Abstract
The association between O3 exposure and preterm birth (PTB) remains unclear. We evaluated associations for three categories of PTB and O3 in Harris County, Texas, during narrow periods of gestation. We computed two sets of exposure metrics during every 4 weeks of pregnancy for 152,214 mothers who delivered singleton, live-born infants in 2005–2007, accounting first for temporal variability and then for temporal and spatial sources of variability in ambient O3 levels. Associations were assessed using multiple logistic regression. We also examined the potential for a fixed cohort bias. In the bias-corrected cohort where associations were somewhat stronger, elevated odds ratios (ORs) per 10 parts per billion increase in O3 exposure (county-level metric) were detected for the fifth (OR=1.08, 95% confidence interval (CI): 1.04–1.12), sixth (OR=1.05, 95% CI=1.01–1.09), and seventh (OR=1.07, 95% CI=1.03–1.10) 4-week periods of pregnancy for late PTB (33–36 completed weeks gestation), the fifth (OR=1.13, 95% CI=1.02–1.25) and seventh (OR=1.15, 95% CI=1.04–1.27) 4-week periods of pregnancy for moderate PTB (29–32 completed weeks gestation), and the fifth (OR=1.21, 95% CI=1.08–1.36) 4-week period of pregnancy for severe PTB (20–28 completed weeks gestation). Conversely, decreased odds were found in the first 4-week period of pregnancy for severe PTB (OR=0.83, 95% CI=0.74–0.94). Associations were slightly attenuated using the spatially interpolated (kriged) metrics, and for women who did not work outside of the home. Our analyses confirm reports in other parts of the United States and elsewhere with findings that suggest that maternal exposure to ambient levels of O3 is associated with PTB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.INTRODUCTION
Houston, the nation’s fourth largest city and the largest city in Texas, is located in Harris County, a county that exceeds the National Ambient Air Quality Standard for ambient levels of ozone (O3) and is therefore designated as a non-attainment area for this air pollutant. All seven surrounding counties, including neighboring Galveston and Brazoria Counties, also share this non-attainment designation.1 Ozone is created in the troposphere as a result of chemical reactions between nitrogen oxides (NOx) and volatile organic compounds in the presence of heat and sunlight. Major sources of NOx and volatile organic compounds in ambient air largely originate from car and truck exhaust, industrial facilities, electric utilities, and chemical solvents.2 With over 15,000 miles of roadway in Harris County, the US Department of Transportation3 estimates that Houstonians travel approximately 93 million vehicle miles per day. Moreover, the population of Harris County has grown by 26% since 2000 to over 4.1 million persons in 20104 thereby increasing on-road emissions from mobile sources. In addition to a network of heavily trafficked roadways, Harris County is home to the nation’s largest petrochemical industrial complex; has two of the four largest refineries in the United States; over 700 establishments that manufacture chemical and plastic products; and leads the nation in production of three major resins (i.e., 36.6% of polyethylene production; 51.7% of polypropylene production; and 41.5% of polyvinyl chloride production). Thus, Houston is often referred to as the “energy capital of the world.”5
There is increasing evidence that ambient air pollution is associated with preterm birth (PTB).6, 7 Unlike other air pollutants for which the evidence has been more consistent, the association between O3 exposure and PTB remains unclear. Of the studies published between 2000 and 2014, five found no association between O3 and PTB8, 9, 10, 11, 12 and nine studies reported positive associations.13, 14, 15, 16, 17, 18, 19, 20, 21 Inconsistencies in study findings may be due to differences in study settings (e.g., regional differences in geography and temperature, as well as pollutant sources), demographic differences in the study population, study designs, modeling approaches (e.g., single pollutant models or multiple pollutant models), and/or exposure assessment methods7, 22, 23 that include variability in the duration of exposure windows that have been examined.23, 24
Because most studies typically use trimesters and/or the entire pregnancy as the exposure periods of interest,6, 7 less is known about effects of air pollutant exposure during narrower gestational periods throughout pregnancy. Second, little is known whether associations would differ based on how early the infant is born because most studies evaluate PTB as a single category (i.e., births with less than 37 completed weeks of gestation). Third, although there is increased emphasis on accounting for spatial and temporal sources of exposure variability in air pollution epidemiology,25, 26 applying spatial interpolation techniques such as kriging has been limited in studies of adverse birth outcomes.15, 27 Only one of these studies investigated O3 exposure.15 Finally, in the air pollution literature, using infant date of birth to define the birth cohort is a typical practice and, yet, it has the potential to introduce selection bias (described as a fixed cohort bias) because the population at risk differs at the beginning and the end of the study period.28 Hence, to address several gaps, we designed a study with threefold objectives: (1) to investigate the association between ambient O3 levels and late (33–36 completed weeks of gestation), moderate (29–32 completed weeks of gestation), and severe (20–28 completed weeks of gestation) PTB in a large urbanized area of the United States; (2) to compare findings using county-level and spatially interpolated estimates of maternal exposure to O3 for every 4 weeks of her pregnancy; and (3) to examine fixed cohort bias, by comparing results using a cohort defined on the basis of infant birth date to a cohort defined on the basis of date of conception.
