Abstract
Background/Objectives:
We aimed to evaluate mitochondrial biogenesis (MB), structure, metabolism and dysfunction in abdominal adipose tissue from male pediatric patients with obesity.
Subjects/Methods:
Samples were collected from five children with obesity (percentile ⩾95) and five eutrophic boys (percentile ⩾5/⩽85) (8–12 years old) following parental informed consent. We analyzed the expression of key genes involved in MB (sirtuin-1 (SIRT1), peroxisome proliferator-activated receptor-γ (PPARγ), PPARγ coactivator-1α (PGC1α), nuclear respiratory factors 1 and 2 (NRF1, NRF2) and mitochondrial transcription factor A (TFAM) and surrogates for mitochondrial function/structure/metabolism (porin, TOMM20, complex I and V, UCP1, UCP2, SIRT3, SOD2) by western blot. Citrate synthase (CS), complex I (CI) activity, adenosine triphosphate (ATP) levels, mitochondrial DNA (mtDNA) content and oxidative stress end points were also determined.
Results:
Most MB proteins were significantly decreased in samples from children with obesity except complex I, V and superoxide dismutase-2 (SOD2). Similarly, CS and CI activity showed a significant reduction, as well as ATP levels and mtDNA content. PPARγ, PGC1α, complex I and V and SOD2 were hyperacetylated compared with lean samples. Concurrently, in samples from children with obesity, we found decreased SOD2 activity and redox state imbalance highlighted by decreased reduced glutathione/oxidized glutathione (GSH/GSSG) ratio and significant increases in protein carbonylation.
Conclusions:
Adipose tissue from children with obesity demonstrates a dysregulation of key modulators of MB and organelle structure, and displays hyperacetylation of key proteins and altered expression of upstream regulators of cell metabolism.
Similar content being viewed by others
Introduction
Obesity is a worldwide public health problem whose pathogenesis has been associated with genetic factors, inappropriate diet and sedentary lifestyle among other causes.1 Obesity was mainly considered as a condition that develops during adulthood. Nowadays, it has become a health problem that appears with greater frequency earlier in life including childhood.2 Children with obesity tend to retain this condition into adulthood, carrying a higher risk of developing obesity-associated type 2 diabetes mellitus and cardiovascular diseases.3 In Mexico, as of 2012, the prevalence of overweight or children with obesity between 5 and 11 years of age is ~37% in males and ~32% in females. At the aggregate level, this roughly represents ~5.7 million of school-age children.4
Adipose tissue is essential for energy storage. It is also a metabolically active tissue with relevant roles in glucose homeostasis and endocrine functions.5 However, obesity can alter adipose tissue functions and lead to an increased release of fatty acids, hormones and proinflammatory molecules that can contribute to promote obesity-associated complications.6, 7 Several studies have shown that with obesity, adipose tissue mitochondrial respiration is decreased, thus evidencing mitochondrial dysfunction.8, 9 Obesity and other metabolic disorders such as type 2 diabetes and metabolic syndrome are also associated with a mitochondrial overload of glucose and fatty acids, yielding an incomplete oxidation of substrates as well as increased generation of intermediate products such as diacylglycerol that stimulate an excess production of reactive oxygen species (ROS).10, 11
Other perturbations associated with mitochondrial dysfunction have been identified in animal models and patients with obesity, including reduced fatty-acid oxidation,12 lower oxidative phosphorylation,13, 14 increased inflammation, ROS generation and oxidative stress,15, 16, 17, 18 reduced mitochondrial mass and mitochondrial biogenesis (MB) genes, structural damage and altered mitochondrial morphology14, 19 as well as mitochondrial protein hyperacetylation.20 Thus, altered mitochondrial structure and function may play an important role in the pathophysiology of obesity-induced disorders.20 However, nothing is known about the nature of such changes in pediatric adipose tissue samples.
The aim of this study was to compare in adipose tissue obtained from lean subjects and children with obesity, their mitochondrial status defined by the assessment of end points reflective of organelle abundance, structure and function.
Materials and methods
Study group
Samples of subcutaneous adipose tissue from the abdominal region were obtained during laparoscopy, after parental informed consent from male pediatric appendicitis patients (8–12 years old) from La Raza National Medical Center in Mexico City. Anthropometric measurements were obtained to classify patients according to their body mass index and percentile reference tables from the US Center for Disease Control. Based on these results, we stratified our sample into two groups, one with five boys with obesity (percentile ⩾95) and a lean (control) group with five eutrophic children (percentile ⩾5/⩽85). Our protocol was approved by La Raza National Medical Center institutional review board. All participants had proper informed consent.
