Abstract
Background:
Body weight and adiposity are heritable traits. To date, it remains unknown whether obesity-associated brain structural alterations are under a similar level of genetic control.
Methods:
For this study, we utilized magnetic resonance imaging data from the Human Connectome Project. Voxel-based morphometry was used to investigate associations between body mass index (BMI) and regional gray matter volume (GMV) in a sample of 875 young adults with a wide BMI range (386 males/489 females; age 28.8±3.7 years; BMI 26.6±5.3 kg m−2) that included 86 pairs of monozygotic twins and 82 pairs of dizygotic twins. Twin data were analyzed by applying the additive genetic, common environmental and residual effects model to determine heritability of brain regions that were associated with BMI.
Results:
We observed positive associations between BMI and GMV in the ventromedial prefrontal cortex and the right cerebellum and widespread negative associations within the prefrontal cortex, cerebellum, temporal lobes and distinct subcortical structures. Varying degrees of heritability were found for BMI-associated brain regions, with the highest heritability estimates for cerebellar GMV and subcortical structures.
Conclusions:
These data indicate that brain regions associated with obesity are subject to differing levels of genetic control and environmental influences. Specific brain regions with high heritability might represent an inherent vulnerability factor for obesity.
Similar content being viewed by others
Introduction
The rising prevalence of obesity remains unchecked in industrialized societies. As of 2011/2012, 70% of the adult US population were estimated to be overweight or obese with a body mass index (BMI) >25 kg m−2.1
Accumulation of excess adipose tissue is generally a result of unhealthy eating patterns and chronically positive energy balance, further promoted by the western ‘obesogenic’ environment, where an abundance of unhealthy energy-dense foods is readily available at any time. Hence, increasing efforts are being made to enhance our understanding of the neurobiological underpinnings that may promote and/or follow unhealthy eating patterns and the development of obesity. Both animal and human studies have documented dysfunctional dopaminergic central pathways and the effects on reward-related and motivated behavior to be key neurophysiological contributors to the behavioral phenotype leading to obesity.2, 3, 4 In addition, a number of neuroimaging studies have found obesity to be associated with functioznal and structural alterations of the brain, including prefrontal cortical, insular and striatal regions critically involved in executive functioning, reward processing and interoception.5, 6, 7, 8, 9, 10, 11, 12 Intriguingly, reduced gray matter volume (GMV) of similar brain regions (that is, insular and prefrontal cortex) was reported in subjects with high risk for obesity, possibly indicating that structural brain differences are predisposing to obesity.13 Similar results were reported by Yokum et al.14 linking prefrontal GMV with future weight gain in a sample of young women; however, reported brain regions of these studies differed with respect to the exact locations. Obesity and BMI have been shown to be highly heritable, generally on a polygenic basis.15, 16 Certain genetic variants are common and carry significant risk of increased adiposity; for example, variations in the FTO gene (that is, fat mass and obesity-associated protein), where those homozygous for the risk allele have an increased risk for obesity (hazard ratio 1.67), on average weighing 3–4 kg more than non-carriers.17 In addition, evidence for a genetically determined behavioral phenotype with altered reward sensitivity (thus favoring unhealthy eating patterns) is provided by multiple studies that have linked genetic variants of the dopamine receptor D2 (DRD2) gene (that is, the Taq1A A1 allele) and of the μ-opioid receptor (OPRM1) to obesity, binge-eating disorder and addictive behavior in general.18, 19
Given evidence of volumetric brain differences and genetic influences, an important question is the degree to which obesity-related structural brain differences are heritable. It is possible that at least some regional brain volume differences reflect central endophenotypes leading to unhealthy eating and excess adiposity. Twin studies provide a powerful approach for heritability analyses of complex traits, such as obesity, by comparing monozygotic (MZ) and dizygotic (DZ) twins. Previous twin studies have shown varying degrees of estimated heritability for regional brain volume, thus indicating differing susceptibilities with respect to genetic and environmental influences.20
In this study, we aimed to investigate associations between regional GMV, body weight (that is, BMI) and the heritability of obesity-associated structural brain alterations, hypothesizing high degrees of heritability—particularly for brain regions that form part in dopaminergic reward pathways. We utilized structural neuroimaging data from the Human Connectome Project (HCP), a large-scale neuroimaging study with open access data that investigates human brain networks and behavioral correlates under consideration of genetic and environmental influences.21 In a first step, we investigated associations between BMI and regional GMV in a sample of 875 healthy young adults who were non-twin siblings, DZ or MZ twin pairs. In a second step, we estimated heritability of BMI and of predefined brain regions that we found to be associated with BMI, by using available twin data only.
