Abstract
Background:
The Mediterranean diet has been consistently associated with reduced mortality risk. Few prospective studies have examined whether the benefits from a Mediterranean diet are equally shared by obese individuals with varying metabolic health.
Objective:
The objective of this study was to investigate the association between Mediterranean diet, metabolic phenotypes and mortality risk in a representative obese US population.
Methods:
Data from 1739 adults aged 20–88 years were analyzed from participants of the National Health and Nutrition Examination Survey III, 1988–1994 followed up for deaths until 31 December 2011 in a prospective cohort analysis. Mediterranean Diet Scores (MDS) were created to assess the adherence to Mediterranean diet. Participants were classified as metabolically healthy obese (MHO) phenotype (0 or 1 metabolic abnormality) or metabolically unhealthy obese (MUO) phenotype (two or more metabolic abnormalities), based on high glucose, insulin resistance, blood pressure, triglycerides, C-reactive protein and low high-density lipoprotein cholesterol.
Results:
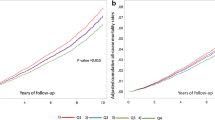
The MHO phenotype (n=598) was observed in 34.8% (s.e., 1.7%) of those who were obese (mean body mass index was 33.4 and 34.8 in MHO and MUO phenotypes, respectively). During a median follow-up of 18.5 years, there were 77 (12.9%) and 309 (27.1%) deaths in MHO and MUO individuals, respectively. In MHO individuals, the multivariable-adjusted hazard ratio (HR) of all-cause mortality in the highest tertile compared with the first tertile of MDS was 0.44 (95% confidence interval (CI), 0.26–0.75; P for trend <0.001), after adjustment for potential confounders. A five-point (1 s.d.) increment in the adherence to MDS was associated with a 41% reduction in the risk of all-cause mortality (HR, 0.59; 95% CI, 0.37–0.94). Similar findings were obtained when we restricted our analyses to those with or without prevalent diabetes mellitus and hypertension. We did not observe mortality risk reduction in either individuals with MUO phenotype or all obese participants combined.
Conclusions:
Adherence to a Mediterranean dietary pattern appears to reduce mortality in the MHO phenotype, but not among the MUO phenotype in an obese population.
Similar content being viewed by others
Introduction
The Mediterranean diet is characterized by high intake of olive oil and polyunsaturated fats, legumes, grains, fish, fruits and vegetables; moderate intake of milk, dairy products and alcohol; and low intake of meat and meat products.1 Multiple epidemiological studies have demonstrated that adherence to the Mediterranean diet is associated with lower risk of cardiometabolic disease in addition to lower risk of all-cause mortality, cardiovascular disease (CVD) and cancer mortality.2, 3, 4
The risk of developing obesity-related metabolic complications corresponds to the degree of obesity.5, 6 However, the existence of these obesity-related metabolic abnormalities varies extensively in obese individuals.7 Despite being outwardly obese, a subset of obese individuals appears to be protected or more resistant to the development of cardiometabolic abnormalities associated with obesity. These individuals with a metabolically healthy obese (MHO) phenotype, namely benign or uncomplicated obesity, demonstrate favorable metabolic characteristics such as normal insulin sensitivity, relatively low visceral fat, lower liver enzyme profiles, no sign of dyslipidemia or hypertension8, 9, 10 and lower risk of CVD,11 compared with their metabolically unhealthy obese (MUO) counterparts.
As healthy dietary behaviors are crucial to reduce obesity-related morbidity and mortality,12 it is important to understand how the beneficial effects of established healthy dietary patterns such as Mediterranean diet differ according to MHO and MUO phenotypes. A few previous studies examined the potential differential association of dietary factors between MHO and MUO phenotypes; however, many of these studies were cross-sectional,13, 14, 15, 16 without consideration of dietary pattern,15 had small sample size17, 18 or with a short follow-up.17, 18, 19, 20 In addition, it is unclear how dietary patterns may influence the natural history of these phenotypes differentially.
Therefore, we tested the hypotheses that Mediterranean diet would have differential benefits in relation to mortality in MHO individuals and MUO individuals, in a nationally representative obese US population.