Study design
Adipose tissue samples were processed to isolate protein extracts. The identification of specific mitochondrial proteins by western blot, citrate synthase (CS) activity, complex I activity, adenosine triphosphate (ATP) content and mitochondrial DNA (mtDNA) levels were used as surrogates of mitochondrial density, structure and function. Total protein acetylation was analyzed by western blot using an antibody against acetylated lysine. Mitochondrial protein acetylation was analyzed after immunoprecipitation with an acetylated lysine antibody by western blots. As indicators of oxidative stress, we evaluated mitochondrial superoxide dismutase-2 (SOD2), ratio of reduced glutathione (GSH)/oxidized glutathione (GSSG) levels and total protein carbonylation.
Western blotting (WB)
WB included analysis of nuclear NAD+ dependent deacetylase sirtuin-1 (SIRT1), energy metabolism co-transcriptional activation factors peroxisome proliferator-activated receptor-γ, (PPARγ) and PPARγ coactivator-1α (PGC1α), nuclear respiratory factors 1 and 2 (NRF1 and NRF2) as well as the mitochondrial transcription factor A (TFAM). We also analyzed the mitochondrial outer membrane protein porin, translocase of outer mitochondrial membrane-20 (TOMM20), oxidative phosphorylation complex I (CI) (subunit NDUFB8) and CV (ATP synthase subunit α), uncoupling proteins 1 and 2 (UCP1, UCP2), mitochondrial NAD+-dependent deacetylase sirtuin-1 and -3 (SIRT) and SOD2. For the analysis, ~50 mg of fat tissue were homogenized with a polytron in 500 μl lysis buffer (1% Triton X-100, 20 mM Tris, 140 mM NaCl, 2 mM EDTA and 0.1% SDS) with protease and phosphatase inhibitor cocktails (P2714 and P2850, Sigma-Aldrich; St. Louis, MO, USA), supplemented with 0.15 mM phenylmethylsulfonyl fluoride, 5 mM Na3VO4 and 3 mM NaF. Homogenates were sonicated for 10 min at 4 °C and centrifuged at 12 000 g for 10 min. Total protein content was measured in the supernatant using the Bradford method. A total of 40 μg of protein was loaded onto a 4–15% precast polyacrylamide gel (Bio-Rad Laboratories Inc.; Hercules, CA, USA) and electrotransferred to a polyvinylidene difluoride membrane using a semidry system. Membranes were incubated for 1 h in blocking solution (5% non-fat dry milk in tris-buffered saline plus 0.1% Tween-20), followed by 3 h of incubation at room temperature with primary antibodies from the following sources: SOD2 (Cell Signaling Technology; Danvers, MA, USA), TOMM20, UCP1, UCP2 and PPARγ (Abcam; Burlingame, CA, USA), PGC1α, NRF1, NRF2, SIRT1, SIRT3 (Cell Signaling Technology; Danvers, MA, USA) and TFAM (Sigma-Aldrich; St. Louis, MO, USA), porin, CV and CI (MitoSciences Inc.; Eugene, OR, USA). β-Tubulin (Cell Signaling Technology; Danvers, MA, USA) protein levels were used as a loading control. All primary antibodies were diluted 1:1000–2000. Membranes were washed (3 × for 5 min) in tris-buffered saline plus 0.1% Tween-20 and incubated for 1 h at room temperature with specific horseradish peroxidase-conjugated secondary antibodies. Membranes were again washed 3 times in tris-buffered saline plus 0.1% Tween-20, and the immunoblots developed using an ECL Plus detection kit (Amersham-GE Healthcare Life Sciences; Chicago, IL, USA). Band intensities were digitally quantified using ImageJ software (http://www.nih.gov).
