Abstract
Biomonitoring has become a standard approach for exposure assessment in occupational and environmental epidemiology. The use of biological effect markers to identify early adverse changes in target organs has also become widely adopted. However, the potential for kidney function to affect biomarker levels in the body and the optimal approach to adjustment of biomarker concentrations in spot urine samples for hydration status are two important but underappreciated challenges associated with biomarker use. Several unexpected findings, such as positive associations between urine nephrotoxicant levels and estimated glomerular filtration rate (eGFR), have been reported recently in research using biomarkers. These and other findings, discussed herein, suggest an impact of kidney glomerular filtration or tubule processing on biomarker levels. This is more commonly raised in the context of decreased kidney filtration, traditionally referred to as reverse causality; however, recent data suggest that populations with normal kidney filtration may be affected as well. Misclassification bias would result if biomarkers reflect kidney function as well as either exposures or early biological effect outcomes. Furthermore, urine biomarker associations with eGFR that differ markedly by approach used to adjust for urine concentration have been reported. Associations between urine measures commonly used for this adjustment, such as urine creatinine, and specific research outcomes could alter observed biomarker associations with outcomes. Research recommendations to address the potential impact of kidney function and hydration status adjustment on biomarkers are provided, including a range of approaches to study design, exposure and outcome assessment, and adjustment for urine concentration.
Similar content being viewed by others
INTRODUCTION
Biomonitoring, involving measurement of exogenous chemicals and/or their metabolites in blood, urine or other biological specimens, for example, blood lead, has become a standard approach to exposure assessment in occupational and environmental epidemiology. The use of biological effect markers to identify early adverse changes in target organs, such as β-2-microglobulin for the kidney, has also become widely adopted. These biomarker tools provide substantial benefits in terms of internal dose and early outcome assessment. However, their use poses certain challenges including: (i) the potential for kidney function to affect biomarker levels in the body, traditionally labeled reverse causality, and (ii) the optimal approach to adjustment for urine concentration, which is required to accurately determine concentration of urinary biomarkers in spot urine samples. Recent research supports the need to reconsider both issues in environmental epidemiology research. Our objectives herein are to: (i) review the potential impact of these challenges on research utilizing biomarkers; (ii) consider techniques to more accurately assess their influence on research using biomarkers; and (iii) provide recommendations for future work that may provide solutions to these challenges.
KIDNEY FUNCTION
The kidney is responsible for maintaining homeostasis in the body by excreting waste products and excess levels of water and essential chemicals. The glomerulus is the functional unit of the kidney. Blood flows continuously through the glomeruli. The ability of the components of filtered blood to enter the glomerular filtrate (urine) is dependent on many factors, including molecular size and charge. As a result, protein binding of a substance influences its filtration. Once filtered, the urinary filtrate is processed by the kidney tubules and this processing includes the reabsorption of essential low molecular weight substances such as amino acids, glucose and electrolytes. The tubules also have the ability to secrete substances via specialized transporters, and this may significantly contribute to the excretion of a given substance. Thus, the concentration of a substance in the blood and urine may be impacted by the glomerular filtration rate (GFR), the ability of the substance to be filtered at the glomerulus, and the ability of the substance to be secreted or reabsorbed by transporters in the kidney tubules.
GFR assesses the net filtration by all of the glomeruli in the kidneys and is the most common measure of kidney function. Because it is labor-intensive and expensive to measure, GFR is most commonly estimated using serum levels of endogenous compounds, such as creatinine, that are freely filtered at the glomerulus. Thus, when GFR is decreased, excretion of endogenously produced creatinine also decreases leading to higher serum levels. However, as creatinine is metabolized from creatine in muscle, differences in muscle mass and meat intake cause variation in creatinine levels that are unrelated to kidney function. For example, women and children generally have lower serum creatinine levels than men because they have less muscle and thus lower endogenous production of creatinine.1 Therefore, equations to estimate GFR have been developed that utilize serum creatinine but also incorporate age, sex and race in an attempt to adjust for differences in muscle mass. Cystatin C and β-2-microglobulin are other endogenously produced serum biomarkers that are freely filtered at the glomerulus that have also been used to estimate GFR, although less commonly than serum creatinine. However, the serum levels of cystatin C and β-2-microglobulin are also impacted by factors other than GFR. Furthermore, the equations developed to estimate GFR based on any of these biomarkers may not fully account for the within- and between-person natural variation in the serum concentrations of these substances. As GFR estimating equations have been primarily developed in populations with chronic kidney disease (CKD), their precision and accuracy is often decreased when applied to populations with more normal kidney function. These limitations must be considered when using endogenous GFR biomarkers or estimated GFR as an outcome in research studies.