METHODS
Study Population
We obtained electronic birth records for infants who were born between the period of 1 January 2005 and 31 December 2007 in the greater Houston area (Harris County, Texas) from the Texas Department of State Health Services. These records included geocoded addresses for the location of the mother’s residence at time of delivery and were linked to the Texas Birth Defects Registry. The University of Texas Health Science Center at Houston Committee for the Protection of Human Subjects and the Texas Department of State Health Services Institutional Review Boards approved our study.
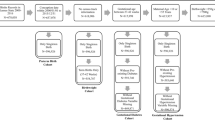
We used SAS version 9.2 (SAS Institute, Cary, NC, USA) and Arc GIS 10 (ESRI, Redlands, CA, USA) to prepare our birth record data for analyses. Among the 222,735 infants who were born in the study area during the years 2005–2007, we excluded the following data: 34,191 deliveries of mothers who lived outside of Harris County; 19,692 birth records with missing birth weight and/or gestational age or where there were large discrepancies between the obstetrical estimate of gestational age and the estimate computed based on the first day of the last menstrual period; 5,665 multiple births; 4,564 deliveries of mothers with missing addresses; and 511 infants with computed gestational ages less than 20 weeks or greater than 43 weeks. We also excluded 5,898 infants with one or more congenital anomalies, based on records from an active, state-wide population-based birth defects registry in Texas. This element of our study design is likely important as it will establish that an association between O3 and PTB is independent of confounding due to congenital anomalies, for which there is evidence of an association with air pollution29, 30, 31 and PTB.32, 33, 34 Following application of the aforementioned exclusion criteria, a total of 152,214 singleton births comprised the study population.
Assessment of PTB
We computed gestational age at birth using information on the first day of the last menstrual period from the birth certificates, which was assigned as the first day of pregnancy. Next, we classified PTBs into three mutually exclusive categories and evaluated each one separately in the statistical analyses: late PTB (33–36 completed weeks of gestation), moderate PTB (29–32 completed weeks of gestation), and severe PTB (20–28 completed weeks of gestation). For all three categories, the control group was full-term infants (37 or more completed weeks of gestation).
Maternal Exposure to O3 during 4-week Gestational Periods and for the Entire Pregnancy
A total of 22 O3 monitors were used in the exposure assessment. Of this total, 16 monitors were operating in Harris County in 2004 and 2005 and 17 monitors were operating in 2006 and 2007. In neighboring counties, there were two monitors in Brazoria County, two in Galveston County (two in the years 2004 and 2007, one in the years 2005 and 2006), and one in Montgomery County. Daily maximum 8-h moving averages of O3, reported in units of parts per million (p.p.m.), were downloaded from the Air Quality System Data Mart of the US Environmental Protection Agency35 during the period of 1 January 2004 to 31 December 2007. All concentrations were converted to parts per billion (p.p.b.).
We computed two sets of exposure metrics during every four weeks of a mother’s pregnancy accounting first for temporal variability alone and then for both temporal and spatial sources of variability in ambient air levels of O3. R programming language (version x64 2.13.0) was used to generate both sets of exposure metrics. For our county-level metrics, daily maximum 8-h values were averaged across all monitoring locations in Harris County. Using these time series of daily air pollutant levels for each of the 1,461 days from 1 January 2004 to 31 December 2007, we computed the mean exposure for each 4-week period of a mother’s pregnancy (i.e., first 4-week period was for 1–4 weeks; second 4-week period was for 5–8 weeks; third 4-week period was for 9–12 weeks; fourth 4-week period was for 13–16 weeks; fifth 4-week period was for 17–20 weeks; sixth 4-week period was for 21–24 weeks; seventh 4-week period was for 25–28 weeks; eight 4-week period was for 29–32 weeks; and ninth 4-week period was for 33–36 weeks).