Immunoprecipitation (IP)
IP was implemented as follows. Adipose tissue (50 mg) was lysed with 250 μl of nondenaturing extraction buffer (0.5%, Triton X-100, 50 mmol l−1 Tris-HCl, pH 7.4, 0.15 mol l−1 NaCl, 0.5 mmol l−1 EDTA) and supplemented with protease and phosphatase inhibitor cocktails plus 2 mmol l−1 Na3VO4 and 1 mmol l−1 NaF. Homogenates were incubated on ice with shaking for 25 min and centrifuged (15 min, 4 °C) at 12 000 g. Total protein content was measured in the supernatant by the Bradford method. A total of 0.5 mg protein was precleared by adding 1 μg of normal rabbit IgG control and 20 μl protein A/G-agarose and mixed for 30 min (4 °C) with subsequent centrifugation at 12 000 g for 10 min at 4 °C. The supernatant was recovered and incubated at 4 °C under mild agitation for 3 h with 10 μl of IP antibody (Ac-Lys, Cell Signaling). Then, 20 μl of protein A/G-agarose was added, and the mixture was incubated overnight at 4 °C with shaking. The IP mixture was centrifuged at 12 000 g for 15 min at 4 °C, and the supernatant was recovered and stored at 4 °C for later analysis. The pellet was washed 3 × with extraction buffer under shaking 15 min and centrifuged at 12 000 g for 15 min at 4 °C. The IP proteins in the pellet and those remaining in the supernatant were boiled 5 min and applied to a precast 4–15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis for WB against CI, CV, SOD2 PPARγ and PGC1α.
CS activity
Adipose tissue samples (25 mg) were homogenized with a polytron in 250 μl of cold extraction buffer (20 mM Tris-HCl, 140 mM NaCl, 2 mM EDTA and 0.1 % sodium dodecyl sulfate) with protease inhibitors (P2714, Sigma-Aldrich), 5 mM Na3VO4 and 3 mM NaF. Homogenates were centrifuged at 10 000 g for 15 min at 4 °C. Supernatants were recovered and used to measure CS as the rate of production of the mercaptide ion based on conversion of acetyl-coenzyme A (CoA) and oxaloacetate into CoA-SH. CoA-SH in the presence of 5,5-disthiobis-2-nitrobenzoic acid produces mercaptide ion. Samples were analyzed in a Beckman DU 730 spectrophotomer (Beckman, Fullerton, CA, USA) at 412 nm.1, 2 All samples were tested in duplicate and measured at room temperature.
CI activity
CI activity was assessed using a precoated microplate with a capture antibody for CI in a kit (Abcam). Protein samples (20 μg per well) were loaded onto the kit microplate wells and once CI was immunocaptured its activity was analyzed at 450 nm using a spectrophotometer (Synergy HT, BioTek Instruments Inc; Winooski, VT, USA) by measuring NADH oxidation to NAD+ following the instructions.
ATP assay
ATP levels were evaluated using an ATP determination kit (Life Technologies; Carlsbad, CA, USA). Approximately 10 mg of tissue was homogenized in 200 μl of cold isolation buffer (5 mM HEPES pH 7.2, 225 mM mannitol, 75 mM sucrose, 1 mM EGTA and protease inhibitors) followed by 5 times syringe passages. Samples were centrifuged at 1500 g for 5 min and supernatants were used to measure ATP using a luminometer (Synergy HT Luminometer, BioTek). All samples were tested in duplicate and measured at room temperature following kit instructions.
Mitochondrial DNA measurements
Total genomic DNA was extracted from adipose tissue using a DNA extraction kit (QIAGEN Inc.; Germantown, MD, USA). Mitochondrial/nuclear DNA ratio was calculated using reference standards from the Step 1 Real-Time PCR (Applied Biosystems; Foster City, CA, USA). We used mtDNA primers: forward 1 (5′-CCTCATCCTAGCAATAATCCCCATCCTCCATATAT-3′) and reverse 1 (5′-ACTTGTCCAATGATGGTAAAA GG-3′) and nuclear DNA primers forward 1 (5′-TGCTGTCTCCATGTTTGATGTATCT-3′) and reverse 1 (5′-TCTCTGCTCCCCACTCTAAGT-3′). Real-time PCR was performed in triplicate.
SOD2 activity
Adipose tissue (20 mg) was homogenized with a polytron in 250 μl of cold buffer (50 mM potassium phosphate, pH 7.4 containing 1 mM EDTA), centrifuged at 10 000 g for 15 min at 4 °C and supernatant (duplicates) used to measure activity using a colorimetric kit (Cayman Chemicals; Ann Arbor, MI, USA).
Protein carbonylation
Approximately 100 mg of adipose tissue were rinsed with phosphate-buffered saline, pH 7.4. Tissue was homogenized in 1 ml of cold buffer (50 mM MES pH 6.7, containing 1 mM EDTA). Homogenates were centrifuged at 10 000 g for 15 min at 4 °C. Supernatant was recovered and incubated at room temperature for 15 min with streptomycin sulfate at a final concentration of 1%. Samples were centrifuged at 6000 g for 10 min at 4 °C. Supernatant was recovered and an aliquot was used to measure protein content using a Bradford assay (Bio-Rad). Carbonyl assay kit (Cayman Chemicals) relied on protein carbonyl functional group derivatization with 2,4-dinitrophenyldrazone forming a Shiff base to produce hydrazone that was measured in spectrophotometer μQuant (BioTek Instruments, Inc.; Winooski, VT, USA) at 360–385 nm.
