect feedback signals might strengthen self-regulation and learning capacity on cardiovascular autonomic function and resulted in long-lasting effects.
Our present study revealed an intense BP reduction by HRV-BF, which was slightly different from our previous studies22, 23, 24 and is consistent with other relaxation therapies involving respiratory training.49, 50 The greater BP reduction in our study may also be attributed to the younger subjects as demonstrated by Nolan et al.45 as well as the dose–response effects related to training repetitions described by Elliott et al.51
In summary, the present findings support the assumption that HRV-BF may have beneficial effects on prehypertensives under stress conditions by reducing the cardiovascular responses and BP as well as improving autonomic sympathovagal modulation, suggesting that HRV-BF may serve as a novel behavioral coping strategy for pathophysiologically stress-related cardiovascular diseases. Further studies are required to investigate the connection between the medullary neural activity in the cardiorespiratory network and the cortical frontal-limbic circuits within the central autonomic network. The nerve–endocrine mechanisms, including the modulation of the hypothalamic–pituitary–adrenal axis and inflammatory factors, should also be investigated.

References
Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 2001; 345: 1291–1297.
Elliott WJ, Black HR . Prehypertension. Nat Clin Pract Cardiovasc Med 2007; 4: 538–548.
Pimenta E, Oparil S . Prehypertension: epidemiology, consequences and treatment. Nat Rev Nephrol 2010; 6: 21–30.
Gu D, Chen J, Wu X, Duan X, Jones DW, Huang JF et al. Prehypertension and risk of cardiovascular disease in Chinese adults. J Hypertens 2009; 27: 721–729.
Rozanski A, Blumenthal JA, Kaplan J . Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 1999; 99: 2192–2217.
Larkin KT . Stress and hypertension. In: Examining the Relation between Psychological Stress and High Blood Pressure. Yale University Press: New Haven, CT, USA, 2005.
Player MS, King DE, Mainous AR, Geesey ME . Psychosocial factors and progression from prehypertension to hypertension or coronary heart disease. Ann Fam Med 2007; 5: 403–411.
Schwartz AR, Gerin W, Davidson KW, Pickering TG, Brosschot JF, Thayer JF et al. Toward a causal model of cardiovascularresponses to stress and the development of cardiovasculardisease. Psychosom Med 2003; 65: 22–35.
Ghiadoni L, Donald DE, Cropley M, Mullen JM, Oakley G, Taylor M et al. Mentalstress induces transient endothelial dysfunction in humans. Circulation 2000; 102: 2473–2478.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572.
Steptoe A, Kivimäki M . Stress and cardiovascular disease: an update on current knowledge. Annu Rev Public Health 2013; 34: 337–354.
Tofler GH, Muller JE . Triggering of acute cardiovascular disease and potential preventive strategies. Circulation 2006; 114: 1863–1872.
Lucini D, Riva S, Pizzinelli P, Pagani M . Stress management at the worksite: reversal of symptoms profile and cardiovascular dysregulation. Hypertension 2007; 49: 291–297.
Lucini D, Di Fede G, Parati G, Pagani M . Impact of chronic psychosocial stress on autonomic cardiovascular regulation in otherwise healthy subjects. Hypertension 2005; 46 (5): 1201–1206.
Lucini D, Mela GS, Malliani A, Pagani M . Impairment in cardiac autonomic regulation preceding arterial hypertension in humans: insights from spectral analysis of beat-by-beat cardiovascular variability. Circulation 2002; 106: 2673–2679.
Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 case and 13648 controls from 52 countries (The INTERHEART study): case-control study. Lancet 2004; 364: 953–962.
Julius S . Sympathetic hyperactivity and coronary risk in hypertension. Hypertension 1993; 21: 886–893.
Brotman D, Golden SH, Wittstein IS . The cardiovascular toll of stress. Lancet 2007; 370: 1089–1100.
Vrijkotte TG, van Doornen LJ, de Geus EJ . Effects of work stress on ambulatory blood pressure, heart rate, and heart rate variability. Hypertension 2000; 35: 880–886.
Genovesi S, Pieruzzi F, Giussani M, Tono V, Stella A, Porta A et al. Analysis of heart period and arterial pressure variability in childhood hypertension: key role of baroreflex impairment. Hypertension 2008; 51: 1289–1294.
Flaa A, Mundal HH, Eide I, Kjeldsen S, Rostrup M . Sympathetic activity and cardiovascular risk factors in young men in the low, normal, and high blood pressure ranges. Hypertension 2006; 47: 396–402.
Xu XY, Gao J, Ling D, Wang TH . Biofeedback treatment of prehypertension: analyses of efficacy, heart rate variability and EEG approximate entropy. J Hum Hypertens 2007; 21: 973–975.
Wang SZ, Li S, Xu XY, Lin GP, Shao L, Zhao Y et al. Effect of slow abdominal breathing combined with biofeedback on blood pressure and heart rate variability in prehypertension. J Altern Complement Med 2010; 16: 1039–1045.
Lin G, Xiang Q, Fu X, Wang S, Wang S, Chen S et al. Heart rate variability biofeedback decreases blood pressure in prehypertensive subjects by improving autonomic function and baroreflex. J Altern Complement Med 2012; 18 (2): 143–152.
Wheat AL, Larkin KT . Biofeedback of heart rate variability and related physiology: a critical review. Appl Psychophysiol Biofeedback 2010; 35: 229–242.
Montano N, Porta A, Cogliati C, Costantino G, Tobaldini E, Casali KR et al. Heart rate variability explored in the frequency domain: a tool to investigate the link between heart and behavior. Neurosci Biobehav Rev 2009; 33: 71–80.