To account for temporal and spatial sources of variability in ambient O3 levels, we applied kriging for optimal linear spatial prediction at unmonitored sites and fit spherical and exponential semivariogram models36 for each day in the 4-year period. Next, we used the resulting time series from both fitted semivariogram models to predict daily ambient O3 concentrations at each mother’s residence at delivery for each day of her pregnancy. As before, we used these data to compute exposure metrics for every 4-week period of a mother’s pregnancy. Because the kriged values generated under both spherical and exponential semivariograms were very close to each other, we conducted the logistic regression analyses using the exposure metrics generated under one (spherical) model.
To ease comparisons with previous studies, we also computed estimates of exposure during a mother’s entire pregnancy using both the county-level and kriged time series.
Potential Confounders
Using information reported on the birth records, the following variables were evaluated as potential confounders. These variables included: mother’s smoking status (no smoking, smoking before pregnancy, and smoking throughout pregnancy); race/ethnicity (non-Hispanic white, Hispanic-foreign born; Hispanic-US born, black, and other); level of education (<12 years, High School Graduate or GED, 13–15 years, and 16+ years); age; body mass index (BMI) before pregnancy (weight in kg divided by height in m2); receipt of Women, Infants, and Children services; trimester prenatal care initiated; parity; and type of payment for delivery (private insurance, Medicaid, self-pay, other/missing). Using US Centers for Disease Control and Prevention guidelines for females,37 mother’s BMI was categorized as being underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), or obese (> 30 kg/m2). For mothers younger than 20 years of age, BMI categories were based on percentile ranges, as per Centers for Disease Control and Prevention guidelines for teenagers. Information about marital status and paternal information reported on the birth certificate was used to create a three-level variable as a proxy for level of support received from the infant’s father: mother married; mother not married but reported information on infant’s father (age, education, or address); and mother not married and did not report information about the age, educational level, or address of the infant’s father. We also considered season of birth and season of conception as potential confounders (summer, June–August; fall, September–November; winter, December–February; and spring, March–May).
Statistical Analysis
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). To assess the potential for multicollinearity, we conducted correlation analyses for all exposure metrics (computed for every 4-week period of pregnancy) and obtained correlation coefficients that ranged from −0.43 to 0.59. We performed logistic regression analyses to evaluate the associations between O3 exposure during pregnancy and PTB. For late PTB, exposure metrics for the first to the eighth 4-week periods of pregnancy were included as independent variables within the model; for moderate PTB, exposure metrics for the first to the seventh 4-week periods of pregnancy were included as independent variables within the model; and for severe PTB exposure metrics for the first to the fifth 4-week periods of pregnancy were included as independent variables within the model.
We controlled for other variables in our analyses if they resulted in a ≥10% change in the estimate of the association between O3 and PTB38 or are known risk factors for PTB (i.e., smoking, race/ethnicity, age, and education).39 Therefore, our final logistic regression models were adjusted for: mother’s smoking status; race/ethnicity; age; level of education; and season of birth.
Evaluating Fixed Cohort Bias
To examine the potential for fixed cohort bias, we re-defined the cohort on the basis of dates of conception by limiting to those births whose conception date was no more than 20 weeks (the shortest gestational age in the cohort) before the cohort started (1 January 2005) and at least 43 weeks (the longest gestational age in the cohort) before the cohort ended (31 December 2007). Our bias-corrected cohort included 132,075 pregnancies that represented 87% of the full study population.
Sensitivity Analysis
Using the kriged exposure metrics, we conducted a sensitivity analysis to address potential misclassification of exposure due to mobility to and from work and included only mothers who listed “homemaker,” “housewife,” “stay at home,” “unemployed,” “none,” “N/A,” or some combination of these as a response to the question about their usual occupation. This sensitivity analysis was conducted on women who comprised the bias-corrected cohort.