Owing to concerns about the insensitivity of GFR for kidney damage, selected urine biomarkers may be measured to detect kidney damage earlier and/or to provide information about the location of damage in the tubules. Microalbuminuria, commonly defined as 30–300 mg/l in a spot sample, is the most commonly used of these markers. Traditionally an indicator of early glomerular damage, it is now thought to reflect proximal tubular damage as well.2 Urine levels of β-2-microglobulin can indicate tubular damage. This low molecular weight protein is almost completely reabsorbed by the proximal tubule after plasma levels are freely filtered at the glomerulus; elevated urinary levels reflect reduced proximal tubule reabsorption. Urinary N-acetyl-β-D-glucosaminidase (NAG) is released from proximal tubular cells. The presence of the NAG-B isoenzyme is thought to indicate tubular necrosis,3 whereas elevated levels of the NAG-A isoenzyme reflect a milder degree of proximal tubule pertubation. Both isoforms contribute to total NAG, which is the form generally measured in research.4 Most urine biomarkers reflect abnormalities of kidney tubule processing rather than solely filtration. CKD is defined as the presence of markers of kidney damage (e.g., microalbuminuria) and/or GFR <60 ml/min/1.73 m2 (kidney function is proportional to kidney size, which is proportional to body surface area, therefore GFR is adjusted to a body surface area of 1.73 m2 to allow comparison of estimated GFR values) for≥3 months.5
REVERSE CAUSALITY
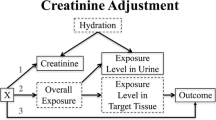
In kidney disease, the excretory capacity is impaired and substances that should be excreted remain in the body instead. Impaired excretion could potentially result in increased biomarker levels in blood and correspondingly lower levels in urine in individuals and populations with CKD. As shown in Figure 1, this could result in negative associations between blood biomarker concentrations and GFR but positive associations between urine biomarker concentrations and GFR. This has been referred to as “reverse causality”. The reverse causality hypothesis, as applied to research on nephrotoxicants, states that increased nephrotoxicant levels in the body reflect reduced excretion secondary to CKD from causes other than injury to the kidney from the nephrotoxicant. This is, therefore, the reverse of concluding that CKD is due to toxicant exposure. Reverse causality has been considered, for example, as an explanation for negative associations between blood lead levels and kidney excretion when observed in populations with lower exposure levels where the potential for lead-related nephrotoxicity remains controversial.6, 7
(a) and (b) Opposite associations of a biomarker in blood and urine with glomerular filtration rate (GFR) consistent with kidney filtration influence. Reverse causation traditionally considers only decreased filtration from chronic kidney disease (low GFR) but biomarker excretion could potentially reflect kidney filtration across the entire range, increasing with kidney filtration in the high range. The traditional approach in which toxicants are modeled as independent variables to determine their impact on GFR is shown in Firgure 1a; the potential impact of GFR on toxicant level is shown in Figure 1b.
Much of the early validation work utilizing biomarkers was conducted in the occupational setting where kidney function is generally in the normal range (the “healthy worker effect”) and exposure levels are generally higher than environmental exposure. As a result, an impact of decreased kidney excretion on biomarker concentrations is likely to be limited. This cannot be assumed when general populations are the focus of research efforts, as is common currently in environmental epidemiology, and where exposure levels are lower and CKD is increasingly common (estimated to affect 11.5% of US adults8).
KIDNEY FILTRATION
Of much greater potential impact, however, is the possibility that biomarkers are affected over the entire range of kidney filtration rather than just with decreased excretion in CKD. Given variations in nephron endowment and other biological factors, the normal range of GFR is quite wide. In adults, depending on age, normal GFR is 90–130 ml/min/1.73 m2.9 When also considering the prevalence of CKD, kidney filtration in the general population covers a wide range before kidney replacement therapy is needed (generally GFR <10 ml/min/1.73 m2). In the broadest definition of reverse causality, biomarker levels could be impacted by any level of kidney filtration capacity. For example, at higher GFR levels, more toxicant could be excreted into urine leaving less in blood and other body stores. This pattern is shown in Figure 1 at higher GFR; notably the directions of the associations are the same as in lower GFR.