Methods
Study population
Data from 1739 adults aged 20–88 years were analyzed from participants of the National Health and Nutrition Examination Survey (NHANES) III, 1988–1994, followed up for deaths until 31 December 2011 in a prospective cohort analysis. In NHANES III, a complex multi-stage stratified clustered probability sampling scheme was applied to achieve a nationally representative sample of the civilian, non-institutionalized US population.
We included 2535 obese (body mass index (BMI)⩾30 kg m−2 based on measured height and weight) adults aged 20 years and older who were eligible for mortality follow-up and had complete data on food frequency questionnaire (FFQ) and 24-h dietary recall, those with complete data on cardiometabolic parameters including fasting glucose, insulin, blood pressure (BP), triglycerides, high-density lipoprotein cholesterol (HDL-C) and high-sensitivity C-reactive protein (hs-CRP). We excluded those who reported a history of myocardial infarction, stroke, congestive heart failure or cancer (other than skin cancer) (n=299). To minimize misclassification, we excluded the participants who reported changing their dietary patterns during the previous 12 months because of pre-existing obesity, high BP, high blood cholesterol, diabetes mellitus or general health (n=415). In addition, we excluded those who reported implausible extreme energy intakes (<1st and >99th percentiles of energy intake per day in adults), those with hs-CRP >10 mg l−1, BMI>60 kg m−2, or pregnant or lactating women (n=82). Finally, a total of 1739 individuals were analyzed.
Assessment of Mediterranean diet
Dietary intake was assessed using the FFQ and the 24-h dietary recall data that were validated by the Nutrition Methodology Working Group.21 Adherence to the Mediterranean diet was assessed using the scoring methodology developed by Panagiotakos et al.22, 23 In this methodology, scores 0–5 or 5–0 were assigned for the weekly consumption of food items assumed to be contributing to or against the Mediterranean dietary pattern, respectively (Supplementary Table S1). This Mediterranean Diet Score (MDS) has been shown to be highly associated with prevalent cardiometabolic diseases, 10-year CVD risk, and inflammation and coagulation markers, in addition to capturing inherent characteristics of Mediterranean dietary pattern.22, 23, 24 It also has been reported that the MDS used in the present study was correlated with widely used indexes of adherence to the Mediterranean diet such as MDS developed by Trichopoulou et al.1 (r=0.64) and MDS used in the PREDIMED study2 (r=0.53).25
The NHANES III FFQ was used to collect information on usual diet during the previous month, but did not include information on portion sizes. We calculated the MDS, assuming that the number of servings per week were equivalent to the number of times that a food item was consumed per week. Potatoes were excluded from our MDS assessment because preparation methods for potatoes in US are quite different from European countries.26 Light to moderate alcohol consumption, one of the Mediterranean diet components, is associated with a reduced risk of cardiovascular morbidity and mortality.27 As the benefit from alcohol consumption mainly comes from alcohol rather than the components of each type of drink,28 we used the summary measure to estimate the amount of alcohol consumed daily using the following assumption: 12.8 g for 12- oz beer, 11 g for 4-oz glass of wine and 14 g for an ounce of liquor based on the questionnaire provided. In addition, assessment of alcohol consumption was modified using gender-specific cutoffs.29 Olive oil consumption was not measured in the NHANES III FFQ. Thus, we approximated olive oil consumption by calculating the ratio of total monounsaturated fatty acids (MUFA) to total saturated fatty acids (SFA)30 using the 24-h dietary recall data, then dividing it into the six even intervals. The possible overall MDS ranged from 0 to 50, with higher values indicating greater adherence to the Mediterranean diet.