Methods
Study population
For this study, data were drawn from the publicly available HCP database (that is, S500 and S900 data releases; www.humanconnectome.org) that included a total of n=875 healthy young to middle-aged adults with structural magnetic resonance imaging (that is, T1-weighted MPRAGE) and BMI data. For additional information on eligibility criteria of the original study, we refer to previously published detailed descriptions.21, 22 The study population was composed of non-twin siblings (n=447), MZ (identical) twins (n=206) and DZ (fraternal) twins (n=222) from n=380 families. Structural magnetic resonance imaging scans from both twin siblings were available for n=86 identical and n=83 fraternal twin pairs. All participants gave written consent and experimental procedures were approved by the institutional review board (IRB no. 201204036; Title: ‘Mapping the Human Connectome: Structure, Function, and Heritability’). For our data analyses, no additional approval was required by the local ethics commitee of the University of Leipzig.
Imaging procedures
Detailed descriptions of structural imaging protocols are provided on the website of the HCP (www.humanconnectome.org) and in previous publications.21 In brief, two separate T1-weighted, high-resolution (0.7-mm isotropic voxels) anatomical images (that is, 3D MPRAGE) were acquired on a customized Siemens 3-T Skyra system (Siemens, Erlangen, Germany) with a 32-channel head coil, using the following parameters: field of view=224 mm, matrix=320, 256 sagittal slices per single slab, TR=2400 ms, TE=2.14 ms, TI=1000 ms, FA=8°, Bandwidth (BW)=210 Hz per pixel, Echo Spacing (ES)=7.6 ms. For this study, we used anatomical images from the first scan only.
Data analysis
Voxel-based morphometry (VBM)23 of T1-weighted MPRAGE images was performed with the Statistical Parametric Mapping package (SPM8, Wellcome Department of Imaging Neuroscience, London, UK; www.fil.ion.ucl.ac.uk/spm) and the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html). Images were preprocessed with default settings of the VBM8 Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) toolbox, including high-dimensional DARTEL normalization algorithms and modulation for non-linear components, which accounts for interindividual differences in total brain volume. First, images were segmented into gray matter (GM), white matter and cerebrospinal fluid, followed by normalization to the DARTEL template in Montreal Neurological Institute (MNI) space (voxel size: 1.5 × 1.5 × 1.5 mm3) and smoothing of GM data with an 8-mm full-width-half-maximum isotropic Gaussian kernel. All images were visually inspected prior to statistical analyses.
Multiple regression analyses of smoothed GM data were employed to investigate associations between regional GMV and BMI. As covariates of no interest, we included age, gender, handedness (Edinburgh Handedness Questionnaire; ranging from −100 (lefthanded) to 100 (righthanded)), race (white/non-white), twin status and zygosity. Analysis of variance was used to test for gender × BMI interaction. In addition, we applied an absolute threshold to exclude voxels with a GM value of <0.1. Results were considered significant at a peak voxel threshold of P<0.05 corrected for the entire brain volume (FWE, family-wise error correction for multiple comparisons on the voxel level). As SPM8 by defaults only displays three peaks within a cluster, we used the current cluster option to explore large clusters (>10 000 voxels).
In order to test whether associations of BMI with GMV are driven by educational background of intelligence, we ran additional post hoc analyses, including years of education (n=874) or measures of fluid intelligence (n=869; that is, correct answers in the Penn Progressive Matrices) as further covariates.