Reduced and oxidized glutathione
Tissue samples (25 mg) were homogenized with a polytron in 250 μl of cold buffer (50 mM potassium phosphate, pH 7, containing 1 mM EDTA) and centrifuged at 10 000 g for 15 min at 4 °C. The supernatants were deproteinated as per the manufacturer’s instructions. Briefly, samples were mixed with an equal volume of 1.25 M metaphosphoric acid and centrifuged at 2000 g for 2 min. Supernatants were recovered and incubated with triethanolamine (TEAM) 4 M and then samples were used to measure GSH and GSSG using a colorimetric detection assay kit according to the manufacturer’s instructions at 405 nm using spectrophotometer μQuant (Bio-Tek Instruments, Inc.) (Cayman Chemicals). All samples were tested in duplicate and measured at room temperature.
Statistical analysis
All data are presented as mean±s.e. unless otherwise noted. Given the limited number of samples examined, we performed Wilcoxon signed-rank tests to ascertain whether any significant difference (two-sided tests) in median values exists across groups for each characteristic. We further plotted our results stratified by group (lean vs obese) in scatterplots, graphing mean and s.e. Statistical significance was defined when P<0.05.
Results
Biopsies of visceral adipose tissue from male pediatric subjects were classified according to the patient’s body mass index as lean or obese (Table 1).
Mitochondrial protein levels
To evaluate the expression of genes involved in MB, we analyzed SIRT1, PPARγ, PGC1α, NRF1, NRF2 and TFAM protein levels by WB. We also analyzed porin, TOMM20, CI and CV, UCP1, UCP2, and SIRT3 as surrogates of mitochondrial function, structure and metabolism (SOD2). As shown in Figures 1 and 2 (right panels, and Supplementary Figure S1), relative to lean subjects, most samples from children with obesity demonstrated a statistically significant reduction in their relative protein levels except for CI, CV and SOD2 vs lean values.
Relative protein levels of mitochondrial biogenesis-associated regulatory factors SIRT1, PPARγ, PGC1α, NRF1, NRF2 and TFAM in adipose tissue of lean and children with obesity. Protein levels were assessed by western blot using specific antibodies. Signals were quantified by densitometry and values normalized using β-tubulin as a loading control. Each column represents the mean±s.e. of n=5 subjects/group. Statistical differences were assessed using the nonparametric Wilcoxon test, *P<0.05.
Relative protein levels of mitochondrial-associated molecules porin, TOMM20, CI, CV, UCP1, UCP2, SIRT3 and SOD2 in adipose tissue from lean and children with obesity. Protein levels were assessed by western blot using specific antibodies. Signals were quantified by densitometry and values normalized using β-tubulin as a loading control. Each column represents the mean±s.e. of n=5 subjects/group. Statistical differences were assessed using nonparametric Wilcoxon test, *P<0.05.
Mitochondrial function
As an indicator of mitochondrial function, we evaluated CS, CI activity and ATP content. CS activity values shown in Figure 3a, CI activity (Figure 3b) and ATP levels (Figure 3c) evidence a statistically significant relative reduction in samples from children with obesity compared with that of lean subjects.
Citrate synthase (CS) (a), complex I activity (b), ATP levels (c) and mitochondrial DNA (mtDNA) content (d) in adipose tissue from lean and children with obesity. CS and CI activity and ATP levels were quantified, and values normalized to total protein. mtDNA content was normalized to nuclear DNA. Each column represents the mean±s.e. of n=5 subjects/group. Statistical differences were assessed using nonparametric Wilcoxon test, *P<0.05.
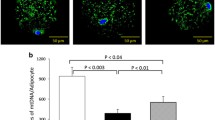
mtDNA content
The mtDNA copy number is often used as an indicator of mitochondrial mass. The mtDNA/nuclear DNA values shown in Figure 3d demonstrate a statistically significant reduction in samples from children with obesity vs lean group values.
Protein acetylation
Obesity-induced hyperacetylation has been described as a hallmark of suppressed protein function.20 As shown in Figure 4a, total protein acetylation levels evidence a significant increase among children with obesity compared with the lean group. Following IP assays, the acetylation levels of PPARγ, PGC1α, CI, CV and SOD2 were significantly increased in samples from children with obesity (Figure 4b) vs lean values.