Assessment of metabolic health
Metabolic health was assessed using the metabolic parameters that were measured under quality control standards of Centers for Disease Control and Prevention. BMI was calculated as kg m−2; height was measured to the nearest 0.1cm and weight to the nearest 0.01 kg. BP was averaged over five separate measurements. Serum glucose was measured using a modified hexokinase enzymatic method. Serum insulin was measured using radioimmnuoassay (Pharmacia Diagnostics, Kalamazoo, MI, USA). HDL-C and triglycerides were measured using a Hitachi 704 analyzer (Boehringer-Mannheim Diagnostics, Indianapolis, IN, USA). Serum hs-CRP concentrations were measured by latex-enhanced nephlometry (Department of Laboratory Medicine, Immunology Division, University of Washington, Seattle, WA, USA). As NHANES III participants did not comply with fasting instruction strictly, 6-h fasting data were used to increase the sample size.31
Metabolic health was defined when the individual had fewer than two cardiometabolic abnormalities (systolic/diastolic BP⩾130/85 mm Hg or antihypertensive medication use, triglycerides⩾150 mg l−1 or on cholesterol-lowering medication, fasting glucose⩾100 mg dl−1 or antidiabetic medication use, homeostasis model assessment of insulin resistance (HOMA-IR=fasting glucose (mg dl−1) × fasting insulin (lU ml−1)/405)>the 90th percentile, hs-CRP>the 90th percentile, and HDL-C<40 mg dl−1 in men or<50 mg dl−1 in women or on cholesterol-lowering medication).32
Assessment of mortality
To identify mortality and cause of death, the National Center for Health Statistics linked information from all participants aged 20 years and older to the National Death Index to 31 December 2011. Therefore, for each participant, follow-up extended from the date of the examination to the date of death or 31 December 2011. The underlying cause listed on the death certificate was applied to determine cause of death that was identified using the underlying Cause of Death-113 groups (international classification of disease (ICD), 10th revision). Total mortality was defined as deaths with any underlying cause of death; CVD and cancer mortality had underlying cause of death codes of ICD-10 I00-I69 and C00-C97, respectively (http://www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes3_linkage.htm).
Assessment of covariates
Demographic variables included age, gender, race or ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American or others), educational attainment (<12 years, 12 years or >12 years of education), living with spouse, level of income based on poverty income ratio (PIR), which is the ratio of household income to the appropriate poverty threshold (low (PIR⩽1.3), middle (1.3<PIR⩽3.5) and high (PIR>3.5)). The potential risk factors for CVD included smoking status (never, former and current) and the presence of family history of CVD. Physical activity was classified based on the recommended levels of physical activity.33 A group with recommended physical activity was defined as those who had self-reported leisure time moderate activity (3⩽metabolic equivalents <6) of five or more times per week or leisure time vigorous activity (metabolic equivalents⩾6) three or more times per week; physically inactive group as those with no reported leisure time physical activity; a group with insufficient physical activity as those who did not meet the criteria for recommended levels of physical activity but not inactive.
Statistical analysis
The statistical analyses were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA), using the appropriate survey procedures to account for the complex sampling design and weights. For the subgroup analysis, domain option was applied in survey procedure to preserve appropriate subsample in the complex sampling design, and it utilized the entire samples to estimate the variance of subpopulations. Continuous variables were presented by mean (s.e.: standard error) and compared using linear regression analyses. Categorical variables were expressed by percentage with s.e. and were compared using Rao-Scott χ2 tests. P value of less than 0.05 was considered statistically significant.
We used Cox proportional hazards regression to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause, CVD and cancer mortality. The proportional hazards assumption of the Cox proportional hazards models was evaluated with log of negative log survival curves based on Kaplan–Meier estimates for MDS tertile group as well as categorical age, gender and race/ethnicity. In addition to crude HRs, we estimated age-, gender- and race/ethnicity-adjusted HRs as well as the multivariable-adjusted HRs including age, BMI and total energy intake as continuous covariates; gender, race/ethnicity, educational attainment, income, living with spouse, smoking status, level of physical activity, family history of CHD as categorical covariates, all of which were identified using the prior literature. Missing values in the covariates were added as a dummy variable in the multivariable models. To assess potential differential association of MDS by age (<65 vs ⩾65 years), sex, race/ethnicity, smoking (ever vs never smoked), conformity to recommended physical activity (yes vs no), BMI (<35 vs ⩾35) and the presence or absence of chronic disease including diabetes mellitus and hypertension, we also stratified our analysis and performed interaction tests using all-cause mortality as the outcome by including the cross-product interaction terms in the Cox proportional hazards models based on Satterthwaite adjusted F test. In addition, we performed sensitivity analyses with an alternative common definition of metabolic health as metabolic syndrome (defined when the individual had fewer than three cardiometabolic abnormalities (systolic/diastolic BP⩾130/85 mm Hg or antihypertensive medication use, triglycerides⩾150 mg l−1 or on cholesterol-lowering medication, fasting plasma glucose⩾100 mg dl−1 or antidiabetic medication use, HDL-C<40 mg dl−1 in men or<50 mg dl−1 in women or on cholesterol-lowering medication, and waist circumference⩾102 cm in men or⩾88 cm in women),34 and after exclusion of subjects who died during the first 5 years of follow-up.