Heritability analyses of BMI-associated brain structures were performed on predefined regions of interests (ROIs). These a priori ROIs were chosen based on previous publications and functional relevance and included the prefrontal cortex, including the ventromedial prefrontal cortex (VMPFC),5, 6, 12, 24 cerebellum,6, 25, 26 temporal6, 25, 27 and insular cortices;6, 25, 28 anterior cingulate cortex6, 25, 29; hippocampal/parahippocampal cortex12, 30; dorsal and ventral striatum;5, 9, 31 midbrain;3, 32 amygdala3, 6, 33 and hypothalamus.3, 5 To do so, masks of the ROIs were generated with the WFU-Pickatlas toolbox (www.fmri.wfubmc.edu). Next results of the whole-brain analyses that were masked for the respective ROI and GM data were extracted by centering a 3-mm sphere on the peak locations34 of the overall regression analysis. Owing to masking procedures, slight differences in the respective peak locations may occur, as compared with the whole-brain analyses. As no associations were found for the hypothalamus and right striatum (except for neighboring extranuclear structures), no heritability analyses were performed for these ROIs.
SAS Software (SAS Institute Inc., version 9.4, Cary, NC, USA) was used for statistical analyses of non-imaging data and for heritability analyses of extracted GM data. For heritability analyses, we applied the additive genetic, common environmental and residual effects (ACE) model, as provided by Feng et al.,35 with additional modifications to fit quantitative data. Heritability of BMI and GM data were estimated in n=336 twins (that is, 86 MZ and 82 DZ twin pairs) from n=168 families using non-linear mixed model analysis (that is, PROC NLMIXED). Estimation of additive genetic and common environment random-effects components (that is, ACE model) was performed by the maximum likelihood method. Heritability was quantified as the ratio between estimated additive genetic variance and the total variance of the trait. Values of GM volumes were log-transformed to approximate a normal distribution prior to analyses. Owing to significant group differences between MZ and DZ, sex and age were included as covariates. A P<0.05 was considered statistically significant.
In addition, we employed the Biological Parametric Mapping Toolbox (BPM; www.ansir.wfubmc.edu) within the SPM5 framework (Wellcome Department of Imaging Neuroscience; www.fil.ion.ucl.ac.uk/spm) to create descriptive correlation maps of twin pair GM data, thus allowing further quantification of concordance of regional GMV associated with BMI in MZ and DZ. This includes voxel-wise correlation within MNI space to generate results for a homologous correlation field. BPM analyses were regionally restricted to an ROI based on the results from our VBM whole-group analyses by using inclusive masks of regional GMV clusters we found to be associated with BMI. Correlational maps were not thresholded in order to illustrate within-twin pair correlations across the entire ROI. In addition, we provide results of thresholded analyses (that is, correlation coefficient of >0.5 and k=50 continuous voxels) as Supplementary Data.
Results
Characteristics of the study population and comparative group statistics of identical and fraternal twin pairs are listed in Table 1. No significant differences were found for BMI, race, education or fluid intelligence, yet identical and fraternal twins differed with respect to total body weight, height, age and gender.
VBM analyses
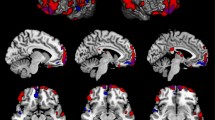
Detailed results of the VBM analyses are listed in Table 2. Using the entire sample of 875 subjects revealed distinct positive associations (Figure 1) within the VMPFC (PFWE<0.001; k=179; MNIxyz (2 mm 21 mm −26 mm)) and the right cerebellum (PFWE=0.003; k=21; MNIxyz (27 mm −45 mm −42 mm)). Negative associations were observed within widespread areas of the brain (Figure 2) with one large cluster comprising bilateral prefrontal and precentral cortices; cerebellum; bilateral temporal lobes, including bilateral hippocampal/parahippocampal region and the bilateral amygdala, the bilateral insula, parts of the occipital cortex, bilateral thalamic regions and adjacent parts of the midbrain (that is, ventral tegmental area) and left dorsal and ventral striatum (PFWE<0.001; k=1179490; MNIxyz (−42 mm 48 mm −14 mm)). In addition, several smaller clusters were found within the prefrontal and cingulate cortex, cerebellum and occipital cortex (for details, please see Table 3). Additional adjustment for educational level (n=874) or fluid intelligence (measured by Penn Progressive Matrices; n=869) did not significantly change these results and separate analyses of n=380 non-siblings revealed a very similar pattern of positive and negative associations (data not shown).