Total protein acetylation in adipose tissue from lean and children with obesity. Acetylation levels of total tissue homogenates (a) were evaluated by western blot using anti Ac-lysine antibody. Acetylation levels of mitochondrial related proteins were determined following immunoprecipitation (IP) using an Ac-lysine antibody and by western blot using antibodies against PPARγ, PGC1α, CI, CV and SOD2 (b). Signals were normalized using β-tubulin as a loading control (a) or total protein input in IP (b). Each column represents the mean±s.e. of n=5 subjects/group. Statistical differences were assessed using nonparametric Wilcoxon test, *P<0.05.
Oxidative stress
SOD2 activity values shown in Figure 5a evidence a statistically significant reduction in samples from children with obesity vs lean group levels. A significant decrease in the GSH/GSSG ratio was also identified in these samples (Figure 5b). Conversely, a significant increase in the amount of total protein carbonylation was noted in samples from boys with obesity (Figure 5c) vs lean values.
Superoxide dismutase 2 (SOD2) activity is diminished, and oxidative stress indicators are altered in adipose tissue from children with obesity. SOD2 activity (a) was quantified, and values normalized to total protein content. The GSH/GSSG ratio (b) and total protein carbonylation (c) values are also noted. Each column represents mean±s.e. of n=5 subjects/group. Statistical differences were assessed using nonparametric Wilcoxon test, *P<0.05.
Discussion
Unique results from this study demonstrate that adipose tissue from children with obesity evidence a dysregulation of key modulators of MB, structure and function that is associated with the hyperacetylation of proteins and altered expression of upstream regulators of cell metabolism. We also demonstrate that in adipose tissue from these children, there is increased oxidative stress relative to lean subjects.
Obesity is an ever increasing serious public health problem leading to the emergence of associated comorbidities such as type 2 diabetes, cardiovascular disease and metabolic syndrome.3 However, the cellular and molecular mechanisms involved in the development of obesity are poorly understood. Adipose tissue mitochondrial dysfunction has been suggested to possibly contribute to the pathogenesis of obesity.
In adipocytes, as in multiple tissues, MB is regulated at the transcriptional level by the action of the key transcription factor PPARγ.21, 22 The dynamic control of MB and respiratory function of mitochondria also depends on the PGC1 family of transcriptional factors.21, 23 The activity of PGC1 factors is by itself also regulated by post-translational modifications elicited by intracellular energy state sensors such as AMP-activated protein kinase and NAD+-dependent deacetylase SIRT1.24 In adipose tissue, SIRT1 can promote metabolic efficiency by stimulating adiponectin production.25 However, in adipose tissue from individuals with obesity, SIRT1 expression is markedly reduced, thus adversely affecting the energy status of adipocytes.19, 23 Under normal conditions, SIRT1 closely interacts with AMP-activated protein kinase and deacetylates multiple lysine residues that are present in PGC1α, and lead to the stimulation of lipid catabolism while suppressing the ‘inflammation’ of white adipose tissue (WAT).19, 26, 27 As a consequence of the reduced expression of SIRT1 and deregulated downstream effectors (that is, PGC1α), the expression and function of MB regulators such as NRF1, NRF2 and TFAM that are involved in the replication and transcription of mtDNA is reduced.28 In this study, a relative decreased expression of these regulatory proteins was noted in adipose tissue of children with obesity, supporting previous reports regarding the alteration of mitochondrial control systems with obesity and potentially accounting for the reduced mtDNA levels noted.15, 18, 29 The reduced levels noted for porin and TOMM20 in WAT from children with obesity coupled with diminished mtDNA and ATP levels strongly suggest reduced mitochondrial content vs lean donors.
We also observed decreases in the expression of the uncoupling proteins UCP1 and UCP2 in adipose tissue of children with obesity. In rodent models, the ablation of UCP1 has obesogenic effects, and thus it has been postulated that the loss of UCP1 function is permissive for the development of obesity.30 UCP2 activity in WAT has been associated with facilitating fat oxidation31, 32 and with reductions in tissue oxidative stress.33, 34 In visceral and subcutaneous adipose tissue, the expression of UCP2 is known to be decreased in subjects with obesity and type 2 diabetes, suggesting that it indeed may play a role in their pathophysiology.27, 35
With obesity, the presence and activity of specific functional components within the mitochondria appear dysregulated.36 CS activity has been used as a surrogate indicator of mitochondrial function.35, 37, 38, 39 In our study, we report a significant decrease in CS activity in the obese group. The reduction in CS activity is similar to that reported by Christe et al.38 who measured both the activity and the expression of the CS in omental adipose tissue and found a significant reduction in samples obtained from adult subjects with obesity.