Results
The MHO phenotype (n=598) was observed in 34.8% (s.e., 1.7%) of those who were obese. MHO individuals with the highest MDS tertile were more likely to have a higher income, lower insulin resistance and higher diastolic BP (Table 1). MUO individuals with the highest MDS tertile tended to be older and not non-Hispanic white; were more likely to have a higher education, higher income, lower proportion of current smokers and recommended physical activity, lower BMI and waist circumference, lower insulin resistance, higher systolic BP, lower triglycerides and higher HDL-C than MUO individuals with other tertiles. Comparing MHO and MUO individuals, MHO individuals tended to be younger, female, not non-Hispanic white and non-smokers, and have a more favorable metabolic status (Table 1).
In both phenotypes, consumption of grains, legumes, fruit, vegetable, fish, ratio of MUFA to SFA tended to increase with increasing tertile of MDS (Table 2). In addition, consumption of red meats and dairy products tended to decrease with increasing tertile of MDS. However, consumption of poultry and alcohol was higher with increasing tertile of MDS only in MUO individuals. Overall, MHO individuals showed higher ratio of MUFA to SFA (P=0.03), and consumed less red meats and dairy products (P=0.001 and 0.02, respectively), resulting in higher MDS for those components, compared with MUO individuals (Table 2). No significant difference in the ratio of polyunsaturated fatty acids to SFA was observed between MHO and MUO individuals (data not shown).
During a median follow-up of 18.5 years, there were 77 and 309 deaths in 598 MHO and 1141 MUO individuals, respectively. Overall, higher mortality risk was observed in MUO compared with MHO individuals after adjusting for potential confounders (HRs were 1.50 (95% CI, 1.12–2.00) for all-cause mortality, 2.50 (95% CI, 1.20–5.21) for CVD mortality and 1.27 (95% CI, 0.81–1.98) for cancer mortality). In the total obese participants, we did not observe a significant association between MDS and risk of all-cause, CVD and cancer mortality (Supplementary Table S2).
HRs of all-cause, CVD and cancer mortality by tertile categories and a five-point (1 s.d.) increment in the MDS in MHO and MUO individuals were shown in Table 3. In MHO individuals, compared with the lowest tertile, HRs in the second and the highest tertiles, respectively, were 0.35 (95% CI, 0.19–0.64) and 0.44 (0.26–0.75) (P for trend <0.001) for all-cause mortality, and 0.13 (0.06–0.26) and 0.23 (0.02–2.10) (P for trend =0.03) for cancer mortality, after multivariable adjustment. A five-point increment in the adherence to MDS was inversely associated with risk of all-cause mortality (HR, 0.59; 0.37–0.94). However, no mortality risk reduction was observed in MUO phenotypes with increasing MDS, and there was no association between MDS and CVD mortality in either group.
In stratified analyses for all-cause mortality with a five-point increase in MDS score, stronger inverse associations were observed in men and those meeting physical activity recommendations (P for interaction=0.002 and 0.008, respectively) (Table 4). In addition, the inverse association between MDS and all-cause mortality in MHO individuals was consistent among individuals with and without prevalent chronic disease including diabetes mellitus and hypertension. In MUO individuals, higher MDS tended to increase mortality risk for those who met physical activity recommendations and decrease mortality risk for those who did not meet physical activity recommendations (P for interaction=0.003).
When we excluded subjects who died in the first 5 years of follow-up, the overall results did not materially change (Supplementary Table S3). In addition, using an alternative definition of metabolic health as metabolic syndrome shows similar results in terms of the reduction of all-cause mortality only in MHO individuals (Supplementary Table S4).