RESEARCH EXAMPLES RELATING TO THE POTENTIAL IMPACT OF KIDNEY FUNCTION ON BIOMARKER LEVELS
If biomarker levels are impacted across the entire kidney filtration range, the implications for research are substantial. Specifically, exposure biomarkers could reflect kidney filtration as well as exposure. Published associations between low-level toxicant dose measures and adverse outcomes, especially those with prospective data, serve to validate the use of biomarkers in environmental epidemiology and provide evidence against a substantial impact of normal kidney filtration. Associations of blood toxicant concentrations with diseases for which CKD increases risk, such as cardiovascular diseases, are less useful to address the reverse causality concern. However, associations with other outcomes are relevant. For example, in prospective Cadmibel study data, urine cadmium levels predicted lung cancer,10 an outcome that is unrelated to kidney function. Similarly, studies showing negative associations between blood lead and children's intellectual function are useful in this regard.11 Biomonitoring data also validate biomarker use in environmental epidemiology. For example, significant positive correlations among urine, blood and kidney cadmium measures were recently reported.12 However, misclassification bias due to a kidney impact on biomarker levels could still reduce the strength of reported associations or underlie an inconsistent body of research.
Moreover, less reassuring data have also been reported. As shown in Table 1, recent publications have reported a range of findings that raise concern regarding the potential for kidney filtration to impact biomarker concentrations. These findings include positive associations between urine toxicant levels and estimated GFR (eGFR) as well as blood and urine toxicant associations in opposite directions with eGFR. Data in children and adolescents suggest that the impact of kidney filtration may occur over the entire GFR range rather than just in the setting of reduced excretion traditionally considered in reverse causality. Furthermore, associations of urine toxicants that differ according to the method used to estimate GFR have been reported. For example, positive associations between urine toxicants and serum creatinine-based eGFR but not serum cystatin-C-based eGFR suggest a biomarker specific impact.
Hyperfiltration presents an additional complexity for interpretation of research on nephrotoxicants. Hyperfiltration is a chronic process reported in humans with diabetes, hypertension and obesity13 and in lead-exposed rodents,14 in which an initial increase in GFR is followed by a subsequent decline indicative of CKD. This process has been implicated to explain positive associations between blood lead levels and GFR measures.15, 16
The kidney may also impact biomarker levels by processes other than glomerular filtration, such as kidney reabsorption of protein-bound toxicants. In a recent study, associations between urine cadmium and lead with kidney effect biomarkers, also measured in urine, were observed at lower levels of urinary albumin but not at higher levels.17 Furthermore, blood cadmium and lead were not associated with the urinary kidney effect biomarkers. The authors hypothesized that urine metal associations were due to similar reabsorption characteristics in the proximal tubule between proteins bound to metals and proteins used as kidney effect biomarkers rather than a nephrotoxic effect on the proximal tubules by the metals. Kidney proximal tubule processing could also impact biomarker levels via competition for transporters. Alteration of biomarker processing, such as via an impact on transporters, is also possible. For example, in vitro data indicate that cadmium increases transport of p-aminohippurate (a classic marker used to assess organic anion transporters) at lower levels but inhibits it at higher levels.18
If biomarker levels do reflect underlying kidney function as well as the exposure or outcome metric they are thought to represent, currently unappreciated misclassification bias would exist in research relying on biomarkers. This has the potential to bias study results to the null, resulting in non- or less significant associations. In order to address this concern, several approaches in future research should be considered. Much of this work would rely on techniques developed in the nephrotoxicant research community but results would apply to biomarker work overall.
RESEARCH RECOMMENDATIONS RELATING TO THE POTENTIAL IMPACT OF KIDNEY FILTRATION ON BIOMARKER LEVEL
Research recommendations to assess the potential impact of reduced filtration (the traditional reverse causality hypothesis) include:
-
Assessment of associations by level of kidney function. Data analysis could focus on eGFR categories,19 but graphical and modeling approaches to determine whether a threshold exists are ultimately needed. For example, Kim and colleagues presented a figure of the adjusted association between blood lead and serum creatinine illustrating that the association was not limited to participants with the lowest GFR.20
-
Research in which exclusion of participants with CKD is performed as a sensitivity analysis. However, the challenges of kidney outcome assessment may limit accurate identification of all participants with CKD.