T-score maps of regional GMV positively associated with BMI ((a) VMPFC; (b) cerebellum) superimposed on high-resolution rendered images of the brain within the sagital, coronal and axial plane (left to right). Maps are thresholded at P<0.05 whole brain corrected on the voxel level (FWE); Crbl, cerebellum; color bar indicates T-score.
T-score maps of regional GMV negatively associated with BMI superimposed on high-resolution rendered images of the brain (a) and on slices within the axial plane (b) with corresponding locations on the z axis below; maps are thresholded at P<0.05 whole brain corrected on the voxel level (FWE); color bar indicates t-score.
Investigating differential effects of overweight and obesity on brain structure in males and females (that is, gender × BMI interaction), only two small clusters were found within the left uncus/inferior temporal gyrus (PFWE=0.016; k=25; MNIxyz (−29 mm 0 mm −50mm)) and the right inferior temporal gyrus (PFWE=0.035; k=5; MNIxyz (32 mm −6 mm −45 mm)) with stronger negative associations of BMI with regional GMV in males compared with females.
Heritability analyses
Detailed results of the heritability analyses are listed in Table 3. Applying the ACE model for heritability analyses yielded high heritability of BMI (that is, h2=0.73). For BMI-associated regional GMV, highest heritability estimates were found for bilateral cerebellar GMV followed by GMV of subcortical structures (that is, bilateral midbrain, left putamen, left nucleus accumbens (NAcc)) and bilateral amygdala. Overall, lower, yet still statistically significant, heritability estimates, were observed for GMV of prefrontal cortical regions (that is, left lateral and right medial orbitofrontal cortices), right VMPFC, bilateral parahippocampus and insula. Non-significant and low heritability estimates were found for GMV of bilateral temporal lobes and the bilateral anterior cingulate cortex.
BPM analyses
Unthresholded correlational maps of regional GMV negatively associated with BMI within identical and fraternal twin pairs are illustrated in Figure 3. Thresholded correlational analyses (that is, r>0.5 and k=50 continuous voxels) showed the strongest within-twin pair correlations for cerebellar GMV, followed by (in descending order) subcortical structures (that is, left caudate, right thalamus); mesiotemporal regions, including the bilateral parahippocampal and lingual gyrus; prefrontal and limbic structures (that is, bilateral cingulate) for identical twins (detailed results in Supplementary Table S1). Within fraternal twin pairs, less extensive and overall weaker correlations were observed for (in descending order) right temporal cortex, cerebellar, occipital, mesiotemporal and prefrontal cortical regions (detailed results in Supplementary Table S2). Brain regions positively associated with BMI showed relatively strong positive correlations within identical twins (that is, r=0.78 for VMPFC), whereas in fraternal twins no suprathreshold correlations were observed (Supplementary Information).
Discussion
Overall, our results show widespread and mostly negative associations of BMI with regional GMV in healthy young adults, particularly within prefrontal, temporal/mesotemporal and cerebellar brain regions as well as distinct subcortical structures with only marginal differences between males and females. In addition, we find varying degrees of heritability for these brain regions, with the highest heritability estimates for cerebellar GMV and subcortical structures.