Brosschot JF, Pieper S, Thayer JF . Expanding stress theory: prolonged activation and perseverative cognition. Psychoneuroendocrinology 2005; 30: 1043–1049.
Lehrer PM, Vaschillo E, Vaschillo B . Resonant frequency biofeedback training to increase cardiac variability: rationale and manual for training. Appl Psychophysiol Biofeedback 2000; 25: 177–191.
Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 1986; 59 (2): 178–193.
Yamamoto Y, Hughson RL . Coarse-graining spectral analysis: new method for studying heart rate variability. J Appl Physiol 1991; 71: 1143–1150.
Mancia G, Bjorn Folkow A . Lecture: the sympathetic nervous system in hypertension. J Hypertens 1997; 15: 1553–1565.
Mancia G, Grassi G, Giannattasio C, Seravalle G . Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 1999; 34: 724–728.
Lucini D, Solaro N, Pagani M . May autonomic indices from cardiovascular variability help identify hypertension? J Hypertens 2014; 32 (2): 363–373.
Peper E, Harvey R, Lin IM, Hana T, Donald M . Is there more to blood volume pulse than heart rate variability, respiratory sinus arrhythmia, and cardiorespiratory synchrony? Biofeedback 2007; 35 (2): 54–61.
Eliasson K, Hjemdahl P, Kahan T . Circulatory and sympatho-adrenal responses to stress in borderline and established hypertension. J Hypertens 1983; 1: 131–139.
Manuck SB . Cardiovascular reactivity in cardiovascular disease: “once more unto the breach”. Int J Behav Med 1994; 1: 4–31.
Schwartz JE, Warren K, Pickering TG . Mood, location, and physical position as predictors of ambulatory blood pressure and heart rate: application of a multi-level random effects model. Ann Behav Med 1994; 16: 210–220.
Carels RA, Blumenthal JA, Sherwood A . Emotional responsivity during daily life: relationship to psychosocial functioning and ambulatory blood pressure. Int J Psychophysiol 2000; 36: 25–33.
Ebrahim S . Ann bowling. In: Handbook of Health Research Methods: Investigation, Measurement and Analysis. Open University Press: New York, NY, USA, 2005.
Billman GE . The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol 2013; 4: 26.
Sun P, Zhou K, Wang S, Li P, Chen SJ, Lin GP et al. Involvement of MAPK/NF-κB signaling in the activation of the cholinergic anti-inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS One 2013; 8 (8): e69424.
Pagani M, Montano N, Porta A, Malliani A, Abboud FM, Birkett C et al. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation 1997; 95 (6): 1441–1448.
Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med 2004; 116 (10): 682–692.
Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology. Circulation 2003; 107 (24): 3109–3116.
Nolan RP, Floras JS, Harvey PJ, Kamath MV, Picton PE, Chessex C et al. Behavioral neurocardiac training in hypertension: a randomized, controlled trial. Hypertension 2010; 55: 1033–1039.
Nolan RP, Kamath MV, Floras JS, Stanley J, Pang C, Picton P et al. Heart rate variability biofeedback as a behavioral neurocardiac intervention to enhance vagal heart rate control. Am Heart J 2005; 149: 1137.
Porges SW . Cardiac vagal tone: a physiological index of stress. Neurosci Biobehav Rev 1995; 19 (2): 225–233.
Porges SW . The poly vagal perspective. Biol Psychol 2007; 74: 116–143.
Elliott WJ, Izzo JL . Device-guided breathing to lower blood pressure: case report and clinical overview. MedGenMed 2006; 8 (3): 23.
Meles E, Giannattasio C, Failla M, Gentile G, Capra A, Mancia G . Nonpharmacologic treatment of hypertension by respiratory exercise in the home setting. Am J Hypertens 2004; 17: 370–374.
Elliot WJ, Izzo JL Jr, White WB, Rosing DR, Snyder CS, Alter A et al. Graded blood pressure reduction in hypertensive outpatients associated with use of a device to assist with slow breathing. J Clin Hypertens (Greenwich) 2004; 6 (10): 553–559.
Acknowledgements
This study was supported by the Fund of Science and Technology projects in Guangdong Province (No. 2010B031600075). We gratefully acknowledge the efforts of all volunteers who participated in this study.
Author Contributions
SJC, PS and THW conceived and designed the experiments. SJC, PS, SW and GPL performed the experiment. SJC, PS and THW analyzed the data. THW contributed reagents/materials/analysis tools. SJC, PS and THW wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Journal of Human Hypertension website
Rights and permissions
About this article
Cite this article
Chen, S., Sun, P., Wang, S. et al. Effects of heart rate variability biofeedback on cardiovascular responses and autonomic sympathovagal modulation following stressor tasks in prehypertensives. J Hum Hypertens 30, 105–111 (2016). https://doi.org/10.1038/jhh.2015.27
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2015.27
- Springer Nature Limited
This article is cited by
-
A Pilot Study of the Biofeedback Training to Reduce Salivary Cortisol Level and Improve Mental Health in Highly-Trained Female Athletes
Applied Psychophysiology and Biofeedback (2023)
-
Methods for Heart Rate Variability Biofeedback (HRVB): A Systematic Review and Guidelines
Applied Psychophysiology and Biofeedback (2023)
-
Heart Rate Variability: A Measure of Cardiovascular Health and Possible Therapeutic Target in Dysautonomic Mental and Neurological Disorders
Applied Psychophysiology and Biofeedback (2022)
-
Resonance frequency is not always stable over time and could be related to the inter-beat interval
Scientific Reports (2021)
-
Awareness of comfort immediately after a relaxation therapy session affects future quality of life and autonomic function: a prospective cohort study on the expectations of therapy
BioPsychoSocial Medicine (2018)