Determining whether filtration across the entire range of kidney function and/or tubular kidney processing impacts biomarker levels requires additional approaches; many are applicable to the classic reverse causality issue of decreased excretion as well. Research recommendations include:
-
Comparison of associations of blood and urine toxicant levels in models of kidney outcomes when biomarkers can be accurately measured in both media, for example, cadmium. If kidney filtration impacts biomarker levels, blood and urine biomarker levels should be associated in opposite directions with eGFR as shown in Figure 1. Specifically, urine biomarker levels would be lower at lower eGFR levels, indicating that less of the biomarker is being excreted into urine. However, blood levels would be higher, again because with impaired kidney excretion, a greater proportion of biomarker dose remains in blood and other body stores in the same manner as observed clinically with normal constituents in the blood in patients needing dialysis. These inconsistent associations should be most apparent in the setting of low-level exposure. In contrast, if both urine and blood biomarker associations increase with decreasing eGFR (Figure 2), nephrotoxicity is suggested. Challenges to this research approach include the fact that many toxicants have a preferred biomonitoring approach and are less well validated when measured in a different biological medium, for example, lead is routinely measured in blood rather than urine. Others, such as perfluorooctanoic acid, are filtered and reabsorbed, so measurable levels are not present in the urine. Urine concentration issues must also be addressed as discussed below. In addition, assessment of kidney outcomes can be challenging and should be examined with a range of approaches and the consistency among results considered.
-
Examination of urine toxicant and blood (or tissue) toxicant relations via correlations and regression modeling. In the cadmium biomonitoring study mentioned above, blood and kidney cadmium levels were positively associated in adjusted models of urine cadmium.12 Blood, urine and kidney cadmium measures were all positively correlated. Thus, these results are not consistent with a kidney filtration effect although analyses by GFR categories would be useful to evaluate consistency across the kidney filtration range. Small sample size may preclude this in these data, however.
-
Comparisons of associations of modeled or predicted exposure measures, when available, and biomarker concentrations in models of kidney outcomes such as discussed above with perfluorooctanoic acid.21
-
Adjustment for GFR measures in studies examining associations between exposure biomarkers and outcomes not thought to be influenced by normal kidney function, for example, neurological outcomes, to determine whether this alters the association.
-
Prospective study designs to assess the temporal relation between biomarker levels and change in kidney function. A recent publication in which lead, cadmium and mercury were measured in stored erythrocytes from existing prospective, population-based studies and participants were linked to a renal registry to identify those who developed end-stage renal disease provides an example of a very efficient approach to such research.22 However, as CKD progresses over many years, prolonged follow-up is necessary to determine the direction of the causal pathway.
-
Examination of associations between external exposure and internal dose levels in the approach used in the initial validation of biomonitoring in the occupational setting. Challenges to this approach in the environmental setting include the fact that historically, in occupational validation work, biomarker levels were higher and airborne exposure was the primary route. Environmentally, multi-route exposure—inhalation, ingestion and, in some cases, dermal—is much more common and exposure via the latter two routes is much harder to accurately measure than is airborne exposure. When attempted, however, examination of associations by kidney filtration level would also be useful.
-
In vitro experiments to increase understanding of protein binding and kidney processing of toxicants.
-
Experimental designs in animal models allow a prospective approach comparing exposed and unexposed animals. However, such research generally involves high toxicant doses in order to see an effect with a cost-effective number of animals. In addition, kidney function may not vary significantly in most healthy animals.
ADJUSTMENT FOR URINE CONCENTRATION
Urine concentration varies throughout the day in relation to hydration status. Therefore, biomarkers measured in spot urine samples (collected at a single time point rather than over a timed collection period) are generally adjusted for differences in urine concentration at the time the specimen was obtained. For example, if the individual providing the sample has just consumed a large drink, urine flow rate will be high, the spot urine sample will be dilute, and the measured biomarker concentration (e.g., μg/l) will be low. Similarly, the opposite is true if less fluid has been ingested prior to specimen collection. Several approaches to adjust for variability in spot urine sample concentrations have been explored but no consensus on the best solution has emerged to date.1, 23, 24, 25 The most common adjustment method used involves dividing the urine biomarker level by urine creatinine. For example, urine albumin in mg/dl divided by urine creatinine in g/dl is expressed as mg/g. However, the use of urine creatinine has a number of limitations. Creatinine is metabolized from creatine in muscle, so muscle mass and diet affect creatinine levels resulting in variation that is unrelated to hydration. A U.S. National Health and Nutrition Examination Survey (NHANES) data analysis recently highlighted differences in urine creatinine levels across broad segments of the general population (levels in men>women>children) reflecting differences in muscle mass and recommended creating separate normal ranges for these groups.1 Creatinine also undergoes substantial processing in the kidney as it is both filtered and secreted which raises the potential for kidney tubule processing to impact creatinine levels. Concerns about the use of creatinine to adjust urine biomarkers have also been discussed recently in the kidney epidemiology literature and adjustment with an estimate of creatinine excretion rate has been advocated.26
Limitations of creatinine as an adjustment approach have led to research using alternative measures of urine concentration.24, 27 Specific gravity is the weight of the urine sample compared with that of an equal volume of distilled water.28 Urine osmolality indicates osmoles of solute per kilogram urine. In normal urine, containing primarily small solutes, these measures will be similar. However, if larger molecules, such as glucose, are present in high concentrations in urine, specific gravity will be disproportionately higher than osmolality as it is affected by solute weight.29 Given the increasing diabetes prevalence globally, osmolality is likely preferable to specific gravity although it is less commonly used and more difficult to measure.