Associations of BMI with GMV
A number of previous studies investigated the relationship between obesity and brain structure, mostly showing negative associations between measures of adiposity and brain GMV, but results varied significantly with respect to the brain regions involved.5, 7, 9, 10, 11 However, two recently published studies with larger samples demonstrated comparable patterns of adiposity-related brain structural alterations. Analyzing two separate community-based samples (1586 adults with a mean age of 46 years and 758 adults with a mean age of 50 years), Janowitz et al.6 showed largely overlapping negative associations between rGMV and waist circumference for both samples that included bilateral frontal, temporal and occipital lobes, somatosensory and motor cortex, insula, cingulate gyrus, hippocampal formation, subcortical structures (that is, thalamus, caudate nucleus, putamen, globus pallidus) and the cerebellum. Similar observations were made in a sample of 617 older subjects (mean age 68 years), additionally linking these structural alterations to impaired memory performance.8 Importantly, results of both studies were not significantly affected by obesity-associated comorbidities, such as type 2 diabetes, hypertension and dyslipidemia. In contrast to these reports with predominantly negative associations, Horstmann et al.5 demonstrated exclusively positive associations between BMI and GMV in the VMPFC, including neighboring parts of NAcc and left putamen, in a sample of 122 healthy young and middle-aged adults. Although disparities between individual studies may be attributed to differences in sample size, age range, gender distribution and comorbidities of the respective study populations, the present analysis of this large sample of young adults confirmed both positive and negative associations reported from these previous studies. Importantly, these results were not driven by intelligence or education. However, minor differences were observed between males and females with stronger associations of BMI with GMV of the inferior and mesial temporal cortex. Both increases of fat mass and fat-free mass (that is, all non-adipose tissues) are found in obese subjects and we previously found strong negative associations of fat-free mass with GMV of similar regions within the temporal cortex.25 Compared with women, men usually have more fat-free mass; hence, gender-related differences in body composition may have contributed to this finding.
Heritability of BMI and BMI-associated GMV
Applying the ACE model for estimations of heritability in MZ and DZ twins, we found high heritability for BMI. The high heritability of BMI in our study (h2=0.72) is comparable to previous studies. A meta-analysis of 88 twin studies found heritability estimates ranging between 0.47 and 0.90 with a median of 0.75, thus closely resembling the results from our analysis.15
Consistent with previous twin studies of brain morphology, our results show a wide range of heritability estimates for BMI-associated GMV (0.21–0.82). Previous volumetric analyses found overall high correlations within MZ twins (n=12) for cerebral and cerebellar volumes, followed by subcortical structures (that is, thalamus, caudate, putamen), while measures of surface morphology showed less concordance between twins, indicating a significant impact of environmental influences on cortical brain regions.36 Analyses of cerebral GM distribution in MZ and DZ twins (n=40) yielded highly varying heritability estimates for distinct frontal, temporal and sensorimotor cortices, again indicating region-specific genetic influences on brain structure and susceptibilities to environmental influences, respectively.37 In our study, the highest heritability estimates of BMI-associated GMV were found for the cerebellum and for distinct subcortical structures (that is, midbrain, left putamen, left NAcc, bilateral amygdala). Numerous structural6, 7, 8, 9, 11, 25, 38 and functional39, 40, 41, 42 imaging studies have highlighted the cerebellum in the context of obesity and eating behavior. Cerebellar function was once believed to be limited to motor, but increasing evidence indicates a far more diverse role, including non-motor cognitive processes in the context of emotion, executive function and addictive behavior.43, 44
The midbrain—and the ventral tegmental area in particular—gives origin to the mesolimbic dopaminergic ‘reward pathway’ via projections to the ventral striatum (that is, NAcc), which is considered a key structure in reward-related and addictive behavior,3 while reward-based learning—processed in the dorsal striatum—may further habituate behavioral patterns that ultimately lead to obesity.45 The amygdala, on the other hand, together with the thalamus, ventral and dorsal striatum, midbrain and operculum, is part of a network that guides cue-potentiated eating behavior (that is, food intake elicited via reward-predicting cues).46
Overall, lower, but still significant, heritability estimates were obtained for prefrontal cortical regions, the bilateral hippocampal region and insula, while heritability estimates for temporal cortical regions and the anterior cingulate were low and did not reach statistical significance. This comparably wide distribution of heritability estimates for BMI-associated GMV has important implications with respect to the neurobiology of obesity. First, highly heritable brain structural correlates of BMI (that is, cerebellar and subcortical structures) represent potential endophenotypes of obesity and—starting from an early age—potentially facilitate weight gain by altered sensitivity to rewarding stimuli. On the other hand, brain regions with moderate-to-low heritability estimates of GMV (that is, frontal, mesiotemporal insular and temporal cortex, anterior cingulate cortex) appear to be more prone to environmental influence. Thus negative associations of BMI within less heritable brain regions are more likely to represent neuroplastic changes that may occur during the time course of the developing obese phenotype. Considering the consistent detection and functional relevance of many of these regions in the context of obesity and eating behavior,12 it seems plausible that obesity and the related behavioral phenotype may be further consolidated by subsequent structural and functional brain changes arising from the negative health effect of obesity.