Discussion
In this prospective analysis of a nationally representative sample of obese US adults, higher adherence to Mediterranean diet was associated with a lower risk of all-cause mortality in the MHO phenotype, after adjustment for potential confounders. We observed a 41% reduction in all-cause mortality with each five-point increment in the MDS among MHO individuals. This association persisted when we restricted our analyses to those with or without diabetes mellitus and hypertension. However, the inverse association between the MDS and mortality was not observed in MUO phenotype. To our knowledge, the present study is the first to evaluate a differential beneficial association of Mediterranean diet on mortality risk reduction in MHO and MUO phenotype.
Several epidemiologic studies have explored the association between Mediterranean diet and the risk of mortality in the obese population in subgroup analyses, showing inconsistent results. Trichopoulou et al.1 showed inverse association of MDS with all-cause mortality in Greek adults who had a BMI⩾28.06. In contrast, George et al.35 showed that there was a weak association of MDS with all-cause mortality and no association with CVD and cancer mortality in US postmenopausal women with BMI⩾30. Mitrou et al.36 also showed that the associations with all-cause mortality were only observed in ever-smoker in middle to older aged US adults with BMI⩾30. On the basis of the findings from these previous studies, the Mediterranean diet might not be a useful indicator in reducing the risk of mortality in obese population owing to the possibility of underreporting of food intake37 and physiological impact of obesity itself on mortality risk.38
However, these studies assumed that all obese individuals were at the same metabolic health and same risk of mortality. Previous studies have demonstrated that MHO individuals are not at increased risk of mortality and CVD compared with their MUO counterparts.39 The mechanisms explaining favorable cardiometabolic, hormonal and inflammatory profiles in MHO individuals remain largely unknown. However, several potential pathophysiologic mechanisms include differences in degree of gene marker expression reflecting adipose cell differentiation,40 the role of key genes in insulin-signaling pathways41 and amino acid homeostasis42 between MHO and MUO individuals.
Our results show that inverse associations of Mediterranean diet on risk of mortality were observed only in MHO individuals, which persisted in our sensitivity analyses. Underlying mechanisms for health benefits of Mediterranean diet are complex, but can be explained by the improvement of cardiometabolic profiles including insulin sensitivity, lipid profiles, BP, endothelial dysfunction, reactive oxidation and inflammatory markers.43 It has been reported that MHO individuals are at increased risk of unfavorable long-term outcomes compared with metabolically healthy normal weight individuals, even with few metabolic abnormalities.44 In addition, one-third of MHO phenotype can be converted to MUO phenotype within a decade because of unhealthy lifestyle.45 Thus, our results suggest that there could be a synergistic physiological mechanism to prevent adverse health outcomes, along with improving the favorable cardiometabolic profiles linking MHO phenotype and the adherence to Mediterranean diet. Furthermore, those who reported meeting physical activity recommendations had more benefits in mortality reduction from Mediterranean diet, representing the possible synergistic effects of healthy lifestyles.46, 47, 48
On the basis of our findings, MUO individuals might not be as responsive to diet because they are already metabolically overburdened.49 Cardiometabolic comorbidities might explain the lack of association between Mediterranean diet and the risk of mortality in MUO individuals. However, a stratified analysis with and without diabetes mellitus and hypertension showed similar results. Interestingly, MUO individuals meeting physical activity recommendations tended to have a higher risk of all-cause mortality, although the effect estimate was imprecise. Although we cannot completely rule out the possibility of reverse causality in this association, these results suggest that healthy lifestyle alone might not be adequate to reverse the deleterious prognosis of MUO individuals. Thus, the MUO individuals may need to be prioritized for intensive weight loss program along with healthy dietary habits to reduce obesity-related comorbidities.50 Furthermore, appropriate therapeutic approaches may be warranted to reduce the mortality risk of MUO individuals. 51