The use of timed, rather than spot urine samples, is another option to address adjustment for urine concentration. In this approach, individuals void and then collect all their urine output for a defined time period that is usually 24 h. Longer urine collections may average out variation, thus obviating the need for adjustment, but are difficult to comply with, resulting in missed samples, and have greater potential for external contamination. Shorter collection periods may reduce the potential for missed samples but may be more influenced by diurnal variations in excretion known to occur over the course of a 24-h period. Timed urine collections were recently recommended in acute kidney injury settings where glomerular filtration is rapidly changing, resulting in changes in creatinine excretion.30 Overnight urine sample, in which the first morning void is collected, has been considered as a practical approach to timed samples.31, 32 Recent NHANES datasets collect time since previous void and void volume data to use in calculation of urine flow rate. Finally, given the limitations of the adjustment methods discussed above, some authors have advocated not adjusting at all.33, 34
An additional complexity that is not commonly considered in discussions of urine concentration adjustment, but one that has primary importance for research on nephrotoxicants, relates to the fact that both the exposure and outcome variables often contain the same or correlated creatinine values. For example, measured creatinine clearance uses both serum and urine creatinine in a timed urine collection and is calculated as follows:
([urine creatinine in mg/dl × urine volume in ml]/serum creatinine in mg/dl)/collection time in minutes
Thus, if exposure biomarkers are measured in the same urine collection used for measured creatinine clearance determinations, the same urine creatinine value will be used in both the exposure and the outcome unless a non-creatinine method of adjustment for urine concentration is used or the collection occurs over a 24-h period where adjustment is not generally performed. When urinary biomarkers are used as the kidney outcome, this occurs as well because both the exposure and outcome are usually measured in the same spot urine sample.
This may create a misleading statistical effect whereby associations between urine creatinine-adjusted toxicants and outcomes containing urine creatinine reflect urine creatinine associations rather than the exposure of concern. This possibility was implicated in a recent report in lead workers35 in which uranium concentration was measured in a 4-h urine collection and three different methods were used to adjust for urine concentration: uranium level directly divided by urine creatinine (μg uranium/g creatinine); urine uranium (μg/l) and creatinine (g/l) included as separate covariates in the regression model;1 and a non-creatinine-based approach using the total amount of uranium excreted during the 4-h urine collection (urine uranium [μg/l] x total urine volume [l] × 4/actual collection time [h] resulting in μg uranium/4 h). In this analysis of 684 workers, higher ln-urine uranium was associated with lower measured creatinine clearance and higher NAG in models that used urine creatinine to adjust for urine concentration but not in models that used total uranium excreted (μg/4 h). This inconsistency was all the more striking as creatinine-adjusted urine uranium (μg/g creatinine) was highly correlated with total uranium excreted (μg/4 h) (Spearman’s r=0.95).
This suggests that, in some instances, associations between toxicant and kidney outcome biomarkers, when both are measured in urine, may be statistical rather than biological. In the lead worker analysis, the same urine creatinine value was used to adjust uranium for urine concentration and to calculate measured creatinine clearance. Furthermore, NAG was directly divided by urine creatinine in the spot urine sample, which was collected immediately prior to the 4-h urine sample; as would be expected, urine creatinine values from both samples were significantly correlated. Thus, inverse and positive associations of urine uranium concentration with measured creatinine clearance and NAG, respectively, are potentially consistent with a statistical association reflecting the use of urine creatinine in exposure and outcome variables.