Corroborative data are provided by longitudinal weight loss studies, demonstrating increases in hippocampal and insular GMV—brain regions with moderate-to-low heritability estimates—after 3 months of strenuous physical exercise in overweight and obese individuals.47 Similar effects were recently demonstrated by Prehn et al.48 during the course of a dietary weight loss intervention with increased GMV of the hippocampus and the inferior frontal gyrus. Analyses of brain structural changes in obese subjects undergoing bariatric surgery yielded increases of GMV in frontal and posterior brain regions. However, these brain structural changes only partially overlapped with preoperative GM differences.49
As no longitudinal data are available for our study population, no final conclusion can be drawn whether these associations represent origin or consequence of obesity. Consequently, we have to acknowledge the cross-sectional nature of this study as important limitation. Other limitations of this study include the use of ROI-based approached of our heritability analyses that may have concealed other potentially relevant brain regions; therefore, these findings must be considered preliminary. Furthermore, our study is limited by the use of BMI as surrogate of adiposity owing to its inability to precisely measure body fat content. Also, the inherent structure of the HCP data set with twin and non-twin siblings may have influenced our results. Nevertheless, we did attempt to adjust for these factors in our analysis and additional analyses of a smaller sample of unrelated subjects yielded very similar results. Obesity is a complex disorder, accompanied by a variety of comorbidities that are not accounted for in our analyses. Nevertheless, our study population is comparably young (mean age=29 years) and did not include subjects with significant health issues. However, the widespread pattern of mainly negative associations between GMV and BMI suggests a relationship between adiposity and brain structure beyond food intake, energy homeostasis and reward-related behavior that could not be accounted for in these analyses.
Conclusions
In this sample of 875 healthy young adults, we found widespread negative associations between BMI and regional GMV comprising the prefrontal cortex, cerebellum, temporal lobes and subcortical structures. Anatomically distinct positive associations were also observed within the right VMPFC and the right cerebellum. Heritability analyses of prespecified regions of interest showed the highest heritability estimates for regional GMV of the cerebellum and subcortical structures and the lowest for regional GMV of the temporal lobes. These findings indicate that brain regions associated with obesity are subject to differing levels of genetic determination and environmental influences. Some characteristics increase initial vulnerability to obesity while others arise from the negative health effects of the condition.
References
Ogden CL, Carroll MD, Kit BK, Flegal KM . Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014; 311: 806–814.
Berridge KC, Ho C-Y, Richard JM, DiFeliceantonio AG . The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res 2010; 1350: 43–64.
Kenny PJ . Reward mechanisms in obesity: new insights and future directions. Neuron 2011; 69: 664–679.
Volkow ND, Wang G-J, Tomasi D, Baler RD . Obesity and addiction: neurobiological overlaps. Obes Rev 2013; 14: 2–18.
Horstmann A, Busse FP, Mathar D, Muller K, Lepsien J, Schlogl H et al. Obesity-related differences between women and men in brain structure and goal-directed behavior. Front Hum Neurosci 2011; 5: 58.
Janowitz D, Wittfeld K, Terock J, Freyberger HJ, Hegenscheid K, Völzke H et al. Association between waist circumference and gray matter volume in 2344 individuals from two adult community-based samples. Neuroimage 2015; 122: 149–157.
Kurth F, Levitt JG, Phillips OR, Luders E, Woods RP, Mazziotta JC et al. Relationships between gray matter, body mass index, and waist circumference in healthy adults. Hum Brain Mapp 2013; 34: 1737–1746.