RESULTS
Table 1 presents characteristics of the full study population (n=152,214) and the bias-corrected cohort (n=132,075). Most of the population, whether in the full sample population or the bias-corrected cohort, was Hispanic-foreign born (33%), had less than a high school education (36%), were married (58–59%), and reported no smoking during pregnancy (96%). There were near equal percentages of women who used Medicaid (36%) as compared with private insurance (36–37%). The mean maternal age at delivery in the full study population was 26.8 years (SD±6.2) and 27.0 years (SD±6.2) in the bias-corrected cohort. The mean pre-pregnancy BMI was 25.7 kg/m2 (SD±5.9) for both groups, although there were nearly equal proportions of women with normal weight (49–50%) as compared with women who were overweight (28%) or obese (18–19%). The mean gestational age was 38.5 weeks (SD±2.1) and approximately 9% of all births were preterm in both groups, which is slightly lower than the national average of 11%.40 Of these PTBs, 85% were late PTBs, 9% were moderate PTBs, and 6% were severe PTBs in both study populations.
The mean level of the 8-h maximum daily average concentrations (n=1,461) of O3 across the greater Houston area for 2004–2007 was 37.6 p.p.b. (SD±16.34). Selected percentiles of the distribution were: 5th percentile, 16.4 p.p.b.; 10th percentile, 19.8 p.p.b.; 25th percentile, 25.5 p.p.b.; 50th percentile, 33.7 p.p.b.; 75th percentile, 47.5 p.p.b.; and 90th percentile, 62.4 p.p.b.
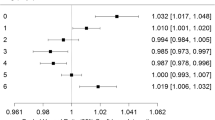
Table 2 presents fully adjusted odds ratios (ORs) for the associations between O3 (county-level metric) and each category of PTB in the full study population. Elevated odds per 10 p.p.b. increase in O3 exposure for late PTB in the full study population were found in the second and seventh 4-week periods of pregnancy. Conversely, an inverse relationship was found in the fourth 4-week periods of pregnancy for both late PTB and moderate PTB.
Results for those pregnancies in the bias-corrected cohort are also presented in Table 2. For late PTB, elevated odds were found in the fifth, sixth, and seventh 4-week periods of pregnancy, as well as the fifth and seventh 4-week periods of pregnancy for moderate PTB. The most significant increase in odds of PTB was found in the fifth 4-week period of pregnancy for severe PTB. In contrast, decreased odds were found for the first 4-week period of pregnancy for severe PTB. Unlike the full study population, an inverse relationship was not found for the fourth 4-week period of pregnancy in late PTB or moderate PTB.
Overall, we found results for the kriged O3 metrics were slightly attenuated when compared with the county-level O3 metrics and these finding are presented in Supplementary Materials, Supplementary Table 1. Also, associations weakened when restricting analyses to the bias-corrected cohort among women who did not work outside of the home (Supplementary Materials, Supplementary Table 2).
When analyzing PTB across the entire pregnancy, increased odds per 10 p.p.b. increase in O3 exposure were found for late PTB (OR=1.21 (95% confidence interval (CI)=1.10–1.32)), moderate (OR=1.31 (95% CI=0.99–1.74)), and severe PTB (OR=1.16 (95% CI=0.82–1.62)), although the results were statistically significant only for preterm infants born between 33 and 36 completed weeks of gestation. Similar results (not shown) were obtained for the exposure metrics obtained using the kriged data.
DISCUSSION
Our study was conducted to examine susceptibility of the fetus to O3 exposure during 4-week windows of a mother’s pregnancy in the greater Houston area, Texas, thus allowing for an evaluation of exposure during narrow gestational periods. As the nation’s fourth largest city and the largest city in Texas, Houston was an ideal location to conduct the study as it is located in a county (Harris County) that is designated by the US Environmental Protection Agency as a non-attainment area for O3. All seven surrounding counties, including neighboring Galveston and Brazoria Counties, also share this non-attainment designation.41 Moderate associations between maternal exposure to ambient levels of O3 and late, moderate, and severe PTB were detected, although they varied in magnitude (and sometimes direction) across gestational periods. We also confirmed increases in the odds of PTB with increasing O3 exposure over the entire pregnancy period. Moreover, our study took into account fixed cohort bias as described by Strand et al.,28 and we report somewhat stronger associations between O3 and PTB when the bias is addressed.