Several dietary intervention studies have explored whether MHO and MUO individuals had the same benefits from CVD risk reduction.18, 19, 52 However, their hypotheses were focused on the short-term effects of energy-restricted diet on change of cardiometabolic parameters in MHO and MUO phenotypes with inconsistent findings. Thus, the interpretation of these results might not be applicable to the present long-term prospective cohort study using a Mediterranean dietary pattern as an exposure. Therefore, more evidence would be necessary based on long-term follow-up studies evaluating the effects of healthy dietary pattern on mortality reduction, considering MHO and MUO phenotypes. 51
Strengths of our study include its prospective study design with nearly 18 years of follow-up for mortality based on the representative US population. In addition, data were collected based on extensive laboratory and physical examinations using standardized protocols to minimize the influence of measurement errors. Furthermore, we were able to replicate the findings using sensitivity analyses. However, our study has limitations. First, we used ‘times per week’ in assessing the consumption frequency instead of ‘servings per week’ in MDS calculation. This approach may cause exposure misclassification, but the direction would be non-differential. In addition, the MDS-scoring methodology used in the present study has not been validated in this population. Second, a ratio of total MUFA to total SFA may not be equal to olive oil consumption as one of MDS components, because the main sources of MUFA are different between the United States and Mediterranean countries. It has been reported that olive oil consumption contributed less than 10% of all monounsaturated fat intake from the Nurses’ Health Study conducted during the similar period as our study.30 In addition, our MDS scoring based on the US data may differ from that of Mediterranean countries owing to difference of dietary patterns and eating behaviors,30 which might contribute to inconsistent results when applied to Mediterranean countries. A previous meta-analysis also showed that the effect of Mediterranean diet on metabolic syndrome was more outstanding in Mediterranean countries than others.3 Third, because of a single measure of diet collected at baseline, we could not account for any changes in dietary intake over time. Also, it is possible that FFQ could not assess accurately individual usual intake, and that single 24-h dietary recalls might be subject to misclassification in a categorical approach to MDS tertiles, all of which may lead to bias in MDS measurements. Despite the limitations of reporting errors, however, it has been known that FFQ data are reproducible and adequately able to rank individuals with regard to food and nutrient intake.53, 54 Finally, there may be residual confounding due to measurement error of self-reported covariates.
In conclusion, our results suggest that higher adherence to Mediterranean diet was associated with a lower risk of all-cause mortality exclusively in the MHO phenotype, based on a nationally representative US adult population. The lack of a beneficial association between adherence to Mediterranean diet with the mortality reduction in MUO individuals may warrant the need of alternative strategies to reduce mortality risk in MUO individuals.
References
Trichopoulou A, Costacou T, Bamia C, Trichopoulos D . Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003; 348: 2599–2608.
Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013; 368: 1279–1290.
Kastorini C-M, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB . The effect of mediterranean diet on metabolic syndrome and its componentsa meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol 2011; 57: 1299–1313.
Sofi F, Cesari F, Abbate R, Gensini GF, Casini A . Adherence to Mediterranean diet and health status: meta-analysis. BMJ 2008; 337: a1344.
Kim CS, Ko SH, Kwon HS, Kim NH, Kim JH, Lim S et al. Prevalence, awareness, and management of obesity in Korea: data from the Korea national health and nutrition examination survey (1998-2011). Diabetes Metab J 2014; 38: 35–43.
Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH . The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009; 9: 88.
Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET . Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab 2004; 89: 2569–2575.
Kim HY, Kim CW, Lee CD, Choi JY, Park CH, Bae SH et al. Can "healthy" normal alanine aminotransferase levels identify the metabolically obese phenotype? Findings from the Korea national health and nutrition examination survey 2008-2010. Dig Dis Sci 2014; 59: 1330–1337.
Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011; 35: 971–981.
Lee SH, Han K, Yang HK, Kim MK, Yoon KH, Kwon HS et al. Identifying subgroups of obesity using the product of triglycerides and glucose: the Korea National Health and Nutrition Examination Survey, 2008-2010. Clin Endocrinol 2015; 82: 213–220.
Ortega FB, Lee DC, Katzmarzyk PT, Ruiz JR, Sui X, Church TS et al. The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur Heart J 2013; 34: 389–397.
Buckland G, Bach A, Serra-Majem L . Obesity and the Mediterranean diet: a systematic review of observational and intervention studies. Obes Rev 2008; 9: 582–593.