Masouleh SK, Arélin K, Horstmann A, Lampe L, Kipping JA, Luck T et al. Higher body mass index in older adults is associated with lower gray matter volume: implications for memory performance. Neurobiol Aging 2016; 40: 1–10.
Pannacciulli N, Del Parigi A, Chen K, Le DSNT, Reiman EM, Tataranni PA . Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage 2006; 31: 1419–1425.
Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K et al. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity 2008; 16: 119–124.
Walther K, Birdsill AC, Glisky EL, Ryan L . Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp 2010; 31: 1052–1064.
Brooks SJ, Cedernaes J, Schiöth HB . Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS One 2013; 8: e60393.
Smucny J, Cornier M-A, Eichman LC, Thomas EA, Bechtell JL, Tregellas JR . Brain structure predicts risk for obesity. Appetite 2012; 59: 859–865.
Yokum S, Ng J, Stice E . Relation of regional gray and white matter volumes to current BMI and future increases in BMI: a prospective MRI study. Int J Obes 2012; 36: 656–664.
Elks CE, den Hoed M, Zhao JH, Sharp SJ, Wareham NJ, Loos RJF et al. Variability in the heritability of body mass index: a systematic review and meta-regression. Front Endocrinol 2012; 3: 29.
Hinney A, Vogel CIG, Hebebrand J . From monogenic to polygenic obesity: recent advances. Eur Child Adolesc Psychiatry 2010; 19: 297–310.
Loos RJF, Bouchard C . FTO: the first gene contributing to common forms of human obesity. Obes Rev 2008; 9: 246–250.
Stice E, Spoor S, Bohon C, Small DM . Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 2008; 322: 449–452.
Ziauddeen H, Farooqi IS, Fletcher PC . Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci 2012; 13: 279–286.
Toga AW, Thompson PM . Genetics of brain structure and intelligence. Annu Rev Neurosci 2005; 28: 1–23.
Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, Ugurbil K . The WU-Minn Human Connectome Project: an overview. Neuroimage 2013; 80: 62–79.
Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TEJ, Bucholz R et al. The Human Connectome Project: a data acquisition perspective. Neuroimage 2012; 62: 2222–2231.
Ashburner J, Friston KJ . Voxel-based morphometry–the methods. Neuroimage 2000; 11: 805–821.
Kringelbach ML . The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 2005; 6: 691–702.
Weise CM, Thiyyagura P, Reiman EM, Chen K, Krakoff J . Fat-free body mass but not fat mass is associated with reduced gray matter volume of cortical brain regions implicated in autonomic and homeostatic regulation. Neuroimage 2013; 64: 712–721.
Zhu J-N, Wang J-J . The cerebellum in feeding control: possible function and mechanism. Cell Mol Neurobiol 2007; 28: 469–478.
Veit R, Kullmann S, Heni M, Machann J, Häring H-U, Fritsche A et al. Reduced cortical thickness associated with visceral fat and BMI. Neuroimage Clin 2014; 6: 307–311.
Frank S, Kullmann S, Veit R . Food related processes in the insular cortex. Front Hum Neurosci 2013; 7: 499.
Weston CSE . Another major function of the anterior cingulate cortex: the representation of requirements. Neurosci Biobehav Rev 2012; 36: 90–110.
Mueller SG, Laxer KD, Cashdollar N, Buckley S, Paul C, Weiner MW . Voxel-based optimized morphometry (VBM) of gray and white matter in temporal lobe epilepsy (TLE) with and without mesial temporal sclerosis. Epilepsia 2006; 47: 900–907.
Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W et al. Brain dopamine and obesity. Lancet 2001; 357: 354–357.
Weise CM, Thiyyagura P, Reiman EM, Chen K, Krakoff J . A potential role for the midbrain in integrating fat-free mass determined energy needs: an H2 (15) O PET study. Hum Brain Mapp 2015; 36: 2406–2415.
Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A . Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res 2009; 198: 149–158.