We applied kriging to capture spatial and temporal variability in assessing maternal exposure to ambient air levels of O3. Yet, our findings using the kriged O3 metric (see Supplementary Materials, Table 1) were slightly attenuated when compared with the results that relied on the county-level O3 metrics. The only other study15 that employed kriging to examine the association between O3 and PTB did so with far fewer (n=3) monitoring stations as compared with our investigation (n=22). However, even with the greater number of monitoring stations available in our study, we recognize that we may not have had a sufficient number to obtain stable estimates of the semivariogram model36 and, thus, may not have captured the local variability in O3 concentrations. Also, it is possible that the spatial average across monitors in the county is a better measure of exposure for an individual than just using the home location because we did not have information about maternal exposures at locations other than her residence.
Causes of preterm labor are multifactorial and vary according to gestational age,42 therefore we examined three mutually exclusive categories of PTB. Our assessment of exposure for each month of pregnancy allowed us to more narrowly assess potentially susceptible exposure windows. In the bias-corrected cohort, we detected positive associations in the later 4-week periods of pregnancy for late PTB, with stronger associations for late PTB and moderate PTB in the fifth and seventh 4-week periods of pregnancy and in the fifth 4-week period of pregnancy for severe PTB. Per 10 p.p.b. increase in O3 exposure, the odds of late PTB increased between 5 and 8% in the fifth through seventh 4-week periods of pregnancy; the odds of moderate PTB increased 13–15% during the fifth and seventh 4-week periods of pregnancy; and the odds of severe PTB increased 21% in the fifth 4-week period of pregnancy. These findings suggest that O3 exposure has the greatest impact on infants born extremely early (20–28 completed weeks of gestation).
Our finding of a protective association in the first 4-week period of pregnancy for severe PTB coupled to the positive association in the fifth 4-week period of pregnancy is difficult to explain. Although other studies have typically not controlled for multiple exposure windows in the same analyses, a few investigations have examined O3 exposure in the first or last month of pregnancy and PTB. Per 1 part per 100 million increase in O3 concentration, Wilhelm and Ritz13 in a Southern California study observed an elevated risk of PTB (relative risk=1.23 (95% CI=1.06–1.42)) in the first month of pregnancy and, in a separate analysis, no risk 6 weeks before birth. In Sydney, Australia, Jaludin et al.21 reported elevated odds of PTB per 1 p.p.b. increase in O3 levels in the first month (OR=1.60 (95% CI=1.27–2.03)); however, interestingly, the same study found a decreased association in the first trimester of pregnancy (OR=0.81 (95% CI=0.67–0.98)).
Although not well understood, a myriad of biological mechanisms have been suggested to explain the association between air pollution and PTB and include oxidative stress, pulmonary and placental inflammation, coagulation, endothelial function, and hemodynamic responses.42, 43 However, as Slama et al.44 state in their findings from the International Workshop on Air Pollution and Human Reproduction, because of the heterogeneous chemical and physical nature of air pollutants, it is unlikely that there is a unique biological mechanism that will explain the air pollutant effects on multifaceted events such as adverse birth outcomes. Further, given that relatively few studies have examined narrow windows of exposure24, 45, 46 additional investigations may help elucidate the underlying biological mechanisms that may be involved in PTB and possibly lead to interventions that improve perinatal health.
To address what has been called fixed cohort bias, Strand et al.28 simulated a retrospective cohort using data from 114,063 singleton births in Brisbane, Australia, between 1 July 2005 and 30 June 2009. Their results indicate that a bias is present when the cohort is defined on the basis of infant’s date of birth rather than date of conception because the population at risk at the beginning of the study period misses pregnancies with shorter gestations, whereas the population at risk at the end of the study period misses pregnancies with longer gestations. Further, this bias is likely important when examining exposures that vary seasonally over the course of a year, as is the case with O3 levels. Whereas Hwang et al.47 found little evidence of fixed cohort bias in a study investigating air pollution (SO2, NO2, CO, O3, and PM10) and stillbirth, we found that this bias had a modest effect on our findings (with somewhat stronger associations detected between O3 exposure and PTB when the cohort was defined on the basis of date of conception rather than on infant’s birth date).