Camhi SM, Whitney Evans E, Hayman LL, Lichtenstein AH, Must A . Healthy eating index and metabolically healthy obesity in U.S. adolescents and adults. Prev Med 2015; 77: 23–27.
Phillips CM, Dillon C, Harrington JM, McCarthy VJ, Kearney PM, Fitzgerald AP et al. Defining metabolically healthy obesity: role of dietary and lifestyle factors. PLoS One 2013; 8: e76188.
Hankinson AL, Daviglus ML, Van Horn L, Chan Q, Brown I, Holmes E et al. Diet composition and activity level of at risk and metabolically healthy obese American adults. Obesity (Silver Spring) 2013; 21: 637–643.
Kimokoti RW, Judd SE, Shikany JM, Newby PK . Food intake does not differ between obese women who are metabolically healthy or abnormal. J Nutr 2014; 144: 2018–2026.
Dalzill C, Nigam A, Juneau M, Guilbeault V, Latour E, Mauriege P et al. Intensive lifestyle intervention improves cardiometabolic and exercise parameters in metabolically healthy obese and metabolically unhealthy obese individuals. Can J Cardiol 2014; 30: 434–440.
Karelis AD, Messier V, Brochu M, Rabasa-Lhoret R . Metabolically healthy but obese women: effect of an energy-restricted diet. Diabetologia 2008; 51: 1752–1754.
Liu RH, Wharton S, Sharma AM, Ardern CI, Kuk JL . Influence of a clinical lifestyle-based weight loss program on the metabolic risk profile of metabolically normal and abnormal obese adults. Obesity (Silver Spring) 2013; 21: 1533–1539.
Hankinson AL, Daviglus ML, Horn LV, Chan Q, Brown I, Holmes E et al. Diet composition and activity level of at risk and metabolically healthy obese American adults. Obesity 2013; 21: 637–643.
United States. National Center for Health Statistics. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–94. US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention: Hyattsville, MD, Washington, DC 1994.
Panagiotakos DB, Pitsavos C, Stefanadis C . Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis 2006; 16: 559–568.
Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C . Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med 2007; 44: 335–340.
Carter SJ, Roberts MB, Salter J, Eaton CB . Relationship between Mediterranean Diet Score and atherothrombotic risk: findings from the Third National Health and Nutrition Examination Survey (NHANES III), 1988-1994. Atherosclerosis 2010; 210: 630–636.
Mila-Villarroel R, Bach-Faig A, Puig J, Puchal A, Farran A, Serra-Majem L et al. Comparison and evaluation of the reliability of indexes of adherence to the Mediterranean diet. Public Health Nutr 2011; 14: 2338–2345.
Fung TT, McCullough ML, Newby P, Manson JE, Meigs JB, Rifai N et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2005; 82: 163–173.
Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA . Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 2011; 342: d671.
Rimm EB, Klatsky A, Grobbee D, Stampfer MJ . Review of moderate alcohol consumption and reduced risk of coronary heart disease: is the effect due to beer, wine, or spirits? BMJ 1996; 312: 731–736.
Liangpunsakul S, Crabb DW, Qi R . Relationship among alcohol intake, body fat, and physical activity: a population-based study. Ann Epidemiol 2010; 20: 670–675.
Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB . Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009; 119: 1093–1100.
Kuk JL, Ardern CI . Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care 2009; 32: 2297–2299.
Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med 2008; 168: 1617–1624.
Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007; 116: 1081–1093.
Hinnouho G-M, Czernichow S, Dugravot A, Batty GD, Kivimaki M, Singh-Manoux A . Metabolically healthy obesity and risk of mortality does the definition of metabolic health matter? Diabetes Care 2013; 36: 2294–2300.
George SM, Ballard-Barbash R, Manson JE, Reedy J, Shikany JM, Subar AF et al. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women's Health Initiative Observational Study: evidence to inform national dietary guidance. Am J Epidemiol 2014; 180: 616–625.
Mitrou PN, Kipnis V, Thiébaut AC, Reedy J, Subar AF, Wirfält E et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP Diet and Health Study. Arch Intern Med 2007; 167: 2461–2468.