Rijsdijk FV, Viding E, De Brito S, Forgiarini M, Mechelli A et al. Heritable variations in gray matter concentration as a potential endophenotype for psychopathic traits. Arch Gen Psychiatry 2010; 67: 406–413.
Feng R, Zhou G, Zhang M, Zhang H . Analysis of twin data using SAS. Biometrics 2009; 65: 584–589.
White T, Andreasen NC, Nopoulos P . Brain volumes and surface morphology in monozygotic twins. Cereb Cortex 2002; 12: 486–493.
Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen V-P, Huttunen M et al. Genetic influences on brain structure. Nat Neurosci 2001; 4: 1253–1258.
Raschpichler M, Straatman K, Schroeter ML, Arelin K, Schlögl H, Fritzsch D et al. Abdominal fat distribution and its relationship to brain changes: the differential effects of age on cerebellar structure and function: a cross-sectional, exploratory study. BMJ Open 2013; 3, pii e001915.
Dimitropoulos A, Tkach J, Ho A, Kennedy J . Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite 2012; 58: 303–312.
Gearhardt AN, Yokum S, Stice E, Harris JL, Brownell KD . Relation of obesity to neural activation in response to food commercials. Soc Cogn Affect Neurosci 2014; 9: 932–938.
Tomasi D, Wang G-J, Wang R, Backus W, Geliebter A, Telang F et al. Association of body mass and brain activation during gastric distention: implications for obesity. PLoS One 2009; 4: e6847.
Tuulari JJ, Karlsson HK, Hirvonen J, Salminen P, Nuutila P, Nummenmaa L . Neural circuits for cognitive appetite control in healthy and obese individuals: an fMRI study. PLoS One 2015; 10: e0116640.
Buckner RL . The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 2013; 80: 807–815.
Strick PL, Dum RP, Fiez JA . Cerebellum and nonmotor function. Annu Rev Neurosci 2009; 32: 413–434.
Burger KS, Stice E . Greater striatopallidal adaptive coding during cue–reward learning and food reward habituation predict future weight gain. Neuroimage 2014; 99: 122–128.
Small DM . Individual differences in the neurophysiology of reward and the obesity epidemic. Int J Obes 2009; 33: S44–S48.
Mueller K, Möller HE, Horstmann A, Busse F, Lepsien J, Blüher M et al. Physical exercise in overweight to obese individuals induces metabolic- and neurotrophic-related structural brain plasticity. Front Hum Neurosci 2015; 9: 372.
Prehn K, Jumpertz von Schwartzenberg R, Mai K, Zeitz U, Witte AV, Hampel D et al. Caloric restriction in older adults-differential effects of weight loss and reduced weight on brain structure and function. Cereb Cortex 2016. e-pub ahead of print 1 February 2016 pii bhw008.
Tuulari JJ . Effects of obesity and weight loss following bariatric surgery on brain function structural integrity and metabolism, 2015. Available at www.doria.fi.
Acknowledgements
Data were provided (in part) by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research and by the McDonnell Center for Systems Neuroscience at Washington University. This study was supported by the IFB AdiposityDiseases, Federal Ministry of Education and Research (BMBF), Germany, FKZ: 01E01001 (http://www.bmbf.de) and the German Research Foundation (DFG; http://www.dfg.de), within the framework of the CRC 1052 Obesity Mechanisms to Project A6 (to BP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Weise, C., Piaggi, P., Reinhardt, M. et al. The obese brain as a heritable phenotype: a combined morphometry and twin study. Int J Obes 41, 458–466 (2017). https://doi.org/10.1038/ijo.2016.222
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.222
- Springer Nature Limited
This article is cited by
-
Viscoelasticity of striatal brain areas reflects variations in body mass index of lean to overweight male adults
Brain Imaging and Behavior (2020)
-
Prefronto-cerebellar neuromodulation affects appetite in obesity
International Journal of Obesity (2019)
-
Neuroanatomical differences in obesity: meta-analytic findings and their validation in an independent dataset
International Journal of Obesity (2019)
-
Preliminary evidence for genetic overlap between body mass index and striatal reward response
Translational Psychiatry (2018)