Our study is not without limitations. Although we evaluated confounding due to multiple risk factors for PTB, we cannot rule out that some residual confounding may still be present. Residential mobility during pregnancy was not assessed and may have introduced exposure misclassification. However, in our previous study that examined exposure misclassification due to residential mobility during pregnancy among Texas mothers participating in the National Birth Defects Prevention study, we found that while 30% of cases (mothers of infants with neural tube defects) and 24% of control mothers moved during pregnancy, the moves tended to be of relatively short distances (median distance moved of 3.5 and 3.7 miles for cases and controls, respectively).48 Further, Chen et al.49 also examined the potential for misclassification of air pollutant exposure in a subgroup of a New York birth cohort and found that of the mothers who moved, the majority of them remained in the same exposure region. Some misclassification of exposure is also likely because potential exposures at locations other than the mother’s residence were not assessed. Our analysis of a subset of mothers who did not work outside the home yielded attenuated findings when compared with the analysis that included all mothers. In the absence of data on daily activity patterns, place or type of work, and time spent commuting, applying metrics developed over broader geographical areas may be preferable to approaches that estimate, for example, residential exposure. Although we could not evaluate alcohol use, environmental tobacco smoke (ETS), or nutritional status as potential confounding variables, results from a nested case–control study11 suggest that effects of air pollution on PTB were not affected by lack of control for these risk factors. Finally, we did not address multiple comparisons; therefore, we recognize that some of our statistically significant findings may have resulted from chance.
Our study offers several advantages over earlier studies because we examined narrower windows of exposure, used a more refined classification of PTB, and addressed what is termed fixed cohort bias. Furthermore, our study benefited in being conducted in one of the most highly monitored regions in the United States that allowed us to compare findings using county-level and spatially interpolated estimates of maternal exposure to O3 for every four weeks of her pregnancy.
CONCLUSIONS
We evaluated associations between maternal exposure to ambient levels of O3 and PTB in the greater Houston area having accounted for more discrete windows of susceptibility than has been examined in previous investigations. Our analyses confirm reports in other parts of the United States and elsewhere with findings that suggest that maternal exposure to ambient levels of O3 is associated with PTB. To better understand the etiology of PTB, we recommend that future studies select a birth cohort by date of conception rather than infant birth date, classify PTB into different categories based on how early the infant is born, and further examine narrow periods of fetal development.
References
Texas Commission on Environmental Quality (TCEQ). Agency Activities: Air Quality (FY2011-2012). 2013; http://www.tceq.texas.gov/publications/sfr/057_12/air. Accessed on 6 September 2013.
Texas Commission on Environmental Quality (TCEQ). Ozone: The Facts. 2013; http://www.tceq.state.tx.us/airquality/monops/ozonefacts.html. Accessed 6 September 2013.
U.S. Department of Transportation, Bureau of Transportation Statistics. Texas Transportation Profile. 2002; http://www.rita.dot.gov/bts/sites/rita.dot.gov.bts/files/publications/state_transportation_statistics/texas/pdf/entire.pdf. Accessed on 17 April 2011.
City of Houston. Houston Facts and Figures. 2013; http://www.houstontx.gov/abouthouston/houstonfacgts.html. Accessed on 6 September 2013.
Greater Houston Partnership. Houston: Energy Powerhouse. 2013; http://www.houston.org/assets/pdf/opportunity/Energy-Data-Sheet.pdf. Accessed on 6 September 2013.
Shah PS, Balkhair T . Air pollution and birth outcomes: A systematic review. Environ Int 2011; 37: 498–516.
Stieb DM, Chen L, Eshoul M, Judek S . Ambient air pollution, birth weight and preterm birth: A systematic review and meta-analysis. Environ Res 2012; 117: 100–111.
Wu J, Wilhelm M, Chung J, Ritz B . Comparing exposure assessment methods for traffic-related air pollution in an adverse pregnancy outcome study. Environ Res 2011; 111: 685–692.
Schifano P, Lallo A, Asta F, De Sario M, Davoli M, Michelozzi P . Effect of ambient temperature and air pollutants on the risk of preterm birth, Rome 2001-2010. Environ Int 2013; 61: 77–87.
Ritz B, Yu F, Chapa G, Fruin S . Effect of air pollution on preterm birth among children born in Southern California between 1989 and 1993. Epidemiology 2000; 11: 502–511.
Ritz B, Wilhelm M, Hoggatt KJ, Ghosh JK . Ambient air pollution and preterm birth in the environment and pregnancy outcomes study at the University of California, Los Angeles. Am J Epidemiol 2007; 166: 1045–1052.