The most common study design used in research on nephrotoxicants assesses associations between urine toxicants and kidney biomarkers, also measured in urine. Most adjust for urine concentration using urine creatinine. Therefore, assessing the potential for statistical rather than biological associations to underlie positive associations in these studies is essential. However, as most such publications have reported both null as well as positive associations, underlying statistical associations are not an invariable result. Also reassuring are reports of similar results when adjusting for urine concentration with either specific gravity or urine creatinine.36
A statistical explanation could also underlie associations between urine creatinine-adjusted toxicants and serum creatinine-based eGFR.1 Most GFR-estimating equations include serum creatinine in the denominator. The commonly used Modification of Diet in Renal Disease (MDRD) equation is calculated as:37
175 × standardized serum creatinine−1.154 x age-0.203 × 1.212 (if black) × 0.742 (if female)
Therefore, positive associations between serum and urine creatinine could result in positive associations between eGFR and urine creatinine-adjusted toxicants. This possibility was implicated in a recent study in which associations of urine cadmium and thallium were positively associated with eGFR when urine creatinine was used to adjust for urine cadmium and thallium concentrations but not when urine osmolality was used as the urine concentration adjustor.38
Importantly, recent research suggests that concern regarding correlated variables is not limited solely to research using kidney outcomes. Using NHANES data, a simulated population was constructed and di-2-ethylhexyl phthalate intake doses were randomly assigned to each individual.39 Therefore, intake doses should not be associated with the study outcomes, which included BMI and waist circumference. As expected, no associations were observed for toxicant dose expressed as excretion rate or urine concentration. However, in the simulation, creatinine-adjusted exposure values were associated with both outcomes. This is particularly concerning as the associations were away from the null, reflecting a differential bias and resulting in observed statistical associations when none actually existed.
RESEARCH RECOMMENDATIONS RELATING TO THE POTENTIAL IMPACT OF ADJUSTMENT FOR URINE CONCENTRATION FUNCTION ON BIOMARKER LEVEL AND/OR ASSOCIATIONS
Approaches using published data and/or incorporated into future research to address this concern include:
-
Comparison of different urine concentration adjustment methods in the same dataset. Examples include urine creatinine, estimated creatinine excretion rate, specific gravity, osmolality, first morning void and/or timed urine collections allowing calculation of analyte excretion. Recent NHANES datasets contain time since previous void and void volume allowing calculation of urine flow rate. Analyte excretion rates (ng/min) can be calculated as the product of the flow rate and the analyte concentration in urine. Similar to the timed urine collections discussed above in the Korean lead worker study35 hydration status is explicitly accounted in excretion rate and additional adjustment, such as with urine creatinine, is not needed. Although the result may not be as accurate as timed urine samples, this NHANES approach is logistically easier. Identification of factors involved when urine concentration adjustment methods are not highly correlated is also needed.
-
Use of a range of kidney outcomes in research including measures not based on serum creatinine. Examples include serum cystatin-C-based eGFR and measured GFR.
CONCLUSIONS
Several challenges, initially noted in research on nephrotoxicants, have significant implications, not only for that research area but potentially also for biomarker use in general. Research at lower exposure levels, common in environmental epidemiology in developed countries, may be the most impacted. The first concern is whether kidney filtration or tubule processing affects biomarker levels. This is more commonly raised in the context of pathologically decreased kidney filtration, that is, reverse causality; however, recent data suggest GFR within the normal range may be involved as well. Research is also needed into the issue of kidney tubule processing such as competition for reabsorption of protein-bound toxicants and biological effect markers that are also proteins. Either process could impact biomarker levels, decreasing their validity for research involving a range of outcomes. Secondly, research to determine the optimal approach for urine concentration adjustment is needed as recent research suggests that urine creatinine, although commonly used, may alter observed associations. Future research in a range of approaches is required to address these concerns.
ABBREVIATIONS
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; NAG, N-acetyl-β-D-glucosaminidase; NHANES, National Health and Nutrition Examination Survey
References
Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL . Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 2005; 113: 192–200.
Comper WD, Russo LM . The glomerular filter: an imperfect barrier is required for perfect renal function. Curr Opin Nephrol Hypertens 2009; 18: 336–342.
Bernard A, Thielemans N, Roels H, Lauwerys R . Association between NAG-B and cadmium in urine with no evidence of a threshold. Occup Environ Med 1995; 52: 177–180.
D'Amico G, Bazzi C . Urinary protein and enzyme excretion as markers of tubular damage. Curr Opin Nephrol Hypertens 2003; 12: 639–643.
National Kidney Foundation K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Guideline 1: definition and stages of chronic kidney disease. Am J Kidney Dis 2002; 39: S1.