Lissner L, Troiano RP, Midthune D, Heitmann BL, Kipnis V, Subar AF et al. OPEN about obesity: recovery biomarkers, dietary reporting errors and BMI. Int J Obes (Lond) 2007; 31: 956–961.
Adams KF, Leitzmann MF, Ballard-Barbash R, Albanes D, Harris TB, Hollenbeck A et al. Body mass and weight change in adults in relation to mortality risk. Am J Epidemiol 2014; 179: 135–144.
Hamer M, Stamatakis E . Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab 2012; 97: 2482–2488.
McLaughlin T, Deng A, Yee G, Lamendola C, Reaven G, Tsao PS et al. Inflammation in subcutaneous adipose tissue: relationship to adipose cell size. Diabetologia 2010; 53: 369–377.
MacLaren R, Cui W, Simard S, Cianflone K . Influence of obesity and insulin sensitivity on insulin signaling genes in human omental and subcutaneous adipose tissue. J Lipid Res 2008; 49: 308–323.
Badoud F, Lam KP, DiBattista A, Perreault M, Zulyniak MA, Cattrysse B et al. Serum and adipose tissue amino acid homeostasis in the metabolically healthy obese. J Proteome Res 2014; 13: 3455–3466.
Widmer RJ, Flammer AJ, Lerman LO, Lerman A . The Mediterranean diet, its components, and cardiovascular disease. Am J Med 2015; 128: 229–238.
Kramer CK, Zinman B, Retnakaran R . Are metabolically healthy overweight and obesity benign conditions? A systematic review and meta-analysis. Ann Intern Med 2013; 159: 758–769.
Soriguer F, Gutierrez-Repiso C, Rubio-Martin E, Garcia-Fuentes E, Almaraz MC, Colomo N et al. Metabolically healthy but obese, a matter of time? Findings from the prospective Pizarra study. J Clin Endocrinol Metab 2013; 98: 2318–2325.
Park YM, Sui X, Liu J, Zhou H, Kokkinos PF, Lavie CJ et al. The effect of cardiorespiratory fitness on age-related lipids and lipoproteins. J Am Coll Cardiol 2015; 65: 2091–2100.
Lee DH, Kim YM, Jekal Y, Park S, Kim KC, Naruse M et al. Low levels of physical activity are associated with increased metabolic syndrome risk factors in korean adults. Diabetes Metab J 2013; 37: 132–139.
Lee S, Kim Y . Effects of exercise alone on insulin sensitivity and glucose Tolerance in obese youth. Diabetes Metab J 2013; 37: 225–232.
Phillips CM . Metabolically healthy obesity: definitions, determinants and clinical implications. Rev Endocr Metab Disord 2013; 14: 219–227.
Fukushima Y, Kurose S, Shinno H, Cao Thu H, Takao N, Tsutsumi H et al. Importance of lean muscle maintenance to improve insulin resistance by body weight reduction in female patients with obesity. Diabetes Metab J 2016; 40: 147–153.
Plourde G, Karelis AD . Current issues in the identification and treatment of metabolically healthy but obese individuals. Nutr Metab Cardiovasc Dis 2014; 24: 455–459.
Janiszewski PM, Ross R . Effects of weight loss among metabolically healthy obese men and women. Diabetes Care 2010; 33: 1957–1959.
Freedman LS, Commins JM, Moler JE, Arab L, Baer DJ, Kipnis V et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am J Epidemiol 2014; 180: 172–188.
Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC . Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992; 135: 1114–1126, discussion 1127-36.
Acknowledgements
We are grateful to Dr Alexandra J White for critical review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Park, YM., Steck, S., Fung, T. et al. Mediterranean diet and mortality risk in metabolically healthy obese and metabolically unhealthy obese phenotypes. Int J Obes 40, 1541–1549 (2016). https://doi.org/10.1038/ijo.2016.114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.114
- Springer Nature Limited
This article is cited by
-
Schizophrenia patients with a metabolically abnormal obese phenotype have milder negative symptoms
BMC Psychiatry (2020)
-
Role of Paced Breathing for Treatment of Hypertension
Current Hypertension Reports (2017)