In human WAT, there is also a dysregulation of other components of the respiratory chain that correlate with the extent of obesity.18, 40 Chattopadhyay et al.41 measured oxidative phosphorylation complex activities and reported reduced CI–IV levels in mitochondria isolated from subcutaneous adipose depots of patients with obesity but no alterations in CS activity. Although CI and CV WB data yielded no differences in samples obtained from children with obesity, the activity of CI was suppressed and associates in our study with reduced CS activity and ATP levels. This observation may also reflect a potential reduction in mitochondrial content.
Lysine acetylation has been implicated in the control of metabolic pathways and in promoting the maintenance of energy homeostasis.42 Complex I, V and SOD2 are inactivated by Lys acetylation.43, 44 PPARγ and PGC1α are also negatively regulated by Lys acetylation.29, 45 Evidence supporting the physiological relevance of Lys acetylation is based on knocking out SIRT3, a nuclear-encoded enzyme localized to the mitochondrial matrix, whose deacetylase function targets key mitochondrial proteins such as SOD2 and CI.46 Studies demonstrated that mitochondrial proteins accumulate in hyperacetylated forms in sirt3−/− animals.43, 47, 48, 49 Disruption of SIRT3 activity in mice, either by genetic ablation or during high-fat feeding, is associated with accelerated development of metabolic abnormalities similar to metabolic syndrome in humans.20, 38 In our study, we report on increases in total protein acetylation levels in WAT of children with obesity. The analysis of acetylation levels of specific proteins evidenced increased levels in CI and CV, SOD2 and in the MB regulatory proteins as PPARγ and PGC1α that were accompanied by decreased SIRT3 protein levels. The concurrence of these events likely contributes to mitochondrial dysfunction in adipocytes from children with obesity, as previous studies have reported in adults.41
Obesity and mitochondrial dysfunction are associated with an elevated production of ROS, partly caused by infiltration of macrophages that produce high levels of superoxide radicals and an increase in NADPH oxidase.50 In our study, we measured components of the antioxidant defense system and oxidative stress levels in WAT. Although we did not identify reductions in SOD2 protein levels, its activity was reduced and correlated with increases in total protein carbonylation. A likely explanation for its reduced activity is the enhanced levels of SOD2 acetylation noted in the IP samples from children with obesity. Of interest is that overexpression of SOD can limit oxidative damage in obese models.51
In our study, we also observed a significant reduction of GSH/GSSG ratio demonstrating a shift of the cellular redox state toward pro-oxidant conditions, similar to those found by Kobayashi et al.52 in WAT of ob/ob mice. We can infer that the decrease in the GSH content follows an overproduction of ROS that must be buffered to reduce oxidative stress. Because of altered cellular redox status and decreased antioxidant systems in adipose tissue of subjects with obesity, damage by ROS can be produced on multiple biomolecules. In adipocytes, ROS oxidize lipids producing hydroperoxides that, in turn, generate reactive aldehydes that covalently modify proteins in a process called carbonylation. As noted above, increased levels of carbonyls were indeed detected in WAT from children with obesity. Similar findings were reported by Frohnert et al.53 who observed a significant positive correlation between total protein carbonylation, body mass index and the content of visceral adipose tissue.
Obesity is a serious public health problem and strategies aimed to ameliorate or control this epidemic represent an urgent need. It is therefore critical to identify the cellular and molecular mechanisms involved in its pathophysiology. In this study for the first time, we report on the altered status of recognized molecular control systems in pediatric samples of WAT that control the metabolic status of fat. Future studies may aim to positively modulate such systems with the goal of mitigating the development of obesity and/or its complications.
References
Lipek T, Igel U, Gausche R, Kiess W, Grande G . Obesogenic environments: environmental approaches to obesity prevention. J Pediatr Endocrinol Metab 2015; 28: 485–495.
Martin A, Saunders DH, Shenkin SD, Sproule J . Lifestyle intervention for improving school achievement in overweight or obese children and adolescents. Cochrane Database Syst Rev 2014; 3: CD009728.
Messiah SE, Lipshultz SE, Natale RA, Miller TL . The imperative to prevent and treat childhood obesity: why the world cannot afford to wait. Clin Obes 2013; 3: 163–171.