Evans M, Elinder CG . Chronic renal failure from lead: myth or evidence-based fact? Kidney Int 2011; 79: 272–279.
Marsden PA . Increased body lead burden—cause or consequence of chronic renal insufficiency? N Engl J Med 2003; 348: 345–347.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612.
National Kidney Foundation. Glomerular Filtration Rate (GFR) FAQ's. page 4http://www.kidney.org/professionals/kdoqi/gfr.cfm Accessed August 4, 2013.
Nawrot T, Plusquin M, Hogervorst J, Roels HA, Celis H, Thijs L et al. Environmental exposure to cadmium and risk of cancer: a prospective population-based study. Lancet Oncol 2006; 7: 119–126.
Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC et al. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ Health Perspect 2005; 113: 894–899.
Akerstrom M, Barregard L, Lundh T, Sallsten G . The relationship between cadmium in kidney and cadmium in urine and blood in an environmentally exposed population. Toxicol Appl Pharmacol 2013; 268: 286–293.
Nenov VD, Taal MW, Sakharova OV, Brenner BM . Multi-hit nature of chronic renal disease. Curr Opin Nephrol Hypertens 2000; 9: 85–97.
Khalil-Manesh F, Gonick HC, Cohen AH, Alinovi R, Bergamaschi E, Mutti A et al. Experimental model of lead nephropathy. I. Continuous high-dose lead administration. Kidney Int 1992; 41: 1192–1203.
de Burbure C, Buchet JP, Leroyer A, Nisse C, Haguenoer JM, Mutti A et al. Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: evidence of early effects and multiple interactions at environmental exposure levels. Environ Health Perspect 2006; 114: 584–590.
Weaver VM, Lee BK, Ahn KD, Lee GS, Todd AC, Stewart WF et al. Associations of lead biomarkers with renal function in Korean lead workers. Occup Environ Med 2003; 60: 551–562.
Chaumont A, Nickmilder M, Dumont X, Lundh T, Skerfving S, Bernard A . Associations between proteins and heavy metals in urine at low environmental exposures: Evidence of reverse causality. Toxicol Lett 2012; 210: 345–352.
Van Kerkhove E, Pennemans V, Swennen Q . Cadmium and transport of ions and substances across cell membranes and epithelia. Biometals 2010; 23: 823–855.
Weaver VM, Kim NS, Lee BK, Parsons PJ, Spector J, Fadrowski J et al. Differences in urine cadmium associations with kidney outcomes based on serum creatinine and cystatin C. Environ Res 2011; 111: 1236–1242.
Kim R, Rotnitsky A, Sparrow D, Weiss S, Wager C, Hu H . A longitudinal study of low-level lead exposure and impairment of renal function. The Normative Aging Study. JAMA 1996; 275: 1177–1181.
Watkins DJ, Josson J, Elston B, Bartell SM, Shin HM, Vieira VM et al. Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environ Health Perspect 2013; 121: 625–630.
Sommar JN, Svensson MK, Bjor BM, Elmstahl SI, Hallmans G, Lundh T et al. End-stage renal disease and low level exposure to lead, cadmium and mercury; a population-based, prospective nested case-referent study in Sweden. Environ Health 2013; 12: 9.
Boeniger MF, Lowry LK, Rosenberg J . Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: A review. Am Ind Hyg Assoc J 1993; 54: 615–627.
Suwazono Y, Akesson A, Alfven T, Jarup L, Vahter M . Creatinine versus specific gravity-adjusted urinary cadmium concentrations. Biomarkers 2005; 10: 117–126.
Aylward LL, Hays SM, Smolders R, Koch HM, Cocker J, Jones K et al. Sources of variability in biomarker concentrations. J Toxicol Environ Health B Crit Rev 2014; 17: 45–61.
Abdelmalek JA, Gansevoort RT, Lambers Heerspink HJ, Ix JH, Rifkin DE . Estimated albumin excretion rate versus urine albumin-creatinine ratio for the assessment of albuminuria: a diagnostic test study from the Prevention of Renal and Vascular Endstage Disease (PREVEND) Study. Am J Kidney Dis 2014; 63: 415–421.
Yassine H, Kimzey MJ, Galligan MA, Gandolfi AJ, Stump CS, Lau SS . Adjusting for urinary creatinine overestimates arsenic concentrations in diabetics. Cardiorenal Med 2012; 2: 26–32.
Wald R . Urinalysis in the diagnosis of kidney disease. In: Basow D (ed). UpToDate. UpToDate: Waltham, MA. 2013.