Liu S, Krewski D, Shi Y, Chen Y, Burnett RT . Association between gaseous ambient air pollutants and adverse pregnancy outcomes in Vancouver, Canada. Environ Health Perspect 2003; 111: 1773–1778.
Wilhelm M, Ritz B . Local variations in CO and particulate air pollution and adverse birth outcomes in Los Angeles County, California, USA. Environ Health Perspect 2005; 113: 1212–1221.
Le HQ, Batterman SA, Wirth JJ, Wahl RL, Hoggatt KJ, Sadeghnejad A et al. Air pollutant exposure and preterm and term small-for-gestational-age births in Detroit, Michigan: Long-term trends and associations. Environ Int 2012; 44: 7–17.
Lee PC, Roberts JM, Catov JM, Talbott EO, Ritz B . First trimester exposure to ambient air pollution, pregnancy complications and adverse birth outcomes in Allegheny County, PA. Matern Child Hlth J. 2013; 17: 545–555.
Hansen C, Neller A, Williams G, Simpson R . Maternal exposure to low levels of ambient air pollution and preterm birth in Brisbane, Australia. BJOG 2006; 113: 935–941.
Ha S, Hu H, Roussos-Ross D, Haidong K, Roth J, Xu X . The effects of air pollution on adverse birth outcomes. Environ Res 2014; 134C: 198–204.
Gray SC, Edwards SE, Schultz BD, Miranda ML . Assessing the impact of race, social factors and air pollution on birth outcomes: A population-based study. Environ Health 2014; 13: 4.
Olsson D, Ekstrom M, Forsberg B . Temporal variation in air pollution concentrations and preterm birth-a population based epidemiological study. Int J Environ Res Public Health 2012; 9: 272–285.
Olsson D, Mogren I, Forsberg B . Air pollution exposure in early pregnancy and adverse pregnancy outcomes: A register-based cohort study. BMJ Open 2013; 3: e001955.
Jalaludin B, Mannes T, Morgan G, Lincoln D, Sheppeard V, Corbett S . Impact of ambient air pollution on gestational age is modified by season in Sydney, Australia. Environ Health 2007; 6: 16.
Dadvand P, Parker J, Bell ML, Bonzini M, Brauer M, Darrow LA et al. Maternal exposure to particulate air pollution and term birth weight: A multi-country evaluation of effect and heterogeneity. Environ Health Perspect 2013; 121: 267–373.
Woodruff TJ, Parker JD, Adams K, Bell ML, Gehring U, Glinianaia S et al. International Collaboration on Air Pollution and Pregnancy Outcomes (ICAPPO). Int J Environ Res Public Health 2010; 7: 2638–2652.
Symanski E, Davila M, McHugh MK, Waller DK, Zhang X, Lai D . Maternal exposure to fine particulate air pollution during narrow gestational periods and newborn health in Harris County, Texas. Matern Child Hlth J 2014; 18: 2003–2012.
Jerrett M, Burnett RT, Ma R, Pope CA, 3rd, Krewski D, Newbold KB et al. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology 2005; 16: 727–736.
Liao D, Peuquet DJ, Duan Y, Whitsel EA, Dou J, Smith RL et al. GIS approaches for the estimation of residential-level ambient PM concentrations. Environ Health Perspect 2006; 114: 1374–1380.
Leem JH, Kaplan BM, Shim YK, Pohl HR, Gotway CA, Bullard SM et al. Exposures to air pollutants during pregnancy and preterm delivery. Environ Health Perspect 2006; 114: 905–910.
Strand LB, Barnett AG, Tong S . Methodological challenges when estimating the effects of season and seasonal exposures on birth outcomes. BMC Med Res Methodol 2011; 11: 49.
Lin YT, Lee YL, Jung CR, Jaakkola JJ, Hwang BF . Air pollution and limb defects: A matched-pairs case-control study in Taiwan. Environ Res 2014; 132: 273–280.
Hansen CA, Barnett AG, Jalaludin BB, Morgan GG . Ambient air pollution and birth defects in Brisbane, Australia. PLoS One 2009; 4: e5408.
Vrijheid M, Martinez D, Manzanares S, Dadvand P, Schembari A, Rankin J et al. Ambient air pollution and risk of congenital anomalies: A systematic review and meta-analysis. Environ Health Perspect 2011; 119: 598–606.