Gutiérrez JP, Rivera-Dommarco JA, Shamah-Levy T, Villalpando-Hernández S, Franco A, Cuevas-Nasu L et al Encuesta Nacional de Salud y Nutrición 2012. Resultados Nacionales. 2a. ed. [Internet]. Instituto Nacional de Salud Publica. 2013.
Boudina S, Graham TE . Mitochondrial function/dysfunction in white adipose tissue. Exp Physiol 2014; 99: 1168–1178.
Arruda AP, Pers BM, Parlakgül G, Güney E, Inouye K, Hotamisligil GS . Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med 2014; 20: 1427–1435.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW . Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796–1808.
Kraunsøe R, Boushel R, Hansen CN, Schjerling P, Qvortrup K, Støckel M et al. Mitochondrial respiration in subcutaneous and visceral adipose tissue from patients with morbid obesity. J Physiol 2010; 588: 2023–2032.
Hansen M, Lund MT, Gregers E, Kraunsøe R, Van Hall G, Helge JW et al. Adipose tissue mitochondrial respiration and lipolysis before and after a weight loss by diet and RYGB. Obesity (Silver Spring) 2015; 23: 2022–2029.
Muoio DM, Neufer PD . Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab 2012; 15: 595–605.
Brownlee M . Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813–820.
Zhang D, Liu Z-X, Choi CS, Tian L, Kibbey R, Dong J et al. Mitochondrial dysfunction due to long-chain Acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci USA 2007; 104: 17075–17080.
Petersen KF, Dufour S, Shulman GI . Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med 2005; 2: 0879–0884.
Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL et al. impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 2007; 56: 1376–1381.
Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 2003; 100: 8466–8471.
Petersen KF, Dufour S, Befroy D, Garcia RSG . Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 2004; 9: 223–224.
Civitarese AE, Ukropcova B, Carling S, Hulver M, DeFronzo RA, Mandarino L et al. Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab 2006; 4: 75–87.
De Pauw A, Tejerina S, Raes M, Keijer J, Arnould T . Mitochondrial (dys)function in adipocyte (de)differentiation and systemic metabolic alterations. Am J Pathol 2009; 175: 927–939.
Costa CDS, Hammes TO, Rohden F, Margis R, Bortolotto JW, Padoin AV et al. SIRT1 transcription is decreased in visceral adipose tissue of morbidly obese patients with severe hepatic steatosis. Obes Surg 2010; 20: 633–639.
Hirschey MD, Shimazu T, Huang J-Y, Schwer B, Verdin E . SIRT3 regulates mitochondrial protein acetylation and intermediary metabolism. Cold Spring Harb Symp Quant Biol 2011; 76: 267–277.
He W, Barak Y, Hevener A, Olson P, Liao D, Le J et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA 2003; 100: 15712–15717.
Sies H . Glutathione and its role in cellular functions. Free Radic Biol Med 1999; 27: 916–921.
Scarpulla RC . Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta Mol Cell Res 2011; 1813: 1269–1278.
Cantó C, Auwerx J . PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 2009; 20: 98–105.
Banks AS, Kon N, Knight C, Matsumoto M, Gutiérrez-Juárez R, Rossetti L et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab 2008; 8: 333–341.
Gillum MP, Kotas ME, Erion DM, Kursawe R, Chatterjee P, Nead KT et al. SirT1 regulates adipose tissue inflammation. Diabetes 2011; 60: 3235–3245.
Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL et al. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol 2009; 29: 1363–1374.
Shoar Z, Goldenthal MJ, De Luca F, Suarez E . Mitochondrial DNA content and function, childhood obesity, and insulin resistance. Endocr Res 2016; 41: 49–56.
Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim S-H, Mostoslavsky R et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J 2007; 26: 1913–1923.
Feldmann HM, Golozoubova V, Cannon B, Nedergaard J . UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 2009; 9: 203–209.
Langin D, Larrouy D, Barbe P, Millet L, Viguerie-Bascands N, Andreelli F et al. Uncoupling protein-2 (UCP2) and uncoupling protein-3 (UCP3) expression in adipose tissue and skeletal muscle in humans. Int J Obes Relat Metab Disord 1999; 23 (Suppl 6): S64–S67.
Thompson MP, Kim D . Links between fatty acids and expression of UCP2 and UCP3 mRNAs. FEBS Lett 2004; 568: 4–9.
Skulachev VP . Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta 1998; 1363: 100–124.
Pecqueur C, Alves-Guerra MC, Gelly C, Lévi-Meyrueis C, Couplan E, Collins S et al. Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J Biol Chem 2001; 276: 8705–8712.