Rose B, Post T . Chapter 13B: Meaning of urine osmolality and pH. In: Basow D (ed). UpToDate. UpToDate: Waltham, MA. 2013.
Waikar SS, Sabbisetti VS, Bonventre JV . Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int 2010; 78: 486–494.
Akerstrom M, Lundh T, Barregard L, Sallsten G . Sampling of urinary cadmium: differences between 24-h urine and overnight spot urine sampling, and impact of adjustment for dilution. Int Arch Occup Environ Health 2012; 85: 189–196.
Akerstrom M, Barregard L, Lundh T, Sallsten G . Variability of urinary cadmium excretion in spot urine samples, first morning voids, and 24 h urine in a healthy non-smoking population: implications for study design. J Expo Sci Environ Epidemiol 2014; 24: 171–179.
Moriguchi J, Ezaki T, Tsukahara T, Furuki K, Fukui Y, Okamoto S et al. Comparative evaluation of four urinary tubular dysfunction markers, with special references to the effects of aging and correction for creatinine concentration. Toxicol Lett 2003; 143: 279–290.
Ikeda M, Ezaki T, Tsukahara T, Moriguchi J, Furuki K, Fukui Y et al. Bias induced by the use of creatinine-corrected values in evaluation of beta2-microgloblin levels. Toxicol Lett 2003; 145: 197–207.
Shelley R, Kim NS, Parsons PJ, Lee BK, Agnew J, Jaar BG et al. Uranium associations with kidney outcomes vary by urine concentration adjustment method. J Expo Sci Environ Epidemiol 2014; 24: 58–64.
Akesson A, Lundh T, Vahter M, Bjellerup P, Lidfeldt J, Nerbrand C et al. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ Health Perspect 2005; 113: 1627–1631.
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247–254.
Weaver VM, Vargas GG, Silbergeld EK, Rothenberg SJ, Fadrowski JJ, Rubio-Andrade M et al. Impact of urine concentration adjustment method on associations between urine metals and estimated glomerular filtration rates (eGFR) in adolescents. Environ Res 2014; 132C: 226–232.
Christensen K, Sobus J, Phillips M, Blessinger T, Lorber M, Tan YM . Changes in epidemiologic associations with different exposure metrics: A case study of phthalate exposure associations with body mass index and waist circumference. Environ Int 2014; 73C: 66–76.
Ferraro PM, Costanzi S, Naticchia A, Sturniolo A, Gambaro G . Low level exposure to cadmium increases the risk of chronic kidney disease: analysis of the NHANES 1999-2006. BMC Public Health 2010; 10: 304.
You L, Zhu X, Shrubsole MJ, Fan H, Chen J, Dong J et al. Renal function, bisphenol A, and alkylphenols: results from the National Health and Nutrition Examination Survey (NHANES 2003-2006). Environ Health Perspect 2011; 119: 527–533.
Shelley R, Kim NS, Parsons P, Lee BK, Jaar B, Fadrowski J et al. Associations of multiple metals with kidney outcomes in lead workers. Occup Environ Med 2012; 69: 727–735.
Acknowledgements
The Oak Ridge Institute for Science and Education Research Participation Program at the National Center for Environmental Assessment, Office of Research and Development, US EPA [#: EPA-ORD/NCEA-RTP-2009-02; Dr. Weaver].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Weaver, V., Kotchmar, D., Fadrowski, J. et al. Challenges for environmental epidemiology research: are biomarker concentrations altered by kidney function or urine concentration adjustment?. J Expo Sci Environ Epidemiol 26, 1–8 (2016). https://doi.org/10.1038/jes.2015.8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jes.2015.8
- Springer Nature America, Inc.
Keywords
This article is cited by
-
Biomonitoring of inorganic arsenic species in pregnancy
Journal of Exposure Science & Environmental Epidemiology (2023)
-
Associations between co-exposure to multiple metals and renal function: a cross-sectional study in Guangxi, China
Environmental Science and Pollution Research (2023)
-
Impact of Dietary Cadmium Intake from Rice on Renal Function Biomarker and Population Toxicokinetic Modelling
Exposure and Health (2023)
-
Variability, Clearance, and Concentration of Multiple Metals and Risk of Kidney Function Abnormality: A New Integrative Metal Exposure Assessment Approach
Exposure and Health (2023)
-
Synergistic impact of co-exposures to whole blood metals on chronic kidney disease in general US adults: a cross‑sectional study of the National Health and Nutrition Examination Survey 2011–2020
Environmental Science and Pollution Research (2023)