Honein MA, Kirby RS, Meyer RE, Xing J, Skerrette NI, Yuskiv N et al. The association between major birth defects and preterm birth. Matern Child Hlth J 2009; 13: 164–175.
Grandi C, Luchtenberg G, Rittler M . The contribution of birth defects to spontaneous preterm birth. Am J Perinat 2007; 24: 487–492.
Shaw GM, Savitz DA, Nelson V, Thorp JM, Jr . Role of structural birth defects in preterm delivery. Paediatr Perinat Ep 2001; 15: 106–109.
U.S. Environmental Protection Agency (EPA). Air Quality System (AQS) Data Mart. 2009; http://www.epa.gov/ttn/airs/aqsdatamart/. Accessed on 28 May 2009.
Waller L . Applied Spatial Statistics for Public Health Data. John Wiley & Sons: Hoboken, NJ. 2004.
U.S. Centers for Disease Control and Prevention (CDC). About BMI for Adults. 2011; http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html (Accessed 22 March 2011).
Rothman KJ, Greenland S, Lash TL . Modern Epidemiology. Third edn. Lippincott Williams & Wilkins: Philadelphia. 2008.
Institute of Medicine of the National Academies (IOM) Preterm Birth: Causes, Consequences, and Prevention. Behrman RE, Stith Butler A (eds). The National Academies Press: Washington, D.C.. 2007.
U.S. Centers for Disease Control and Prevention (CDC). Preterm Birth. 2014; http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm.Accessed on 29 January 2015.
U.S. Environmental Protection Agency (EPA). Currently Designated Nonattainment Areas for all Criteria Pollutants. 2013; http://www.epa/gov/oaqps001/greenbk/ancl.html. Accessed on 12 February 2014.
Institute of Medicine of the National Academies (IOM). Biological pathways leading to preterm birth. Preterm Birth: Causes, Consequences, and Prevention. The National Academies Press: Washington, D.C.. 2007.
Kannan S, Misra DP, Dvonch JT, Krishnakumar A . Exposures to airborne particulate matter and adverse perinatal outcomes: A biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect 2006; 114: 1636–1642.
Slama R, Morgenstern V, Cyrys J, Zutavern A, Herbarth O, Wichmann HE et al. Traffic-related atmospheric pollutants levels during pregnancy and offspring's term birth weight: A study relying on a land-use regression exposure model. Environ Health Perspect 2007; 115: 1283–1292.
Rappazzo KM, Daniels JL, Messer LC, Poole C, Lobdell DT . Exposure to fine particulate matter during pregnancy and risk of reterm birth among women in New Jersey, Ohio, and Pennsylvania, 2000-2005. Environ Health Perspect 2014; 122: 992–997.
Warren JL, Fuentes M, Herring AH, Langlois PH . Air pollution metric analysis while determining susceptible periods of pregnancy for low birth weight. ISRN Obstet Gynecol 2013; 2013: 387452.
Hwang BF, Lee YL, Jaakkola JJ . Time-dependent exposures and the fixed-cohort bias: Hwang et al. respond. Environ Health Perspect. 2011; 119: a423–a423.
Lupo P, Symanski E, Chan W, Mitchell L, Canfield M, Langlois P . Differences in exposure assignment between conception and delivery: The impact of maternal mobility. Paediatr Perinat Ep 2010; 24: 200–208.
Chen L, Bell EM, Caton AR, Druschel CM, Lin S . Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environ Res 2010; 110: 162–168.
Acknowledgements
This study was supported by grant number 5R21ES017347 from the National Institute of Environmental Health Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Journal of Exposure Science and Environmental Epidemiology website
Supplementary information
Rights and permissions
About this article
Cite this article
Symanski, E., McHugh, M., Zhang, X. et al. Evaluating narrow windows of maternal exposure to ozone and preterm birth in a large urban area in Southeast Texas. J Expo Sci Environ Epidemiol 26, 167–172 (2016). https://doi.org/10.1038/jes.2015.32
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jes.2015.32
- Springer Nature America, Inc.
Keywords
This article is cited by
-
Environmental Chemicals and Preterm Birth: Biological Mechanisms and the State of the Science
Current Epidemiology Reports (2017)