Mahadik SR, Lele RD, Saranath D, Seth A, Parikh V . Uncoupling protein-2 (UCP2) gene expression in subcutaneous and omental adipose tissue of Asian Indians. Adipocyte 2012; 1: 101–107.
Joseph A-M, Joanisse DR, Baillot RG, Hood DA . Mitochondrial dysregulation in the pathogenesis of diabetes: potential for mitochondrial biogenesis-mediated interventions. Exp Diabetes Res 2012; 2012: 642038.
Srere PA . [1] Citrate synthase. [EC 4.1.3.7. Citrate oxaloacetate-lyase (CoA-acetylating)]. Methods Enzymol 1969; 13: 3–11.
Christe M, Hirzel E, Lindinger A, Kern B, von Flüe M, Peterli R et al. Obesity affects mitochondrial citrate synthase in human omental adipose tissue. ISRN Obes 2013; 2013: 1–8.
Vieira VJ, Valentine RJ . Mitochondrial biogenesis in adipose tissue: can exercise make fat cells ‘fit’? J Physiol 2009; 587: 3427–3428.
Kaaman M, Sparks LM, van Harmelen V, Smith SR, Sjölin E, Dahlman I et al. Strong association between mitochondrial DNA copy number and lipogenesis in human white adipose tissue. Diabetologia 2007; 50: 2526–2533.
Chattopadhyay M, GuhaThakurta I, Behera P, Ranjan KR, Khanna M, Mukhopadhyay S et al. Mitochondrial bioenergetics is not impaired in nonobese subjects with type 2 diabetes mellitus. Metabolism 2011; 60: 1702–1710.
Norvell A, McMahon SB . Rise of the rival. Science 2010; 327: 964–965.
Qiu X, Brown K, Hirschey MD, Verdin E, Chen D . Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 2010; 12: 662–667.
Tao R, Coleman MC, Pennington JD, Ozden O, Park S-H, Jiang H et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 2010; 40: 893–904.
Fernandez-Marcos PJ, Auwerx J . Regulation of PGC-1, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 2011; 93: 884S–890S.
Lombard DB, Alt FW, Cheng H-L, Bunkenborg J, Streeper RS, Mostoslavsky R et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 2007; 27: 8807–8814.
Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010; 464: 121–125.
Ahn B-H, Kim H-S, Song S, Lee IH, Liu J, Vassilopoulos A et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA 2008; 105: 14447–14452.
Shimazu T, Hirschey MD, Hua L, Dittenhafer-Reed KE, Schwer B, Lombard DB et al. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab 2010; 12: 654–661.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112: 1821–1830.
Liu Y, Qi W, Richardson A, Van Remmen H, Ikeno Y, Salmon AB . Oxidative damage associated with obesity is prevented by overexpression of CuZn- or Mn-superoxide dismutase. Biochem Biophys Res Commun 2013; 438: 78–83.
Kobayashi H, Matsuda M, Fukuhara A, Komuro R, Shimomura I . Dysregulated glutathione metabolism links to impaired insulin action in adipocytes. Am J Physiol Endocrinol Metab 2009; 296: E1326–E1334.
Frohnert BI, Sinaiko AR, Serrot FJ, Foncea RE, Moran A, Ikramuddin S et al. Increased adipose protein carbonylation in human obesity. Obesity (Silver Spring) 2011; 19: 1735–1741.
Acknowledgements
This work was supported by CONACyT (SALUD-2009-01-111494). FV is a co-founder and stockholder in Cardero Therapeutics Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Zamora-Mendoza, R., Rosas-Vargas, H., Ramos-Cervantes, M. et al. Dysregulation of mitochondrial function and biogenesis modulators in adipose tissue of obese children. Int J Obes 42, 618–624 (2018). https://doi.org/10.1038/ijo.2017.274
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.274
- Springer Nature Limited
This article is cited by
-
Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long-COVID
npj Science of Food (2024)
-
The potential of therapeutic strategies targeting mitochondrial biogenesis for the treatment of insulin resistance and type 2 diabetes mellitus
Archives of Pharmacal Research (2024)
-
White adipose tissue mitochondrial bioenergetics in metabolic diseases
Reviews in Endocrine and Metabolic Disorders (2023)
-
Childhood overeating is associated with adverse cardiometabolic and inflammatory profiles in adolescence
Scientific Reports (2021)
-
Maternal obesity during pregnancy leads to adipose tissue ER stress in mice via miR-126-mediated reduction in Lunapark
Diabetologia (2021)